Abstract
Transcutaneous electrical nerve stimulation (TENS) reduces hyperalgesia and pain. Both low frequency (LF) and high frequency (HF) TENS, delivered at the same intensity (90% motor threshold (MT)) daily, result in analgesic tolerance with repeated use by the 5th day of treatment. Thecurrentstudytestedif 1) increasingintensityby 10% per daypreventsthedevelopmentoftolerance to repeated TENS, and 2) iflowerintensity TENS (50 % MT) produces an equivalentreduction in hyperalgesia when compared to 90% MT TENS. Sprague-Dawley rats with unilateral knee joint inflammation (3% carrageenan) were separated according to the intensity of TENS used: Sham, 50% LF, 50% HF, 90% LF, 90% HF, and increased intensity by 10% per day (LF and HF). The reduced mechanical withdrawal threshold following the induction of inflammation was reversed by application of TENS applied at 90% MT and increasing intensity for the first 4 days. On the 5th day, the groups that received 90% MT intensity showed tolerance. Nevertheless, the group that received an increased intensity on each day still showed a reversal of the mechanical withdrawal threshold with TENS. These results show that the development of tolerance can be delayed by increasing intensity of TENS.
Keywords: TENS, intensity, hyperalgesia, pain, opioid, frequency
Introduction
Transcutaneous electrical nerve stimulation (TENS) is used for pain control and is the application of electrical current through electrodes attached to the skin. TENS is a neurostimulation modality that was initially proposed based on the “gate-control”theory of pain.32 However, recent studies show that TENS produces its analgesic effects through release of endogenous opioids in the central nervous system.21, 44 Clinically, TENS can be delivered at different frequencies: low frequency (2-10Hz) or high-frequency (50-100Hz). High- and low-frequency TENS analgesia is mediated by different opioid receptors, with low-frequency TENS producing analgesia through μ-opioid receptors and high-frequency TENS producing analgesia through δ-opioid receptors.21, 44It is well-known that repeated opioid administration produces analgesic tolerance.7, 9, 25, 49 Similarly, repeated TENS administration, high or low frequency, also produces analgesic tolerance.3 This analgesic tolerance occurs at spinal μ- and δ-opioid receptors for low and high frequency TENS, respectively.3
Prevention or delaying tolerance to repeated TENS is clinically useful. Prior work from our laboratory shows pharmacological blockade of spinal receptors involved in opioid tolerance also block tolerance to TENS. Specifically, intrathecal blockade of N-methyl-D-aspartate (NMDA) receptors19 or of cholecystokinin (CCK) receptors13 prevents the development of opioid tolerance. However, non-pharmacological strategies may be more clinically useful for prevention of opioid tolerance. For example, we previously show that alternating frequencies between low and high during treatment delays the onset of tolerance by one week.10
Clinically, intensity of stimulation has recently been shown to play a critical role in TENS effectiveness with lower intensities of stimulation producing minimal analgesia and higher intensities producing greater analgesia.1, 5, 6, 33, 35, 39, 51 Our prior animal studies show TENS given at 90% motor threshold or just above motor threshold produces a strong analgesic effect in animals with joint inflammation.3,43 However, it is not clear if lower intensities would also produce this effect. Further subjects will continuously increase intensity throughout the duration of the stimulation. We propose that continuously increasing intensity of stimulation daily will prevent the development of analgesic tolerance and that lower intensity TENS will be ineffective. The current study tested if: 1) increasing intensity by 10% per day prevents the development of tolerance to repeated TENS, and 2) if lower intensity TENS produces an equivalent reduction in hyperalgesia when compared to 90% mechanical threshold TENS.
Material and methods
Animals
Male Sprague-Dawley rats (N=35), weighing 225–300 g, kept at 12 h dark—light cycle with free access to standard rat chow and water, were used for the experiments. All experiments were approved by University of Iowa Animal Care and Use Committee and were carried out according to the guidelines of the National Institutes of Health.
Behavioral Testing
Before the behavioral tests were applied, animals were acclimated to the testing room and procedures 2x per day for two days. Animals were acclimated to the testing room for thirty minutes after transport to the laboratory from the animal care facilities. They were then placed in transparent Lucite cubicles (24.6 × 7.5 × 7.5 cm3) on an elevated mesh platform for twenty minutes to acclimate (paw withdrawal threshold test). Animals were also acclimated in a gardener’s glove for five minutes (muscle withdrawal threshold test).
For the paw withdrawal thresholds mechanical stimuli with von Frey filaments were applied to the plantar area of the hind paw, as previously described.4, 13, 16, 44 Briefly, different bending forces were progressively applied perpendicularly to the plantar aspect of the hind paw. Each filament had one trial that consisted of two consecutive applications of the filament. The lowest bending force at which the rat withdrew its paw from one of the two applications was recorded as the paw withdrawal threshold for mechanical hyperalgesia. This testing method has shown significant statistical test-retest reliability.44
For muscle withdrawal thresholds, the animal was restrained in a gardener’s glove, the experimenter extended one hind limb, and the knee joint was compressed by using a pair of calibrated forceps.30, 42, 52 The tip of the modified forceps was used for compression. The contact area of the forceps is approximately 30 mm2. Compression was stopped when the animal withdrew the limb forcefully or when it vocalized. The maximum compression force applied at withdrawal was recorded as the baseline compression threshold in mN for the knee joint of the corresponding limb. Three trials spaced five minutes apart were averaged to obtain one reading at each time point.
Induction of Inflammation
After baseline behavioral measurements, rats were deeply anesthetized with 3-5% isoflurane for approximately five minutes and a solution of 3% carrageenan (0.1 ml, pH 7.4) in sterile saline was injected into the left knee joint to induce inflammation. Following intra-articular injection of carrageenan, the rats were returned to their cages and allowed to recover for 24 hours. Within 24 hours, the animals exhibited signs of inflammation such as; edematous, warm knee joints, and also behavioral signs such as; guarding and decreased weight bearing on the inflamed limb. We previously show that this model produces an acute inflammatory response that converts to a chronic inflammation by one week and lasts through 9 weeks.36 The mechanical hypersensitivity of the paw becomes maximal within 24h and lasts through 8 weeks.36 We previously used this model to examine tolerance to TENS over 2 weeks and showed stable hypersensitivity measures in the sham TENS group.10
Application of TENS
Active or sham TENS was administered to the inflamed knee joint under light isoflurane anesthesia (1-2% isoflurane) for twenty minutes, for ten days. Before the application of TENS, every day the animals were shaved and their skin was cleaned with 70% alcohol. Pre-gelled electrodes (3.02 cm in diameter modified down to approximately 1.2 cm in diameter) were placed on the medial and lateral aspects of the inflamed knee joint. Following twenty minutes of administration of TENS, rats were removed from anesthesia, the use of TENS discontinued and the pre-gelled electrodes removed.
There were three TENS treatment groups. 1) The sham TENS group was anesthetized and electrodes were placed on their shaved knee joint, but they did not receive TENS treatment (control group). 2) Low frequency TENS group (frequency 4 Hz), subdivided in three different intensities 50% MT, 90% MT and daily increasing intensity. 3) high frequency TENS group (100 Hz), subdivided in three different intensity 50% MT, 90% MT and daily increasing intensity. For the increasing intensity group, initial intensity started at 90% MT on Day 1 and was increased by 10% each day as follows: Day 1 - ‘90% of motor threshold’; Day 2 ‘90% of motor threshold + 10%’; Day 3 ‘(90% of motor threshold + 10%) +10%’; Day 4 ‘((90% of motor threshold + 10%) +10%) +10%’, and so on. Other treatment parameters were kept constant pulse-width (100 μs) and modulation (normal).3, 43 The intensity was determined by increasing the intensity until a visible motor contraction was elicited, and if the animal was of the subgroup with 50% MT, the intensity was decreased by half.
Experimental Design
Baseline paw and joint withdrawal thresholds bilaterally before and 24h after induction of knee joint inflammation. Rats were then lightly anesthetized with 1% to 2% isoflurane for placement of the TENS electrodes and application of TENS for twenty minutes. Withdrawal thresholds were reassessed immediately after TENS and recovery from the anesthesia (10–15min after removal of TENS). The same procedure (assessment of withdrawal thresholds, TENS application, and reassessment of withdrawal thresholds) was done on each day for ten days. Importantly, 3 rats always were anesthetized with the same vaporizer; at least 1 rat receiving the sham TENS treatment and 1 rat receiving the active TENS treatment were anesthetized at the same time. This procedure ensured that there always were animals in the sham TENS treatment group that received the same dose of anesthesia. Further the tester was blinded to the experimental group. A separate study participant applied TENS.
Data analysis
Differences between groups (different frequencies) and subgroups (different intensities) of treatment were tested with a two-way ANOVA for dependent samples across time (before inflammation, before TENS, and after TENS, on each day of treatment). Parametric paired T-test was used to analyze changes in mechanical withdrawal threshold of the hind paw and the calibrated forceps (knee joint withdrawal threshold) at each time point (before and after TENS stimulation on the same day). Post-hoc testing between groups was performed with a Tukey’s test. P value <0.05 was considered significant.
Results
Twenty-four hours following injection of carrageenan into the knee joint, the paw and muscle withdrawal threshold to mechanical stimuli were reduced ipsilaterally in all groups and subgroups. There were no significant differences between groups after induction of inflammation (Figure 2A, 2B, 3A and 3B).
Figure 2.
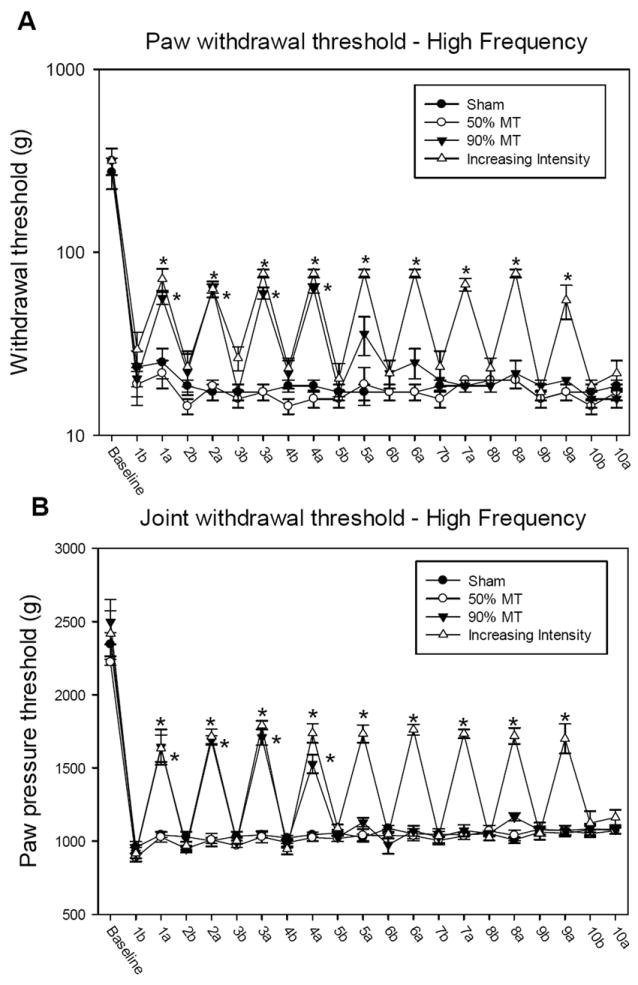
Graph representing mechanical withdrawal threshold of the paw (A) and knee joint (B) in animals that received high frequency TENS. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (baseline), before (b), and after application of TENS (a). Data are represented as means ± SEM. *Significantly different between before and after TENS stimulation (p<0.005).
Figure 3.
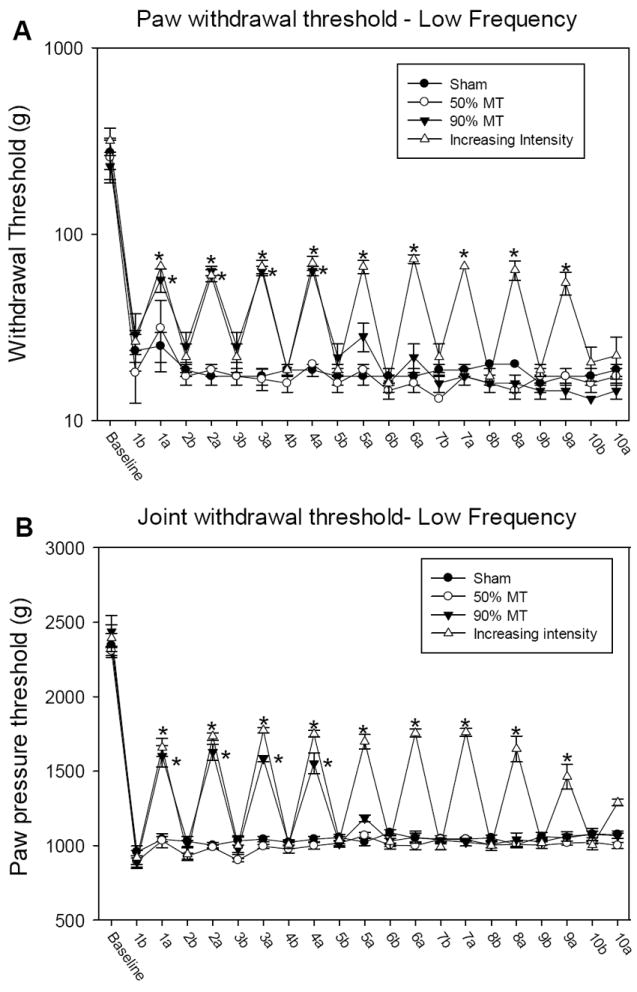
Graph representing mechanical withdrawal threshold of the paw (A) and knee joint (B) in animals that received low frequency TENS. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (baseline), before (b), and after application of TENS (a). Data are represented as means ± SEM. *Significantly different between before and after TENS stimulation (p<0.005).
Daily intensity of TENS for each group is summarized in Figure 1. The intensity of TENS remained constant for all days applied for the 50% MT and 90% MT intensities. Further, there is a daily increase in intensity in the group where intensity was increased by 10% each day. The groups were significantly different from each other with increasing intensity > 90% MT > 50% MT > sham (p<0.001).
Figure 1.
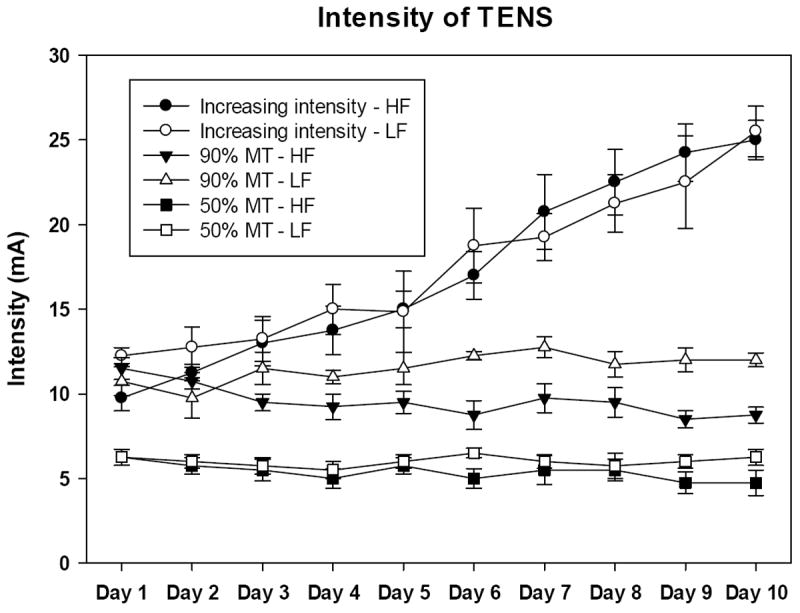
Graph representing the daily intensity of TENS from animals receiving low and high frequency at different intensities. Data are represented as means ± SEM.
The sham TENS group and high frequency TENS group with 50% MT intensity showed no change in the reduced paw withdrawal thresholds in all days analyzed. However, the 90% MT (p=0.003 and p=0.002) and increasing intensity (p<0.001 for both) groups were significantly greater than the sham and 50% MT groups (Figure 4A). Similarly, for joint withdrawal thresholds the90% MT and increasing intensity TENS were significantly higher than sham (p=0.0001 and p=0.001) and 50% MT intensity TENS (p=0.0001 and p=0.0001) (Figure 4B).
Figure 4.
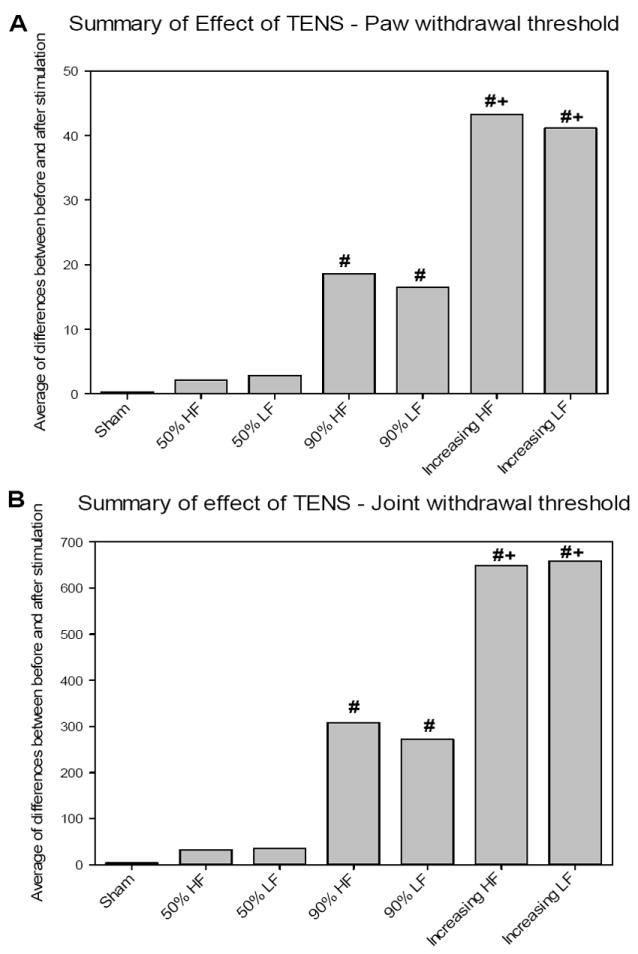
Graph representing the summary of effect of TENS in mechanical withdrawal threshold of the paw (A) and knee joint (B) from animals receiving Low and High Frequency of TENS treatment. #Significantly different from Sham and 50% of MT (p<0.005).+Significantly different from 90% of MT (p<0.005).
The decreased paw withdrawal threshold following induction of inflammation was reversed by application of TENS on the first four days in both the high and low frequency group with 90% MT intensity, as well as the group with increasing intensity TENS. Nevertheless, after four days of TENS application, in the groups that received 90% MT intensity, TENS was ineffective and thus there was a development of analgesic tolerance. (Figure 2A, 2B, 3A and 3B) The low and high frequency TENS groups that received TENS with a 10% increase in intensity daily showed a significant increase in mechanical withdrawal threshold of the paw and muscle after TENS session, until ninth day (p<0.001). As shown in Figures 2A, 2B, 3A and 3B, on tenth day there was no difference between before and after TENS application (p<0.001).
Discussion
The current study shows that we can delay the onset of tolerance to repeated application of TENS by continuously increasing the intensity of stimulation. We further show that low intensities of stimulation, 50% MT, have no analgesic effect. The current study shows the same pattern of analgesia and development of tolerance to repeated application of TENS delivered at the same intensity each day as we have shown previously.3, 10, 12, 19 Our prior studies were able to show prevention of tolerance with both pharmacological and non-pharmacological approaches. Specifically, we show that blockade of NMDA glutamate or cholecystokinin receptors during TENS treatment prevents the onset of tolerance after repeated TENS.13, 19 We further showed that alternating frequency of stimulation in the same session, or between sessions, also delays the onset of tolerance to repeated application of TENS.10 It may be that combining alternating or mixed frequencies of TENS with increasing intensity will delay the onset of tolerance even farther. As patients routinely use TENS on a daily basis, and clinical trials generally give just one frequency of stimulation, it will become increasingly important to find effective strategies to alleviate tolerance. Having multiple strategies for prevention of tolerance available to clinicians is critically important for maintaining long-term efficacy in patient populations.
Development of Tolerance
Clinically, understanding if TENS produces tolerance and if there is cross tolerance between TENS and pharmacological treatments is important for effective delivery. For example, in human subjects we previously show that repeated daily application of TENS delivered at the same intensity each day produces analgesic tolerance by the fifth day, thus, confirming and validating animal data.29 Increasing intensity each day could be one way to overcome the development of tolerance to repeated TENS, as we show in the current pre-clinical study. Similar to animal studies, humans subjects with chronic pain that were opioid tolerant showed a reduced effectiveness of low frequency TENS, but not high frequency TENS.26 This data again confirms and validates the prior animal studies that show low frequency TENS, but not high frequency TENS, is ineffective in animals with morphine tolerance.45 We suggest understanding the mechanisms of opioid tolerance and how to alleviate opioid tolerance will not only assist in the delivery of opioid pharmaceutical agents, but will also prove useful for the effective delivery of TENS.
The mechanisms of analgesic tolerance are not completely understood, and a number of neurotransmitters and receptors have been described.14, 23, 28 Prior studies in our laboratory, show that TENS produces effects by activation of PAG, RVM and spinal cord using opioid receptors in these central sites 11, 21, 44 and others show peripheral activation of opioid receptors in low frequency TENS analgesia.41 We also previously show that tolerance to low frequency and high frequency TENS occurs at central opioid receptors in the spinal cord with low-frequency TENS showing cross tolerance to μ-opioid receptor agonists and high frequency TENS showing tolerance to δ-opioid receptor agonists.3 We further show a lack of cross-tolerance between frequencies, between low frequency TENS and δ-opioid agonists, and between high frequency TENS and m-opioid agonists.3 Similarly, there is a lack of cross-tolerance between spinally administered μ- (morphine) and δ-opioid (D-Ala2-D-Leu5 enkephalin) agonists suggesting that the tolerance produced at μ- and δ-opioid receptors occur independent of each other.22,46 Thus, tolerance to low-frequency TENS occurs at spinal μ-opioid receptors and that to high frequency TENS occurs at spinal δ-opioid receptors; preventing tolerance prevents the cross-tolerance in the spinal cord to opioid agonists.
Intensity of stimulation is critical
The current study also shows that a low intensity, 50% MT, has no analgesic effect when compared to a sham TENS while 90% MT intensity produces a full reversal of hyperalgesia. This suggests there is a dose (intensity)-dependent effect for application of TENS, that TENS must be given at an adequate intensity to reduce pain intensity.1, 33, 38, 39 Recently, we demonstrated a dose-dependent effect of TENS in healthy human subjects similar to the current study. Specifically, sub-sensory and sensory threshold TENS have no significant analgesic effect while a strong but comfortable intensity has a significant analgesic effect.33 We also showed previously that increasing intensity every five minutes within a single session of TENS has a greater analgesic effect than the initial strong but comfortable sensation.29 Similarly, Bjordal and colleagues performed a systematic review of the literature and showed that when TENS is given at adequate intensities to people with postoperative pain or osteoarthritic it is more effective, and when given at inadequate intensities it is ineffective.1, 2
TENS is a sensory modality that acts directly on the nervous system by activating Aβ primary afferent fibers that subsequently leads to a reduction in central nociceptive cell activity.15, 31 Both low and high frequency TENS at sensory intensity clearly activate Aβ primary afferent fibers. We expect that increasing the intensity increases the number of primary afferent fibers activated. This larger number of afferent fibers activated on each day could result in a greater release of opioids. This is supported by work by Janko and colleagues that show, using microneurography of peripheral nerve activity during TENS, that progressively increasing the intensity increased the amplitude of nerve activity.20 Higher intensities might also activate Aδ primary afferent fibers since Aδ primary afferent fibers are activated at intensities of at least twice motor threshold. Our increasing intensity group reached this level between days 8 and 10 and thus we could have activated additional analgesic systems that are primarily activated by noxious stimulation.37 In human subjects, intensities up to 20 mA activate large Aβ afferent fibers and some small nociceptive Aδ afferent fibers as determined with microneurography.20 Further increasing intensity to produce a motor contraction activates Aδ nociceptors. 27,34 Activation of nociceptive afferent fibers could further increase activation of the PAG-RVM pathway as well as activate diffuse noxious inhibitory control (DNIC) pathways.17, 18, 50 DNIC is an alternate opioid-mediated analgesia pathway activated in response to noxious stimulation.8 Thus, the increasing intensity group likely increased the number of large diameter fibers activated and also activated Aδ afferent fibers in sufficient quantities to bring in other analgesic systems.
It is possible that different activation patterns occur after tissue inflammation, and that this changes across the time depending on the severity of inflammation and swelling around the joint, and if the inflammation is acute or chronic. In support, higher electroacupuncture intensities (e.g. 6 V or 20–30 mA) are required to induce effective analgesia in uninjured animals 40 when compared to electroacupuncture intensities in inflamed animals (i.e. 3 mA).24 Current may be transported to deeper tissues easier with more fluid around the joint, i.e. in the early phases of inflammation, and less well in animals with lower levels of inflammation in the chronic phases or in uninjured animals. Alternatively, enhanced excitability may occur as a result of increased excitability in the pain modulatory circuitry, either spinal or supraspinal.12, 47, 48
In summary, the findings presented in this novel study show that increasing intensity of TENS daily by just 10% can delay the onset of analgesic tolerance to repeated treatment. We further show that lower intensities of stimulation are ineffective. Future studies should confirm strategies to prevent tolerance in human subjects and use adequate intensities to test effectiveness. Similarly, clinicians should be aware of the potential development of analgesic tolerance to repeated use of TENS, and simple non-pharmacological strategies aimed at manipulating TENS parameters that can be employed to prevent this analgesic tolerance could be employed.
Perspective.
Our results showed that increasing intensity in both frequencies of TENS was able to prevent analgesic tolerance. Results from this study suggest that increasing intensities could be a clinical method to prevent analgesic tolerance and contribute to the effective use of TENS in reducing inflammatory pain and future clinical trials.
Acknowledgments
This study was supported by AR052316, AR061371 and the Carver College of Medicine at the University of Iowa. Dr. Sluka serves as a consultant for DJO, Inc.
Footnotes
Disclosures:
TENS units were donated by DJO, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7(2):181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 2.Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51. doi: 10.1186/1471-2474-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102(1-2):195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 4.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 5.Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain. 2002;99(1-2):253–262. doi: 10.1016/s0304-3959(02)00118-5. [DOI] [PubMed] [Google Scholar]
- 6.Chesterton LS, Foster NE, Wright CC, Baxter GD, Barlas P. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain. 2003;106(1-2):73–80. doi: 10.1016/s0304-3959(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 7.Contet C, Filliol D, Matifas A, Kieffer BL. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54(3):475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 8.de Resende MA, Silva LF, Sato K, Arendt-Nielsen L, Sluka KA. Blockade of opioid receptors in the medullary reticularis nucleus dorsalis, but not the rostral ventromedial medulla, prevents analgesia produced by Diffuse Noxious Inhibitory Control in rats with muscle inflammation. J Pain. 2011;12(6):687–697. doi: 10.1016/j.jpain.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLander GE, Portoghese PS, Takemori AE. Role of spinal mu opioid receptors in the development of morphine tolerance and dependence. J Pharmacol Exp Ther. 1984;231(1):91–96. [PubMed] [Google Scholar]
- 10.Desantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89(4):754–760. doi: 10.1016/j.apmr.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep. 2008;12(5):338–343. doi: 10.1007/s11916-008-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10(6):492–499. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSantana JM, da Silva LF, Sluka KA. Cholecystokinin receptors mediate tolerance to the analgesic effect of TENS in arthritic rats. Pain. 2010;148(1):84–93. doi: 10.1016/j.pain.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairbanks CA, Wilcox GL. Spinal plasticity of acute opioid tolerance. J Biomed Sci. 2000;7(3):200–212. doi: 10.1007/BF02255467. [DOI] [PubMed] [Google Scholar]
- 15.Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS) Pain. 1994;58(3):309–315. doi: 10.1016/0304-3959(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 16.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81(7):984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 17.Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26(1):126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004;110(1-2):419–426. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Hingne PM, Sluka KA. Blockade of NMDA receptors prevents analgesic tolerance to repeated transcutaneous electrical nerve stimulation (TENS) in rats. J Pain. 2008;9(3):217–225. doi: 10.1016/j.jpain.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janko M, Trontelj JV. Transcutaneous electrical nerve stimulation: a microneurographic and perceptual study. Pain. 1980;9(2):219–230. doi: 10.1016/0304-3959(80)90009-3. [DOI] [PubMed] [Google Scholar]
- 21.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298(1):257–263. [PubMed] [Google Scholar]
- 22.Kalso EA, Sullivan AF, McQuay HJ, Dickenson AH, Roques BP. Cross-tolerance between mu opioid and alpha-2 adrenergic receptors, but not between mu and delta opioid receptors in the spinal cord of the rat. J Pharmacol Exp Ther. 1993;265(2):551–558. [PubMed] [Google Scholar]
- 23.Kissin I, Brown PT, Robinson CA, Bradley EL., Jr Acute tolerance in morphine analgesia: continuous infusion and single injection in rats. Anesthesiology. 1991;74(1):166–171. doi: 10.1097/00000542-199101000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Lao L, Zhang RX, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020(1-2):18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- 25.Lee CY, Perez FM, Wang W, Guan X, Zhao X, Fisher JL, Guan Y, Sweitzer SM, Raja SN, Tao YX. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain. 2011;15(7):669–675. doi: 10.1016/j.ejpain.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain. 2011;12(2):213–221. doi: 10.1016/j.jpain.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Levin MF, Hui-Chan C. Are H and stretch reflexes in hemiparesis reproducible and correlated with spasticity? J Neurol. 1993;240(2):63–71. doi: 10.1007/BF00858718. [DOI] [PubMed] [Google Scholar]
- 28.Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121(3):232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Liebano RE, Rakel B, Vance CG, Walsh DM, Sluka KA. An investigation of the development of analgesic tolerance to TENS in humans. Pain. 2011;152(2):335–342. doi: 10.1016/j.pain.2010.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luis-Delgado OE, Barrot M, Rodeau JL, Schott G, Benbouzid M, Poisbeau P, Freund-Mercier MJ, Lasbennes F. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J Pain. 2006;7(1):32–39. doi: 10.1016/j.jpain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137(1):94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- 32.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 33.Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum-Gardner E, Johnson MI, Rakel BA, Sluka KA, Walsh DM. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12(8):929–935. doi: 10.1016/j.jpain.2011.02.352. [DOI] [PubMed] [Google Scholar]
- 34.Nardone A, Schieppati M. Influences of transcutaneous electrical stimulation of cutaneous and mixed nerves on subcortical and cortical somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1989;74(1):24–35. doi: 10.1016/0168-5597(89)90048-8. [DOI] [PubMed] [Google Scholar]
- 35.Olsen MF, Elden H, Janson ED, Lilja H, Stener-Victorin E. A comparison of high- versus low-intensity, high-frequency transcutaneous electric nerve stimulation for painful postpartum uterine contractions. Acta Obstet Gynecol Scand. 2007;86(3):310–314. doi: 10.1080/00016340601040928. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 104. 2003;3:567–77. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. J Pain. 2005;6(10):673–680. doi: 10.1016/j.jpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4(8):455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 39.Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, Miller CA, Polehna AC, Ruggle RC, Vance CG, Walsh DM, Sluka KA. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11(3):230–238. doi: 10.1016/j.jpain.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romita VV, Suk A, Henry JL. Parametric studies on electroacupuncture-like stimulation in a rat model: effects of intensity, frequency, and duration of stimulation on evoked antinociception. Brain Res Bull. 1997;42(4):289–296. doi: 10.1016/s0361-9230(96)00264-x. [DOI] [PubMed] [Google Scholar]
- 41.Sabino GS, Santos CM, Francischi JN, de Resende MA. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J Pain. 2008;9(2):157–163. doi: 10.1016/j.jpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain. 2005;6(1):41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77(1):97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 44.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289(2):840–846. [PubMed] [Google Scholar]
- 45.Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain. 2000;4(2):185–193. doi: 10.1053/eujp.2000.0172. [DOI] [PubMed] [Google Scholar]
- 46.Stevens CW, Yaksh TL. Studies of morphine and D-ala2-D-leu5-enkephalin (DADLE) cross-tolerance after continuous intrathecal infusion in the rat. Anesthesiology. 1992;76(4):596–603. doi: 10.1097/00000542-199204000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11(9):1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- 48.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136(3):331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tortorici V, Morgan MM, Vanegas H. Tolerance to repeated microinjection of morphine into the periaqueductal gray is associated with changes in the behavior of off- and on-cells in the rostral ventromedial medulla of rats. Pain. 2001;89(2-3):237–244. doi: 10.1016/s0304-3959(00)00367-5. [DOI] [PubMed] [Google Scholar]
- 50.Villanueva L. Diffuse Noxious Inhibitory Control (DNIC) as a tool for exploring dysfunction of endogenous pain modulatory systems. Pain. 2009;143(3):161–162. doi: 10.1016/j.pain.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Tang J, White PF, Naruse R, Sloninsky A, Kariger R, Gold J, Wender RH. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth Analg. 1997;85(2):406–413. doi: 10.1097/00000539-199708000-00029. [DOI] [PubMed] [Google Scholar]
- 52.Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods. 2002;115(1):107–113. doi: 10.1016/s0165-0270(02)00011-0. [DOI] [PubMed] [Google Scholar]


