The paper supports the view that ethylene plays a significant role in maintaining tomato pollen thermotolerance. Interfering with the ethylene signalling pathway or reducing ethylene levels and increased tomato pollen sensitivity to heat stress. On the other hand, increasing ethylene levels before heat-stress improved pollen quality.
Abstract
Background and aims
Exposure to higher-than-optimal temperatures reduces crop yield and quality, mainly due to sensitivity of developing pollen grains. The mechanisms maintaining high pollen quality under heat-stress conditions are poorly understood. Our recently published data indicate high heat-stress-induced expression of ethylene-responsive genes in tomato pollen, indicating ethylene involvement in the pollen heat-stress response. Here we elucidated ethylene's involvement in pollen heat-stress response and thermotolerance by assessing the effects of interfering with the ethylene signalling pathway and altering ethylene levels on tomato pollen functioning under heat stress.
Methodology
Plants of the ethylene-insensitive mutant Never ripe (Nr)—defective in an ethylene response sensor (ERS)-like ethylene receptor—and the corresponding wild type were exposed to control or heat-stress growing conditions, and pollen quality was determined. Starch and carbohydrates were measured in isolated pollen grains from these plants. The effect of pretreating cv. Micro-Tom tomato plants, prior to heat-stress exposure, with an ethylene releaser or inhibitor of ethylene biosynthesis on pollen quality was assessed.
Principal results
Never ripe pollen grains exhibited higher heat-stress sensitivity, manifested by a significant reduction in the total number of pollen grains, reduction in the number of viable pollen and elevation of the number of non-viable pollen, compared with wild-type plants. Mature Nr pollen grains accumulated only 40 % of the sucrose level accumulated by the wild type. Pretreatment of tomato plants with an ethylene releaser increased pollen quality under heat stress, with an over 5-fold increase in the number of germinating pollen grains per flower. Pretreatment with an ethylene biosynthesis inhibitor reduced the number of germinating pollen grains following heat-stress exposure over 5-fold compared with non-treated controls.
Conclusions
Ethylene plays a significant role in tomato pollen thermotolerance. Interfering with the ethylene signalling pathway or reducing ethylene levels increased tomato pollen sensitivity to heat stress, whereas increasing ethylene levels prior to heat-stress exposure increased pollen quality.
Introduction
Exposure to higher-than-optimal temperatures reduces the yield and quality of many crops, including cereals, grain legumes and vegetable crops (Kinet and Peet 1997; Maestri et al. 2002; Prasad et al. 2003, 2006). This problem is crucial in tomato because, to avoid insect-transmitted virus infections, a significant proportion of production is achieved in greenhouses, where the daily mean temperatures are high, especially during the warmer seasons. Short waves of high temperature may also be detrimental. We have shown that in tomato, developing pollen grains are most sensitive to both mild chronic and short-term acute heat-stress (HS) conditions (Firon et al. 2006; Pressman et al. 2006): heat-tolerant tomato genotypes exhibiting higher yield under HS produced larger numbers of high-quality pollen grains under these conditions compared with all tested heat-sensitive genotypes (Firon et al. 2006). However, the physiological and molecular bases for pollen grains' heat-stress response (HSR) and heat tolerance (thermotolerance) are poorly understood (Pressman et al. 2008; Zinn et al. 2010).
Recently, using two high-throughput transcriptomic approaches—cDNA amplified fragment length polymorphism and the Affymetrix GeneChip® (Affymetrix Inc., Santa Clara, CA, USA) Tomato Genome Array—we identified genes that are associated with pollen HSR and may contribute to pollen thermotolerance (Frank et al. 2009). Contradicting the previous notion that pollen is unable to mount a ‘significant’ HSR, our results (corroborated by reverse transcriptase–polymerase chain reaction and immunoblot analyses) revealed high HS regulation of 11 members of the small heat-shock protein (HSP) gene family, HSP70 and HSP90 family members, the HS transcription factors A2 (HSFA2) and HSFA3, the reactive oxygen species (ROS) scavenger ascorbate peroxidase (APX) and factors other than the classical HS-responsive genes. Remarkably, our recently published data indicated HS induction of several ethylene-responsive genes in developing tomato pollen grains, including ethylene-responsive gene 5 (ER5), ER21, LeJERF1 and ER24, as well as the gene encoding 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (exhibiting 4-, 63-, 9-, 156- and 6-fold induction following HS, respectively; Frank et al. 2009), pointing to the involvement of ethylene in pollen HSR.
To the best of our knowledge, data on the involvement of ethylene in pollen development and pollen HSR are scarce (De la Torre et al. 2006). The involvement of ethylene in petunia anther and pollen development was recently demonstrated (Kovaleva et al. 2010), suggesting the need for tight control of ethylene levels at the different developmental stages. Ethylene was also recently implicated as one of the factors involved in in-vitro maturation of Nicotiana tabacum pollen (Chibi and Mattilla 2010).
With respect to vegetative tissues, evidence for the involvement of ethylene in plant thermotolerance has been reported in numerous studies. For example, pretreatment of a cool-season grass (Agrostis stolonifera var. palustris) with ACC (a precursor of ethylene) prior to the exposure of plants to HS increased their heat tolerance (Larkindale and Huang 2004). Similarly, exogenous addition of ACC was shown to protect Arabidopsis against heat-induced oxidative damage (Larkindale and Knight 2002). In addition, Larkindale et al. (2005) showed that the Arabidopsis ethylene-signalling mutants ein2 and etr1 are defective in basal thermotolerance. Suzuki et al. (2008) suggested that a member of the transcriptional coactivator multiprotein bridging factor 1 (MBF1) gene family in Arabidopsis—MBF1c—may act as a regulator of thermotolerance upstream of salicylic acid and ethylene (Suzuki et al. 2008). Testing the effect of the ethylene biosynthesis and signalling inhibitors aminoethoxyvinylglycine (AVG) and silver thiosulfate, respectively, on heat-stressed wild-type and transgenic plants over-expressing (OE) MBF1c demonstrated these inhibitors' suppression of HS tolerance in the MBF1c OE plants (Suzuki et al. 2005). The authors suggested that MBF1c expression enhances the tolerance of transgenic plants to heat by perturbing or partially activating the ethylene-response signal transduction pathway (Suzuki et al. 2008).
The objective of the present study was to determine whether ethylene has a role in tomato pollen thermotolerance. The effects of interfering with the ethylene signalling pathway (using plants of a tomato mutant defective in the ethylene receptor, Never ripe (Nr); Klee 2004, and references therein), and altering ethylene levels (by pretreating plants with an ethylene releaser or an inhibitor of ethylene biosynthesis), on tomato pollen quality under HS conditions were tested. Results pointed to ethylene's involvement in increasing pollen quality under HS.
Materials and methods
Plant material, growth conditions and HS application
The following tomato (Solanum lycopersicum) lines were used: Nr, a tomato mutant defective in the ethylene receptor; a semidominant ethylene receptor mutant (Klee 2004, and references therein) and its background line Pearson (a gift from Professor H. Klee, University of Florida, USA); and the tomato cultivar Micro-Tom (Meissner et al. 1997), which is small, has a short life cycle and produces a relatively high number of flowers. Plants were grown in two temperature-controlled greenhouses at The Volcani Center in Bet Dagan, Israel, under natural light conditions (day length of 13.5–14 h) and day/night temperatures of 28/22 ± 2 °C (designated control conditions) for 2 months. These environmental conditions have been found to ensure high pollen quality (Pressman et al. 2002). Then one of the greenhouses, containing Nr and Pearson plants, was set to day/night temperatures of 32/26 ± 2 °C (designated mild chronic HS conditions; MCHS) and the plants were kept, in both greenhouses, for three additional months, with continuous production of flowers and fruits. Mature pollen grains were sampled from plants growing under the two temperature regimes after at least 14 days of exposure to MCHS, to determine the number of viable (stained purple) and non-viable (stained green) pollen grains, using Alexander dye (Alexander 1980). Data were collected during four summer seasons (2008–2011).
Micro-Tom plants grown at day/night temperatures of 28/22 ± 2 °C were exposed to short-term HS conditions (STHS; 50 °C for 2 h) at specific flower developmental stages (detailed below). During the heat treatment, to avoid drought stress and wilting, plants were kept in a humid environment. Short-term HS was applied to flower buds 3 and 0 days before flower opening, corresponding to developmental stages A-3 (early binucleate stage) and A (anthesis) (Sawhney and Bhadula 1988; Pressman et al. 2002). Following calibration of effective concentrations, 1 μL L−1 (1 p.p.m.) ethephon (2-chloroethyl phosphonic acid), an ethylene-releasing compound, or 0.3 mM AVG, was applied to plants containing flower buds 4 days (between vacuolated and early binucleate stage of microspore development) and 1 day before flower opening, by soaking the plants in the respective solution for 18 or 4 h, respectively, before exposure to STHS. In the control treatment, plants were treated with an equal amount of water. Each treatment consisted of at least six replicates.
To determine whether tomato pollen has the capacity for acquired thermotolerance (ATT), plants bearing inflorescences with flowers at different developmental stages were exposed for 1 h to 32 °C, followed by 1 h recovery at 25 °C, prior to exposing them to STHS. To determine pollen quality, microspores were allowed to mature and pollen grains were collected at anthesis from the HS and control plants. Total number, and the number of viable, non-viable and germinating pollen grains, were determined as described by Pressman et al. (1998) and detailed below.
Determination of pollen quality/performance
At least six flowers at first day of anthesis were sampled from each treatment. One anther was removed from each flower and placed in a microfuge tube containing 0.5 mL of germination solution consisting of 100 g L−1 sucrose, 2 mM boric acid, 2 mM calcium nitrate, 2 mM magnesium sulfate and 1 mM potassium nitrate, and the tubes were shaken well to release the pollen grains. The tubes were placed in an incubator at 25 °C for 4 h, after which a drop of Alexander dye was added to the solution. This method enables the number of germinating grains to be recorded while simultaneously analysing the number of non-viable (stained green) and viable (stained purple) pollen grains. Drops of the solution were mounted in a haemocytometer and the grains were counted with a light microscope, eight fields for each sample. This procedure was repeated three times and average results were calculated.
Isolation of pollen grains for carbohydrate analysis
Anthers collected from at least 50 flowers were dissected according to Aouali et al. (2001). Pollen grains were obtained by slicing the anthers transversely and vortexing them in cold, sucrose-free germination solution. The solution was then filtered through cheesecloth to remove the anther walls, and pollen grains were separated by centrifugation for 10 min at 8000 g. The released pollen grains were immediately suspended in 80 % ethanol at 75 °C for 30 min. The carbohydrate concentrations in pollen samples were analysed according to Hubbard et al. (1990) and Stoop and Pharr (1994). Freeze-dried or ethanol-suspended samples were extracted three times in hot 80 % (v/v) ethanol. The supernatant was dried in vacuo at 40 °C and resolubilized in water. Soluble sugars were determined by HPLC with a Fast Carbohydrate column (Bio-Rad, Hercules, CA, USA) operated at 85 °C with deionized, degassed water as eluent (Stoop and Pharr 1994) and detection by a refractometer. The insoluble residue that remained after ethanolic extraction was resuspended in 2 mL of 30 mM HCl and boiled for 30 min. After cooling, the pH was adjusted to 4.5 with KOH. The gelatinized starch was digested for 60 min at 50 °C with ∼36 units of amyloglucosidase from Aspergillus oryza (Hubbard et al. 1990). The reaction mixture was incubated at 25 °C for 30 min and absorbance at 340 nm was measured.
Data analysis
All pollen quality results are the means of at least six biological replicates. The results of starch and carbohydrate analyses are the means of three experiments; in each experiment, samples were prepared from pollen collected from 50 flowers. Data are presented as mean ± SE. Multiple comparison Tukey's honestly significant difference (HSD) test was used to test for mean values that are significantly different (α = 0.05).
Results
A mutation in the ERS-like ethylene receptor affects pollen quality under HS conditions
A tomato mutant defective in ethylene signalling was used to determine ethylene's involvement in modulating pollen number and quality under HS conditions. Tomato mutant Nr plants, carrying a semi-dominant mutation in the ethylene response sensor (ERS)-like ethylene receptor and exhibiting a number of pleiotropic effects indicative of ethylene insensitivity throughout the plant (Lanahan et al. 1994), and plants of the corresponding wild-type line ‘Pearson’ were exposed to MCHS conditions (32/26 °C day/night) for at least 2 weeks, and pollen quality was evaluated. Heat stress affected the pollen quality of both wild-type and Nr mutant plants, causing a 1.75- and 2.5-fold reduction in the number of viable pollen grains per flower, respectively (Table 1). However, the HS-exposed Nr plants showed a significantly lower number of viable pollen grains than the HS-exposed wild-type plants, manifested by a 1.4-fold relative reduction (Table 1). In addition, following exposure to MCHS, there was a significantly higher elevation in the number of non-viable pollen grains in the Nr mutant compared with the wild-type plants (resulting in 9.5 × 103 and 4.9 × 103 non-viable pollen grains per flower, respectively; Table 1). The total number of pollen grains under MCHS conditions was significantly lower in Nr compared with wild-type flowers (reaching 37.7 × 103 and 49.5 × 103, respectively; Table 1). Thus, pollen of the Nr mutant plants was more sensitive to HS conditions.
Table 1.
Effect of heat stress on pollen number and quality of wild-type and Nr mutant tomato plants. Mature pollen grains were collected from wild-type (Pearson) and ethylene receptor mutant (Nr) tomato plants which were exposed for at least 14 days to either control (28/22 °C day/night) or mild chronic heat stress (MCHS; 32/26 °C day/night) conditions. Pollen quality is presented as mean values (n = 4 independent experiments carried out in four summer seasons, at least six biological replicates each) of number of total, viable and non-viable pollen grains per flower.
| Treatment | Number per flower (×103) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total |
Viable |
Non-viable (NV) |
% NV of total |
|||||
| Pearson | Nr | Pearson | Nr | Pearson | Nr | Pearson | Nr | |
| Control | 81.6a | 73.7a | 77.8a | 70.1a | 3.75b | 3.6b | 4.6 | 4.9 |
| MCHS | 49.5b | 37.7c | 44.6b | 28.3c | 4.95b | 9.5a | 10 | 25 |
In each pollen-quality category (total, viable, non-viable), values followed by different letters are significantly different by multiple comparison Tukey's HSD test (α = 0.05).
Pollen carbohydrate metabolism is affected by the mutation in the ERS-like ethylene receptor
In both tomato lines, under both control (28/22 °C day/night) and MCHS (32/26 °C day/night) temperatures, starch levels were lowest in the mature pollen grains at anthesis (flower developmental stage A; Table 2). Mild chronic HS conditions caused a more than 2-fold reduction in starch levels of microspores at 5 days before anthesis (developmental stage A-5) in both tomato lines. No significant differences were detected in starch levels of maturing and mature pollen grains between wild-type and Nr plants exposed to either control or MCHS conditions (Table 2).
Table 2.
Effect of heat stress on starch concentration in pollen grains at different stages of flower development from wild-type and Nr mutant tomato plants. Pollen grains were collected from wild-type (Pearson) and ethylene receptor mutant (Nr) tomato plants exposed for at least 14 days to either control (28/22 °C day/night) or mild chronic heat stress (MCHS; 32/26 °C day/night) conditions. Starch concentrations (mg g DW−1) are presented as mean values of three experimental replicates (n = 3 independent experiments; pooled from at least 50 flowers each). A–n = flower buds n days before anthesis (A = flower opening).
| Pearson |
Nr |
|||||||
|---|---|---|---|---|---|---|---|---|
| A-5 | A-3 | A-1 | A | A-5 | A-3 | A-1 | A | |
| Control | 438.6ab | 327.7ab | 214.0ab | 67.1b | 480.0a | 360.5ab | 264.8ab | 96.5b |
| MCHS | 208.2abc | 324.3ab | 234.3abc | 75.2c | 174.1bc | 227.8abc | 369.0a | 101.7c |
Values in a row followed by different letters are significantly different (α = 0.05) by multiple comparison Tukey's HSD test.
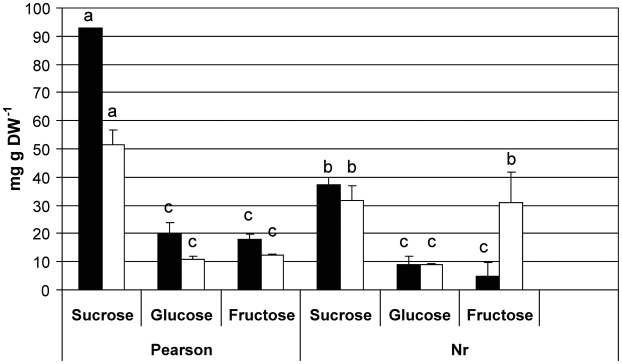
Regarding soluble sugar levels, mature pollen grains (stage A) of Nr had more than 2-fold lower levels of sucrose than those of wild-type plants grown under control conditions (37.4 and 93 mg g dry weight (DW)−1, respectively; Fig. 1). The results thus indicate an effect of perturbation in the ethylene signalling pathway on pollen carbohydrate metabolism. Exposing the plants to HS conditions caused ∼45 % reduction in sucrose levels in pollen of the wild-type plants and no change in the pollen levels of the mutant (reaching 51.4 and 31.7 mg g DW−1, respectively; Fig. 1). Significantly higher fructose levels were detected in mature Nr pollen grains (stage A) following exposure of the plants to MCHS (Fig. 1).
Fig. 1.
Effect of heat stress on levels of sucrose, glucose and fructose in pollen grains of wild-type and Nr mutant tomato plants. Mature pollen grains at anthesis were collected from wild-type (Pearson) and ethylene receptor mutant (Nr) tomato plants which were exposed for at least 14 days to either control (28/22 °C day/night) or mild chronic heat stress (MCHS; 32/26 °C day/night) conditions. Carbohydrate concentrations are presented as mean values ± SE (n = 3 independent experiments; pooled from at least 50 flowers each). In each category (control, black bars; MCHS, white bars), bars with different letters are significantly different by multiple comparison Tukey's HSD test (α = 0.05).
Application of an ethylene releaser to tomato plants increases pollen quality under HS
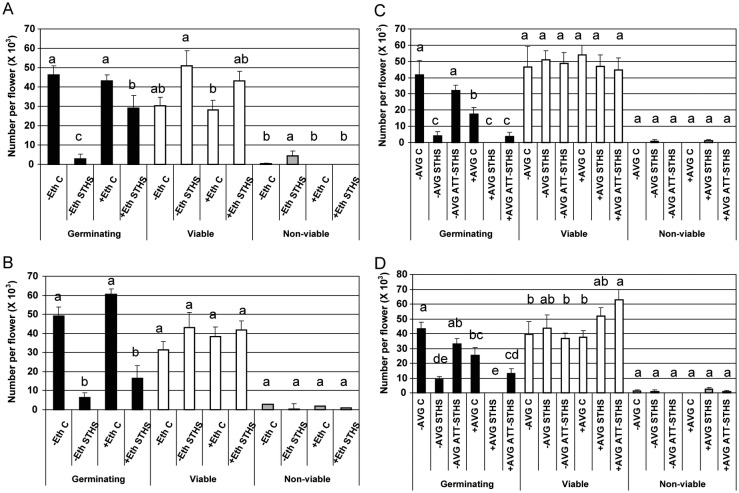
Exposing tomato (cv. Micro-Tom) plants to STHS conditions (2 h at 50 °C) at pollen developmental stages A-3 (3 days before anthesis) and A (anthesis) caused a 16.8- and 7.7-fold reduction, respectively, in the number of germinating pollen grains (Fig. 2A and B, respectively). Treatment of ‘Micro-Tom’ plants during the maturation stage of pollen development (stage A-3) with ethephon for 18 h prior to exposing the plants to STHS conditions resulted in an ∼10-fold increase in the number of germinating pollen grains per flower (Fig. 2A; 2.8 × 103 and 29 × 103 germinating pollen grains per flower in non-treated and ethephon-treated plants, respectively). Ethephon pretreatment at flower developmental stage A-3, prior to STHS, also caused a significant reduction in the number of non-viable pollen grains (from 4.25 × 103 to zero; Fig. 2A). Ethephon pretreatment had a beneficial effect on pollen functioning following HS conditions, even when administered at the mature pollen stage (stage A; Fig. 2B), although the effect was not statistically significant, causing an increase of ∼2.6-fold in the number of germinating pollen grains per flower (Fig. 2B; 6.4 × 103 and 16.5 × 103 germinating pollen grains per flower in non-treated and ethephon-treated plants, respectively).
Fig. 2.
Effect of ethephon and AVG pretreatments of tomato plants on pollen quality following exposure of the plants to short-term heat stress (STHS). (A) and (B) Tomato plants (Micro-Tom) were either pretreated with ethephon (+Eth; 1 p.p.m. for 18 h before HS application) or not pretreated (−Eth). (C) and (D) Tomato plants were either pretreated with AVG (+AVG; 0.3 mM for 4 h before HS application) or not pretreated (−AVG). Short-term heat stress conditions were 2 h exposure to 50 °C. Control (C)—plants maintained at 25 °C. STHS treatment was applied (A) and (C) 3 days before flower opening—developmental stage A-3, or (B) and (D) at anthesis—developmental stage A. ATT—acquired thermotolerance conditions were 1 h exposure to 32 °C followed by 1 h recovery at 25 °C. Acquired thermotolerance conditions were applied prior to HS exposure. Mature pollen grains were collected and pollen quality determined. Data are presented as mean values ± SE (n = 12 biological replicates; collected in two experiments) of number of germinating, viable and non-viable pollen grains per flower. In each pollen-quality category (germinating, black bars; viable, white bars; non-viable, grey bars), bars with different letters are significantly different by multiple comparison Tukey's HSD test (α = 0.05).
Tomato pollen has the capacity for ATT and this capacity is affected by the application of an ethylene biosynthesis inhibitor
To determine whether tomato pollen grains have the capacity for ATT, plants of cv. Micro-Tom were exposed to mild HS (1 h at 32 °C, followed by 1 h recovery at 25 °C) prior to exposure to STHS conditions (2 h at 50 °C) and the effect on pollen quality was tested. The ATT treatment caused an over 3-fold increase in the number of germinating pollen grains compared with non-treated controls following exposure of the plants to STHS conditions, at both stages of flower development (Fig. 2C and D; increase from 4.2 × 103 and 9.0 × 103 to 32 × 103 and 33 × 103 at developmental stages A-3 and A, respectively). These results thus show that maturing and mature pollen grains (at developmental stages A-3 and A, Fig. 2C and D, respectively) have the capacity for ATT.
Pretreating plants with the ethylene biosynthesis inhibitor AVG before exposing them to STHS conditions caused a reduction in the number of germinating pollen grains (from 4.2 × 103 and 9 × 103 to zero, at developmental stages A-3 and A, respectively, as demonstrated in Fig. 2C and D, respectively), and affected the capacity for ATT (reduction from 32 × 103 and 33 × 103 to 3.7 × 103 and 13 × 103 germinating pollen grains), at developmental stages A-3 and A, respectively, as demonstrated in Fig. 2C and D, respectively. It should be noted that AVG pretreatment, applied at either stage A-3 or A of pollen development, affected pollen quality, even under optimal growing conditions (Fig. 2C and D), indicating the involvement of endogenous ethylene in pollen development and germination.
Discussion
Impaired pollen development under high-temperature conditions has been implicated in reduced yields in a large number of crop systems (Stone 2001; Firon et al. 2006; Prasad et al. 2006; Mukesh et al. 2007). In tomato, developing pollen grains are highly sensitive to HS (Pressman et al. 2002, 2006; Firon et al. 2006). Despite the anticipated frequency of HS problems and despite accumulated data on the high HS sensitivity of developing pollen grains, data on the mechanisms involved in pollen HSR and thermotolerance are very limited (Pressman et al. 2008). The results presented in this study indicate that perturbation of the tomato ethylene signalling pathway, by a mutation in the ERS-like ethylene receptor, increases the sensitivity of pollen grains to MCHS conditions, as manifested by a reduction in the total number of pollen grains produced, an increase in the number of non-viable pollen grains and a significant reduction in the number of viable pollen grains in Nr mutant plants (Table 1). Previous studies analysing this mutant indicated that the Nr mutation inhibits tomato fruit ripening, and influences fruit morphology, seed number, ascorbate accumulation, carotenoid biosynthesis, ethylene evolution and expression of many genes during fruit maturation (Alba et al. 2005). The presented results, together with the known activity of Nr in regulating ethylene signalling, suggest a role for ethylene and the ethylene signalling pathway in maintaining higher pollen quality under HS conditions.
We previously demonstrated that a higher quality of pollen in heat-tolerant tomato genotypes is associated with higher levels of starch accumulated during pollen maturation and higher levels of sucrose in the mature pollen grains (Firon et al. 2006). We therefore tested the levels of starch, as well as sucrose, glucose and fructose, in maturing and mature pollen grains, respectively, of wild-type and Nr mutant plants. Starch levels did not differ significantly between pollen of wild-type and Nr plants (Table 2). However, the Nr mutation was shown to affect pollen carbohydrate metabolism by causing an over 2-fold reduction in the sucrose levels accumulated in mature pollen grains (Fig. 1). Since starch levels did not differ between wild-type and Nr maturing pollen, the lower sucrose levels accumulated in mature Nr pollen grains might be due to lower sugar transport to the developing pollen and lower sucrose synthesis, rather than a direct result of starch degradation.
The significantly lower sucrose levels detected in mature pollen grains of Nr mutant plants might contribute to their higher sensitivity to HS. High sucrose content in pollen has previously been suggested to be associated with the acquisition of germination capacity and desiccation tolerance, as it provides the necessary osmolality for cell expansion (Hoekstra and van Roekel 1988) or is related to pollen longevity (Speranza et al. 1997). Buitink and Leprince (2004) suggested that sucrose participates in the formation of intracellular glass, which protects membranes during dehydration. Vesprini et al. (2002) suggested a role for cytoplasmic pollen carbohydrates in resistance to low-temperature exposure. Indeed, we previously showed, in pepper, that both pollen quality and yield are correlated with pollen sucrose levels, being affected by different temperature regimes (with low night temperatures decreasing mature pollen sucrose levels, pollen germination capacity and yield; Pressman et al. 2006). Kaplan et al. (2004) pointed out a relationship between HS and cold-stress responses at the metabolite level, suggesting the possible involvement of signalling molecules and compatible solutes in temperature-stress tolerance.
It is interesting to note that exposing tomato plants to HS caused a 32-fold increase in expression of an ethylene-dependent gravitropism-deficient and yellow–green-like (ATEGY3) gene (homologous to Arabidopsis AT1G17870, 2E-43; Frank et al. 2009) in developing tomato microspores. Molecular studies have revealed that a member of this gene family is involved in controlling the size and number of plastids, and encodes a metalloprotease (Guo et al. 2008). This gene may link the HS-regulated ethylene response to pollen amyloplast biology and carbohydrate metabolism.
The involvement of ethylene in maintaining tomato pollen quality under HS is further demonstrated by: (i) the 2.6- to 10-fold increase in the number of germinating pollen grains and (ii) the significant decrease in the number of non-viable pollen grains, due to pretreatment of the plants with an ethylene releaser, prior to exposing them to STHS. This beneficial effect of ethylene pretreatment was manifested at two different stages of pollen development: 3 days before flower opening (stage A-3) and at the mature pollen stage (A), suggesting ethylene's involvement in thermotolerance of developing/maturing pollen grains as well as in pollen germination, respectively. We obtained similar results with developing pollen of Nicotiana sylvestris, another member of the Solanaceae (N. Firon et al., ARO, Israel, unpubl. res.).
Thermotolerance is generally divided into acquired (i.e. the ability to acquire tolerance to otherwise lethal HS) and basal (i.e. the inherent ability to survive temperatures above optimal growth temperatures) (Suzuki et al. 2008 and references therein). The capacity for ATT may be achieved by elevating expression levels of ‘protective’ genes prior to HS exposure (Larkindale and Vierling 2008). The results, summarized in Fig. 2C and D, indicate that tomato pollen has the capacity for ATT, and that pretreating the plants with mild HS before exposing them to STHS significantly increases the number of germinating pollen grains. This suggests the activation of genes or proteins that may protect pollen germination capacity under HS.
The established ATT conditions were used to determine whether ethylene is involved in basal or acquired thermotolerance, or both, in pollen. Pretreatment of tomato plants with the ethylene biosynthesis inhibitor AVG indicated an 8.5- and 2.5-fold reduction in pollen ATT capacity at developmental stages A-3 and A, respectively, and complete inhibition of pollen germination following exposure to STHS conditions. Taken together, the results indicate involvement of ethylene in both basal and acquired thermotolerance.
The results presented in this work indicate that interfering with the ethylene signalling pathway or reducing ethylene levels increases tomato pollen sensitivity to HS, whereas increasing ethylene levels prior to HS exposure increases pollen quality. It is thus suggested that ethylene increases pollen tolerance to HS, thereby improving pollen functioning under those conditions.
In addition, involvement of ethylene in the normal course of pollen development and germination is suggested based upon the results showing a reduction in pollen quality under optimal growing conditions following pretreatment of the plants with AVG. The role of ethylene synthesis in the development and germination of tobacco pollen has previously been shown (De la Torre et al. 2006 and references therein).
Conclusions and forward look
Perturbation of the tomato ethylene signalling pathway by a mutation in the ERS-like ethylene receptor was found to affect pollen carbohydrate metabolism—manifested by a reduction in mature pollen sucrose levels—and to increase pollen sensitivity to HS conditions. Increasing ethylene levels by applying an ethylene releaser prior to exposing the plants to HS increased pollen thermotolerance, manifested by an increase in the number of germinating pollen grains. On the other hand, application of an inhibitor of ethylene biosynthesis affected basal as well as acquired thermotolerance in pollen, as reflected by a reduced number of germinating pollen grains. The accumulated data thus point to ethylene's involvement in tomato pollen thermotolerance. Additional work is needed to identify and characterize the mechanisms involved. The scavenging of ROS that accompany the HSR, and accumulation of compatible solutes, such as sucrose, may be part of such mechanisms. The established pollen ATT conditions may be further used for the identification of molecular mechanisms involved in pollen ATT, including pollen-expressed components of the ethylene signalling pathway, by employing next-generation sequencing methods at the pollen cDNA level.
Sources of funding
This work was supported in part by the European FP7-PEOPLE-2011-ITN (Initial Training Networks) programme, Solanaceae Pollen Thermotolerance, SPOT-ITN, Number PITN-GA-2011–28922.
Contributions by the authors
All authors contributed to the research by participating in setting up the experiments and performing the measurements. The senior author was responsible for the data analysis and preparation of the manuscript. All authors reviewed and contributed comments to the manuscript.
Conflict of interest statement
None declared.
References
- Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. The Plant Cell. 2005;17:2954–2965. doi: 10.1105/tpc.105.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP. A versatile stain for pollen, fungi, yeast and bacteria. Stain Technology. 1980;55:8–13. doi: 10.3109/10520298009067890. [DOI] [PubMed] [Google Scholar]
- Aouali N, Laporte P, Clement C. Pectin secretion and distribution in the anther during pollen development in Lilium. Planta. 2001;213:71–79. doi: 10.1007/s004250000469. [DOI] [PubMed] [Google Scholar]
- Buitink J, Leprince O. Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology. 2004;48:215–228. doi: 10.1016/j.cryobiol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Chibi F, Mattilla AJ. The involvement of ethylene in in vitro maturation of mid-binucleate pollen of Nicotiana tabacum. Journal of Experimental Botany. 2010;45:529–532. [Google Scholar]
- De la Torre F, Del Carmen Rodríguez-Gacio M, Matilla AJ. How ethylene works in the reproductive organs of higher plants. A signaling update from the third millennium. Plant Signaling and Behavior. 2006;1:231–242. doi: 10.4161/psb.1.5.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N, Peet MM, Pharr DM, Zamski E, Rosenfeld K, Althan L, Pressman E. Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Scientia Horticulturae. 2006;109:212–217. [Google Scholar]
- Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones and sugars in the heat-stress response. Journal of Experimental Botany. 2009;60:3891–3908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Gao X, Li H, Zhang T, Chen G, Huang P, An L, Li N. EGY1 plays a role in regulation of endodermal plastid size and number that are involved in ethylene-dependent gravitropism of light-grown Arabidopsis hypocotyls. Plant Molecular Biology. 2008;66:345–360. doi: 10.1007/s11103-007-9273-5. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, van Roekel T. Desiccation tolerance of Papaver dubium L. pollen during its development in the anther: possible role of phospholipid composition and sucrose content. Plant Physiology. 1988;88:626–632. doi: 10.1104/pp.88.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NL, Pharr DM, Huber SC. Role of sucrose phosphate synthase in sucrose biosynthesis in ripening bananas and its relationship to the respiratory climacteric. Plant Physiology. 1990;94:201–208. doi: 10.1104/pp.94.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology. 2004;136:4159–4168. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet JM, Peet MM. Tomato. In: Wien HC, editor. The physiology of vegetable crops. Wallingford: Commonwealth Agricultural Bureau (CAB) International; 1997. pp. 207–258. [Google Scholar]
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiology. 2004;135:660–667. doi: 10.1104/pp.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaleva LV, Dobrovolskaya A, Voronkov A, Rakitin V. Ethylene is involved in the control of male gametophyte development and germination in petunia. Journal of Plant Growth Regulation. 2010;30:64–73. [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. The Never ripe mutation blocks ethylene perception in tomato. The Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Huang B. Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. Journal of Plant Physiology. 2004;161:405–413. doi: 10.1078/0176-1617-01239. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR. Protection against heat stress induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiology. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiology. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Molecular Biology. 2002;48:667–681. doi: 10.1023/a:1014826730024. [DOI] [PubMed] [Google Scholar]
- Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, Elkind Y, Levy AA. A new model system for tomato genetics. Plant Journal. 1997;12:1465–1472. [Google Scholar]
- Mukesh J, Prasad PVV, Boote KJ, Hartwell AL, Chourey PS. Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench) Planta. 2007;227:67–79. doi: 10.1007/s00425-007-0595-y. [DOI] [PubMed] [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH, Jr, Thomas JMG. Supra-optimal temperatures are detrimental to peanut (Arachis hypogaea L.) reproductive processes and yield at ambient and elevated carbon dioxide. Global Change Biology. 2003;9:1775–1787. [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH., Jr Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agricultural and Forest Meteorology. 2006;139:237–251. [Google Scholar]
- Pressman E, Moshkovitch H, Rosenfeld K, Shaked R, Gamliel B, Aloni B. Influence of low night temperatures on sweet pepper flower quality and the effect of repeated pollinations, with viable pollen, on fruit set. Journal of Horticultural Science and Biotechnology. 1998;73:131–136. [Google Scholar]
- Pressman E, Peet MM, Pharr DM. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany. 2002;90:631–636. doi: 10.1093/aob/mcf240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman E, Harel D, Zamski E, Shaked R, Althan L, Rosenfeld K, Firon N. The effect of high temperatures on the expression and activity of sucrose cleaving enzymes during tomato (Lycopersicon esculentum) anther development. Journal of Horticultural Science and Biotechnology. 2006;81:341–348. [Google Scholar]
- Pressman E, Shaked R, Firon N. Tomato response to heat stress: focus on pollen grains. Plant Stress. 2008;1:216–227. [Google Scholar]
- Sawhney VK, Bhadula SK. Microsporogenesis in the normal and male-sterile stamenless-2 mutant of tomato (Lycopersicon esculentum) Canadian Journal of Botany. 1988;866:2013–2021. [Google Scholar]
- Speranza A, Calzoni GL, Pacini E. Occurrence of mono- or disaccharides and polysaccharide reserves in mature pollen grains. Sexual Plant Reproduction. 1997;10:110–115. [Google Scholar]
- Stone P. The effects of heat stress on cereal yield and quality. In: Basra AS, editor. Crop responses and adaptations to temperature stress. Binghampton, NY: Food Products Press; 2001. pp. 243–291. [Google Scholar]
- Stoop JMH, Pharr DM. Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiology. 1994;106:503–511. doi: 10.1104/pp.106.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R. Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiology. 2005;139:1313–1322. doi: 10.1104/pp.105.070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. Journal of Biological Chemistry. 2008;283:9269–9275. doi: 10.1074/jbc.M709187200. [DOI] [PubMed] [Google Scholar]
- Vesprini JL, Nepi M, Cresti L, Guarnieri M, Pacini E. Changes in cytoplasmic carbohydrate content during Helleborus pollen presentation. Grana. 2002;41:16–20. [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. Temperature stress and plant sexual reproduction: uncovering the weakest links. Journal of Experimental Botany. 2010;61:1959–1968. doi: 10.1093/jxb/erq053. [DOI] [PMC free article] [PubMed] [Google Scholar]