Abstract
Background
Corticosteroids play a major role in the treatment of many diseases of the posterior ocular segment. Systemically or topically administered steroids usually do not attain therapeutic concentrations in the retina, as they must first cross the blood-retina barrier. Intravitreal application is a useful alternative means of achieving therapeutic concentrations in the posterior segment but must be repeated every few weeks, because drugs given in this way have a short half-life. Intraocular sustained-release implants have been now developed in order to prolong the effect of intravitreal drugs and to lessen the need for repeated application. Macular edema is a typical indication for intravitreal steroid treatment.
Methods
Selective review of the literature.
Results
Various intravitreal corticosteroid implants have been evaluated in prospective, randomized clinical trials in recent years, and some have been approved for clinical use. Implants are either longer-acting and non-resorbable (fluocinolone acetonide implants) or shorter-acting and resorbable (dexamethasone implants). Major adverse effects of intravitreal corticosteroids include the induction or worsening of cataracts and elevated intraocular pressure. The likelihood of a complication varies from implant to implant and depends on the duration of action of the particular one used.
Conclusion
Intravitreal corticosteroid implants are a new option in the treatment of diseases of the posterior ocular segment. Long-term results are not yet available. The optimal treatment for these diseases will need to be the focus of further clinical research.
In the industrialized world, diseases of the posterior segment of the eye such as age-related macular degeneration (AMD), diabetic retinopathy, retinal vein occlusion, and uveitis are second only to glaucoma as a leading cause of visual impairment and blindness (e1, e2). The retinal blood supply is separated from the nervous tissue of the retina by the so-called blood-retina barrier, which regulates the exchange of water, proteins, and ions between the retina and the systemic circulation (1). A further property of the blood-retina barrier is that it blocks the passage of medications into the interior of the eye (2): Systemically administered drugs must be given in high doses in order to reach an effective concentration in the posterior ocular segment. As a result, the ability to treat the eye with systemically administered drugs is often limited by side effects that arise in organs at high doses. Effective drug concentrations at the choroid and retina cannot be achieved with eye drops, either. Some diseases can disrupt the blood-retina barrier in such a way as to permit drugs to cross it more easily, but a dysfunctional barrier can also be a cause of macular edema. Modifying the blood-retina barrier to ease the crossing of drugs into the interior of the eye is an approach with very little clinical application because of the side effects that result from increased permeability of the vascular walls (3).
For these reasons, drugs for the treatment of diseases of the posterior ocular segment, such as AMD and macular edema of various causes, are now mainly given by a technique called “intravitreal operative medication” (IVOM), which yields effective local drug concentrations while markedly reducing systemic side effects. IVOM is, however, an invasive procedure that must be performed under sterile conditions. Infections in the interior of the eye (endophthalmitis) after IVOM are very rare, with reported rates ranging from 0.02% (12 in 60 322 procedures) to 0.26% (4 in 1553 procedures) (e3, e4). There is a low risk of other complications, such as the formation of holes in the retina (0.017% [e5]), retinal detachment (0.03% ([6]), and hemorrhage into the vitreous body (0.02% [e5] to 0.03% [6]).
More common than these complications of the injection procedure per se are the the specific side effects of individual drugs, such as intraocular hypertension and cataract formation after the intravitreal application of triamcinolone (5, 6). Corticosteroids can, alternatively, be given by subconjunctival, parabulbar, or retrobulbar injection; in this way, the globe of the eye is not penetrated, but the therapeutic benefit (both anatomical and functional) is inferior to that of IVOM, despite the higher frequency of injection (7), and there may also be systemic side effects, including hyperglycemia. In view of the risks of IVOM, it would be desirable to prolong the effect of intravitreal medication for as long as possible after each injection, so that the injections need not be given as frequently. This would both lower the risk of complications and make the treatment less onerous, so that patients would be more willing to undergo it.
Intravitreal corticosteroids are now used clinically to treat either macular edema due to retinal vein occlusion or uveitis (Table 1). In April/May 2012, an implant containing 0.19 mg of fluocinolone acetonide was approved for the treatment of diabetic macular edema in Austria and the United Kingdom. Macular edema reflects the deposition of liquid in the outer plexiform and inner nuclear layers with swelling of the retinal Müller cells. This, in turn, is due to retinal capillary leakage near the fovea, whose multiple causes include increased release of vascular endothelial growth factor (VEGF) and inflammatory processes (8 – 10).
Table 1. Intravitreal implants.
| Implant | Active drug | Approved indication for intravitreal use | Resorbable? | Implantable without suture? |
| Ganciclovir | Ganciclovir 4.5 mg | Cytomegalovirus (CMV) retinitis | No | No |
| Fluocinolone acetonide | Fluocinolone acetonide 0.59 mg | Chronic non-infectious uveitis | No | No |
| Fluocinolone acetonide | Fluocinolone acetonide 0.19 mg | USA: application for approval rejected by the FDA (2011) Europe: positive evaluation (2/2012), approval in Austria (4/2012) and the U.K. (5/2012) | No | Yes |
| Triamcinolone acetonide | Triamcinolone acetonide 40 mg/mL | Europe: not approved | Yes, | Yes, |
| USA: uveitis or intraocular inflammation not responding to topical steroids, temporal arteritis, sympathetic ophthalmia, intraoperative visualization of the vitreous humor | not an implant but a crystalline suspension | injection of the suspension | ||
| Dexamethasone | Dexamethasone 700 µg | Macular edema after BRVO and CRVO; inflammation of the posterior segment of the eye (non-infectious uveitis) | Yes | Yes |
BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; FDA, Food and Drug Administration
Among other effects, corticosteroids stabilize endothelial tight junctions and inhibit the synthesis of VEGF, prostaglandins, and inflammatory cytokines (11). In a clinical trial, systemic corticosteroid treatment was found to reduce macular edema and improve visual acuity transiently in some patients with macular edema due to central retinal vein occlusion (CRVO) (9). Once the steroid medication was discontinued, however, the macular edema returned. Systemic corticosteroids were, therefore, not recommended as a therapeutic option in this situation, in view of their limited efficacy and unfavorable side-effect profile (12). This remains true today.
In recent years, intravitreal anti-VEGF treatment with pegaptanib, bevacizumab, or ranibizumab has been evaluated in randomized, controlled trials as an alternative to intravitreal corticosteroids for the treatment of various diseases of the posterior ocular segment, including AMD, venous occlusion, and diabetic macular edema (e5– e12). Bevacizumab and ranibizumab were found to have a stronger therapeutic effect than pegap-tanib. Pegaptanib and the antibody fragment ranibizumab are currently approved for the intravitreal anti-VEGF treatment of exudative AMD. Ranibizumab has also been approved for the treatment of diabetic macular edema and of macular edema after venous occlusion with foveal involvement. Anti-VEGF drugs, which are intended to counteract the increased vascular permeability caused by VEGF, must be injected through the pars plana of the ciliary body directly into the vitreous humor of the eye. This route of administration is the same as that of steroid depot preparations. Anti-VEGF drugs must be given at much shorter intervals than steroids (almost monthly); on the other hand, they do not cause lens opacification or intraocular hypertension. The various anti-VEGF drugs differ widely in cost. Bevacizumab, the cheapest one, has not been approved for intraocular use. In Germany, there is still no recognized billing code for the introduction of drugs into the eye, even though intravitreal treatment has been practiced for years. This is inconvenient for patients, as all such treatments must be preceded by an application to the health-insurance carrier for assumption of the costs.
Intravitreal depot systems
The first intravitreal drug systems were developed for the local treatment of CMV (human cytomegalovirus) retinitis in HIV patients and were approved by the Food and Drug Administration (FDA) in 1996 for clinical use in the USA. The ganciclovir implant consists of 4.5 mg of ganciclovir in a tablet coated with polyvinyl alcohol (PVA) and hydrophobic ethylene vinyl acetate (EVA). It initially releases 1 µg of ganciclovir per hour and has therapeutic effects over a period of eight months.This is a non-degradable, relatively large implant that must be sutured to the wall of the globe in the area of the pars plana of the ciliary body through a 5.5-mm incision (3).
Fluocinolone acetonide implants
The first intravitreal implant containing cortisone has a non-degradable matrix similar to that of the ganciclovir implant and contains 0.59 mg of fluocinolone acetonide (FA). The FDA approved it in 2005 for the treatment of macular edema due to non-infectious uveitis; it has not been approved in Europe. This implant is surgically introduced into the eye through a 3.5 mm incision in the pars plana of the ciliary body and sutured to the wall of the globe. The safety and efficacy of FA implants were studied in a prospective, multicenter trial with 3 years of follow-up, in which a total of 278 patients with non-infectious posterior uveitis received 110 0.59-mg implants and 168 2.1-mg implants. FA implants were found to lower the rate of recurrence of uveitis and stabilize visual acuity over a period of three years. They had marked side effects, however, including cataracts that required surgery in almost all of the eyes that had received implants, and intraocular hypertension requiring surgery in many of them (3, 13).
In view of these side effects, FA implants were compared, in a further trial, with the standard treatment for patients with non-infectious uveitis, i.e., systemic corticosteroids and further immunosuppression if necessary. There was no significant difference in the improvement of visual acuity brought about by the two treatments. The patients who received FA implants had lower rates of active uveitis and of treatment for systemic infection, but they had much higher rates of cataract surgery (80%; hazard ratio [HR] = 3.3, p <0.0001), procedures to lower intraocular pressure (61%, HR = 4.2, p <0.0001), and glaucoma (17%, HR = 4.2, p =0.0008). The authors concluded that both forms of treatment have a role to play in the treatment of non-infectious uveitis, and that the choice between them should be made individually for each patient (14).
Another recent, prospective, multicenter trial concerned the efficacy and safety of 0.59-mg FA implants versus standard treatment (laser therapy or observations) for patients with diabetic macular edema. The FA implants brought about a greater improvement of visual acuity, but only at the cost of cataract progression in nearly all of the treated patients, and the need for surgical procedures to lower intraocular pressure in more than 30% of them (15).
Similar results with respect to both efficacy and complications were found 3 years after treatment in a prospectively followed interventional case series of patients with macular edema after CRVO (16).
Thus, for all of the diseases treated in these trials, 0.59-mg FA implants were found to be an effective treatment, but with two serious and very frequent side effects: cataracts in almost all patients, and the need for surgery to lower intraocular pressure in many of them.
0.19-mg fluocinolone acetonide implants
A smaller, non-resorbable implant containing 0.19 mg of FA can be injected through a 25-gauge (0.46-mm) needle into the vitreous body of the eye without the need for a suture. The safety and efficacy of implants with two different concentrations of FA (release rates: 0.2 and 0.5 µg per day) were studied and compared with sham injections in a prospective, placebo-controlled, double-blind phase III trial on patients with persistent diabetic macular edema despite at least one laser treatment. Both types of implant improved visual acuity, but the lower-dose implant had fewer side effects, particularly with respect to intraocular pressure (17). In the fall of 2011, the FDA rejected an application for approval of the 0.19-mg FA implant for the treatment of diabetic macular edema. In Europe, however, this implant was positively evaluated in a decentralized drug-approval process in February 2012 and then approved for use in Austria and the United Kingdom in April/May 2012.
Triamcinolone acetonide
Triamcinolone acetonide is not a steroid implant, but rather a glucocorticoid that, because of its structure, stays at a therapeutic concentration in the eye for several weeks after intravitreal injection as a suspension. Though not approved in Europe, intravitreal triamcinolone (IVTA) has been approved in the USA, where it is in regular clinical use (18). In the multicenter SCORE trial (“Standard Care versus Corticosteroid for Retinal Vein Occlusion”), 271 patients with macular edema after CRVO were given either 1 or 4 mg of triamcinolone acetonide, without preservative, by the intravitreal route. A therapeutic benefit was found, making this the first effective treatment ever described for macular edema after CRVO (9, 19). The lower dose had fewer complications. The authors concluded that IVTA at a dose of 1 mg should be considered for all patients with CRVO who meet the SCORE inclusion criteria (19).
On the other hand, it was found in another clinical trial that IVTA is no better than the standard treatment (macular grid-laser treatment) for macular edema due to branch retinal vein occlusion (BRVO), while conferring the additional risk of intraocular hypertension (20).
Dexamethasone implants
Like fluocinolone, dexamethasone is a highly potent corticosteroid; it is about 30 times more potent than cortisone (21). Its half-life after injection into the human vitreous body is only 5.5 hours, so that repeated injections at short intervals are needed for the treatment of macular edema (22). The dexamethasone implant, developed to prolong the duration of action of the drug, consists of 700 µg of dexamethasone in a biodegradable poly(lactic-co-glycolic acid) (PLGA) matrix. The intravitreal concentration of the active drug is high for the first 60 days after implantation and lower for a longer period thereafter. The implant is delivered in a single-use applicator; it is operatively introduced through the pars plana of the ciliary body into the vitreous body by means of a 22-gauge (0.64-mm) injection canula (Figure 1). The dexamethasone implant was approved by the European Medicines Agency (EMA) in July 2010 for the treatment of macular edema after venous occlusion, and in June 2011 for the treatment of non-infectious uveitis.
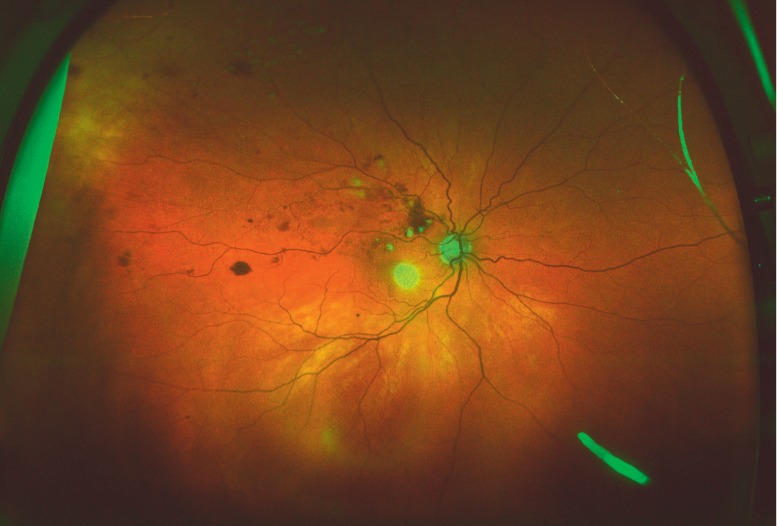
Figure 1.
The dexamethasone implant (700 µg). Wide-angle ophthalmoscopy of a patient with macular edema due to branch retinal vein occlusion. The implant can be seen at the lower right edge of the image as an oblong, bright structure in the vitreous body
The safety and efficacy of a single treatment, or two treatments, with dexamethasone implants over a period of 12 months were studied in two identically designed, prospective, multicenter trials on a total of 1267 patients with either CRVO or BRVO (Figure 2). In the two treatment groups, an improvement of visual acuity by 15 letters or more occurred significantly more rapidly and more often than in the control group (29% compared to 11% at 60 days, p <0.001). After two treatments with the 700-µg implant, lens opacification occurred in 29.8% of the treated phakic eyes (90 of 302), compared with 5.7% in the control group (5 of 88). Intraocular hypertension was most commonly found 60 days after implantation; among patients who received two 700-µg implants, this occurred in 12.6% after the first treatment, and in 15.4% after the second (23, 24).
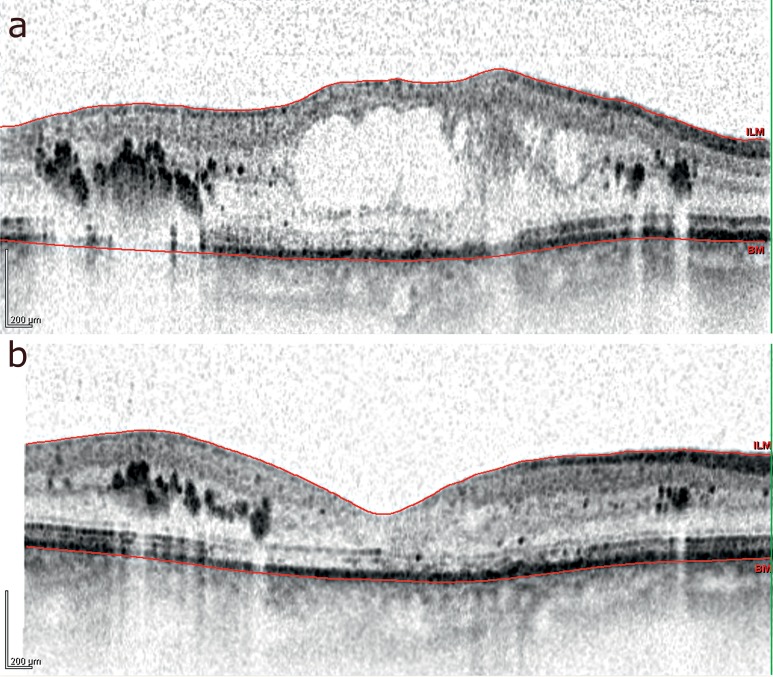
Figure 2.
Optical coherence tomography demonstrating the results of treatment in a patient with macular edema after central retinal vein occlusion.
before implantation
3 months after implantation of a 700-µg dexamethasone implant: note the markedly reduced thickness of the central portion of the retina
The HURON phase III trial concerned the safety and efficacy of dexamethasone implants in the treatment of non-infectious intermediate or posterior uveitis. The treated patients benefited from the treatment over the entire duration of follow-up and had a significantly higher rate of improved visual acuity (by 15 letters or more) than the control group. The intraocular pressure rose to 25 mmHg or higher on follow-up examination in fewer than 10% of patients; lens opacification occurred in 7% (4/55) of patients in the control group and 15% (9/62) of patients in the 700-µg group (25).
The 700-µg dexamethasone implant was found to be more effective than the 350-µg implant, with a similar rate of side effects. Accordingly, the 700 µg implant has been approved for the treatment of macular edema after venous occlusion and in patients with uveitis. According to currently available data, treatment with these implants is safe and their therapeutic benefit lasts several months after each implantation. Clinical experience has shown that lens opacification is more likely when multiple implantations are used to prolong the benefit (Table 2).
Table 2. Summary of data from clinical trials of intravitreal implants.
| Indications, type of study, duration of follow-up | Inclusion criteria | Endpoints | Results |
| Fluocinolone acetonide implant, 0.59 mg | |||
| Non-infectious posterior uveitis (13) Prospective, randomized multicenter trial 3 years | Recurrent non-infectious posterior uveitis, Status post corticosteroid treatment | Comparison of the 0.59-mg (n=110) and 2.1-mg (n=168) FA implants and untreated opposite eyes with respect to safety and effectiveness | Visual acuity improvement ≥3 lines after 3 years: 0.59-mg group: 23% (22/94) [untreated opposite eyes: 6% (5/90)] (p <0.01) undesired effects: rise in intraocular pressure by ≥10mmhg 0.59-mg group: 67% (74/110) [untreated opposite eyes: 23% (25/109)] |
| Diabetic macular edema (15)Prospective, randomized multicenter trial 3 years | Persistent or recurrent diabetic macular edema Visual acuity 0.05–0.4 | Comparison of the 0.59-mg FA implant (n=127) with standard treatment (n=69) (focal/grid laser photocoagulation or observation) (randomized 2:1) with respect to safety and effectiveness | Visual acuity improvement ≥15 letters at 6, 12, 24, and 36 months FA implant: 16.8%, 16.4%, 31.8%, 31.1% Control group: 1.4% (p=0.0012), 8.1% (p=0.1191), 9.3% (p=0.0016), 20.0% (p=0.1566) Rise in intraocular pressure to ≥30 mmHg FA implant: 61.4% (78/127) Control group: 5.8% (4/69) |
| Fluocinolone acetonide implant, 0.19 mg | |||
| Diabetic macular edema (17) Prospective, randomized, placebo-controlled double-blind phase III trial 2 years | Persistent diabetic macular edema despite laser therapy | Comparison of the 0.2-µg/d (n=375) and 0.5-µg/d (n=393) FA implants and sham treatment (n=185), with possible laser therapy and/or retreatment after 12 months (randomized 2:2:1) with respect to safety and effectiveness | Visual acuity improvement ≥15 letters at 24 months FA implant 0.2 µg/d: 28.7% Control group: 16.2% (p=0.002) Rate of filtrating glaucoma surgery FA implant 0.2 µg/d: 3.7% Control group: 0.5% |
| Dexamethasone implant, 700 µg | |||
| Persistent macular edema (e13) Prospective, randomized multicenter phase II trial 6 months | Persistent macular edema despite treatmentVisual acuity 0.1–0.5 | Comparison of the 350-µg (n=105) and 700-µg (n=105) dexamethasone implants and observation (n=105) (randomized 1:1:1) with respect to safety and effectiveness | Visual acuity improvement ≥15 letters at 3 months DEX implant 700 µg: 18% (19/105) Control group: 6% (6/105) (p=0.006) Rise in intraocular pressure by ≥ 10mmHg at some point within 6 months (not necessarily requiring surgical treatment) DEX implant 700 µg: 17% (17/101) Control group: 3% (3/100) |
| Macular edema after CRVO and BRVO (23. 24) Prospective, randomized multicenter phase III trial 6 months, randomized and blinded 6 further months, open trial with retreatment when indicated (700 µg implant) | Macular edema after BRVO (duration 6 weeks – 12 months), after CRVO (duration 6 weeks – 9 months) Visual acuity 0.1–0.4 | Comparison of the 350-µg (n=412) and 700-µg (n=421) dexamethasone implants and observation (n=423) (randomized 1:1:1) with respect to safety and effectiveness | Time to visual acuity improvement ≥15 letters Significantly shorter in the treatment groups than in the control group (p<0.001) percentage of patients with visual acuity improvement ≥15 letters (days 30 – 90 after implantion) significantly higher in the treatment groups than in the control group (p <0.001) rise in intraocular pressure by ≥10mmhg 60 days after implantion dex implant 700 µg: 12.6% (after 1 st implantion) |
| Non-infectious intermediate or posterior uveitis (25) Prospective, randomized multicenter phase III trial 6 months | Non-infectious intermediate or posterior uveitis Visual acuity 0.03–0.63 | Comparison of the 350-µg (n=76) and 700-µg (n=77) dexamethasone implants and observation (n=76) (randomized 1:1:1) with respect to safety and effectiveness | Regression of vitreous haze (Vitreous Haze Score 0) at 8 weeksDEX implant 700 µg: 47% (36/77) Control group: 12% (9/76) (p <0.001) percentage of patients with visual acuity improvement ≥15 letters significantly higher in the treatment groups than in the control group [p <0.001 (700-µg group), p ≤0.027 (350-µg group)] rise in intraocular pressure to ≥25 mmhg after implantion dex implant 700 µg: 7.1% control group: 4.2% (p >0.05) |
BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; FA, fluocinolone acetonide; DEX, Dexamethasone
Overview
A number of steroid depot systems have been clinically tested recently, and some are now in clinical use. They are a major addition to the armamentarium of treatments for diseases of the posterior segment of the eye. The systems discussed here are implanted in markedly different ways: the 0.59-mg FA depot system is introduced into the eye through a relatively long incision and then sutured to the wall of the globe, while the 0.19-mg FA implant and the dexamethasone implant are injected into the eye, and the injection site seals itself without the need for a suture.
A prolonged corticosteroid effect can lead to cataract progression and intraocular hypertension. The various steroid depot systems presented here seem to differ in the frequency of these complications, probably mainly because of their different durations of effect. Clinical trials have shown that longer-acting, non-biodegradable implants are effective against the diseases that they are given to treat, but with an accompanying high risk of these complications. Biodegradable implants have a more favorable side-effect profile, but also a much shorter duration of effect. If they have to be injected repeatedly, they, too, can cause intraocular hypertension (which usually responds to drug treatment) and accelerate the opacification of the lens. No studies have yet been performed on the long-term course of patients who are treated repeatedly with steroid implants, and thus no definitive statement can be made about the potential long-term side effects. Clinical experience to date does does not lead us to suspect that long-term intravitreal steroid therapy will have any further, rare, dangerous side effects.
Key Messages.
Diseases of the posterior segment of the eye present a pharmacotherapeutic challenge. The dose of systemic corticosteroids needed to achieve a therapeutically effective intraocular concentration would be likely to cause systemic side effects.
Intravitreal operative medication (IVOM) is the direct surgical introduction of drugs into the vitreous humor of the eye. IVOM yields effective local drug concentrations with a low rate of systemic side effects.
The potential adverse effects of intravitreal treatment are of two types: those that arise from the surgical procedure per se, and those that reflect a drug effect—in particular, lens opacification and intraocular hypertension after the intravitreal application of corticosteroids.
Intravitreal depot systems have been developed to prolong the effect of drugs given by the intravitreal route and thereby reduce the frequency of intravitreal application.
The trials that have been conducted to date have shown intravitreal steroid depot systems to be highly effective. Side effect profiles vary depending on the type of implant.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Nentwich has received payment for authorship of a publication as well as reimbursement of congress participation fees and expenses for travel and accommodation from Allergan.
Prof. Ulbig serves as a consultant for Novartis, Allergan, and Pfizer. He has received reimbursement of congress participation fees from Novartis and Pfizer and reimbursement of travel and accommodation expenses, as well as payment for giving lectures at conferences organized by Novartis, Allergan and Pfizer, from Novartis, Allergan and Pfizer. He has received payment for carrying out clinical trials on behalf of Novartis.
References
- 1.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2010;21:3–9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 2.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Fischer N, Narayanan R, Loewenstein A, Kuppermann BD. Drug delivery to the posterior segment of the eye. Eur J Ophthalmol. 2010;21:20–26. doi: 10.5301/EJO.2010.6051. [DOI] [PubMed] [Google Scholar]
- 4.Moshfeghi AA, Rosenfeld PJ, Flynn HW, Jr., et al. Endophthalmitis after intravitreal anti-vascular endothelial growth factor antagonists: a six-year experience at a university referral center. Retina. 2011;31:662–668. doi: 10.1097/IAE.0b013e31821067c4. [DOI] [PubMed] [Google Scholar]
- 5.Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010;21:178–183. doi: 10.1097/ICU.0b013e328338679a. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(57-65) doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2005;139:972–982. doi: 10.1016/j.ajo.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 8.Scholl S, Augustin A, Loewenstein A, Rizzo S, Kupperman B. General pathophysiology of macular edema. Eur J Ophthalmol. 2010;21:10–19. doi: 10.5301/EJO.2010.6050. [DOI] [PubMed] [Google Scholar]
- 9.Glacet-Bernard A, Coscas G, Zourdani A, Soubrane G, Souied EH. Steroids and macular edema from retinal vein occlusion. Eur J Ophthalmol. 2010;21:37–44. doi: 10.5301/EJO.2010.6053. [DOI] [PubMed] [Google Scholar]
- 10.Kollias AN, Ulbig MW. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107(5):75–83. doi: 10.3238/arztebl.2010.0075. quiz 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero-Vanrell R, Cardillo JA, Kuppermann BD. Clinical applications of the sustained-release dexamethasone implant for treatment of macular edema. Clin Ophthalmol. 2011;5:139–146. doi: 10.2147/OPTH.S15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohner EM, Laatikainen L, Oughton J. The management of central retinal vein occlusion. Ophthalmology. 1983;90:484–487. doi: 10.1016/s0161-6420(83)34527-9. [DOI] [PubMed] [Google Scholar]
- 13.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 14.Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized Comparison of Systemic Anti-inflammatory Therapy Versus Fluocinolone Acetonide Implant for Intermediate, Posterior, and Panuveitis: The Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson PA, Comstock TL, Ip M, Callanan D, et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580–1587. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Jain N, Stinnett SS, Jaffe GJ. Prospective study of a fluocinolone acetonide implant for chronic macular edema from central retinal vein occlusion thirty-six-month results. Ophthalmology. 2012;119:132–137. doi: 10.1016/j.ophtha.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635. doi: 10.1016/j.ophtha.2010.12.028. e2. [DOI] [PubMed] [Google Scholar]
- 18.Bandello F, Iacono P, Battaglia Parodi M. Treatment options for diffuse diabetic macular edema. Eur J Ophthalmol. 2010;21:45–50. doi: 10.5301/EJO.2010.6054. [DOI] [PubMed] [Google Scholar]
- 19.Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winterhalter S, Ruokonen P, van der Velden KH, Pleyer U, Joussen AM. Intravitreal implants: drug carriers and carriers of hope? Ophthalmologe. 2011;108:222–229. doi: 10.1007/s00347-010-2264-y. [DOI] [PubMed] [Google Scholar]
- 22.Gan IM, Ugahary LC, van Dissel JT, van Meurs JC. Effect of intravitreal dexamethasone on vitreous vancomycin concentrations in patients with suspected postoperative bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2005;243:1186–1189. doi: 10.1007/s00417-005-1182-1. [DOI] [PubMed] [Google Scholar]
- 23.Haller JA, Bandello F, Belfort R, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. doi: 10.1016/j.ophtha.2010.03.032. e3. [DOI] [PubMed] [Google Scholar]
- 24.Haller JA, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Lowder C, Belfort R, Jr., Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- e1.Finger RP, Fimmers R, Holz FG, Scholl HP. Incidence of blindness and severe visual impairment in Germany: projections for 2030. Invest Ophthalmol Vis Sci. 2011;52:4381–4389. doi: 10.1167/iovs.10-6987. [DOI] [PubMed] [Google Scholar]
- e2.Finger RP, Fimmers R, Holz FG, Scholl HP. Prevalence and causes of registered blindness in the largest federal state of Germany. Br J Ophthalmol. 2011;95:1061–1067. doi: 10.1136/bjo.2010.194712. [DOI] [PubMed] [Google Scholar]
- e3.Fong DS, Custis P, Howes J, Hsu JW. Intravitreal bevacizumab and ranibizumab for age-related macular degeneration a multicenter, retrospective study. Ophthalmology. 2010;117:298–302. doi: 10.1016/j.ophtha.2009.07.023. [DOI] [PubMed] [Google Scholar]
- e4.Gragoudas ES, Adamis AP, Cunningham ET, Jr., Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- e5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- e6.Bressler NM, Chang TS, Fine JT, Dolan CM, Ward J. Improved vision-related function after ranibizumab vs photodynamic therapy: a randomized clinical trial. Arch Ophthalmol. 2009;127:13–21. doi: 10.1001/archophthalmol.2008.562. [DOI] [PubMed] [Google Scholar]
- e7.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- e10.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- e12.Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–1602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- e13.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125:309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]