Abstract
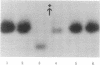
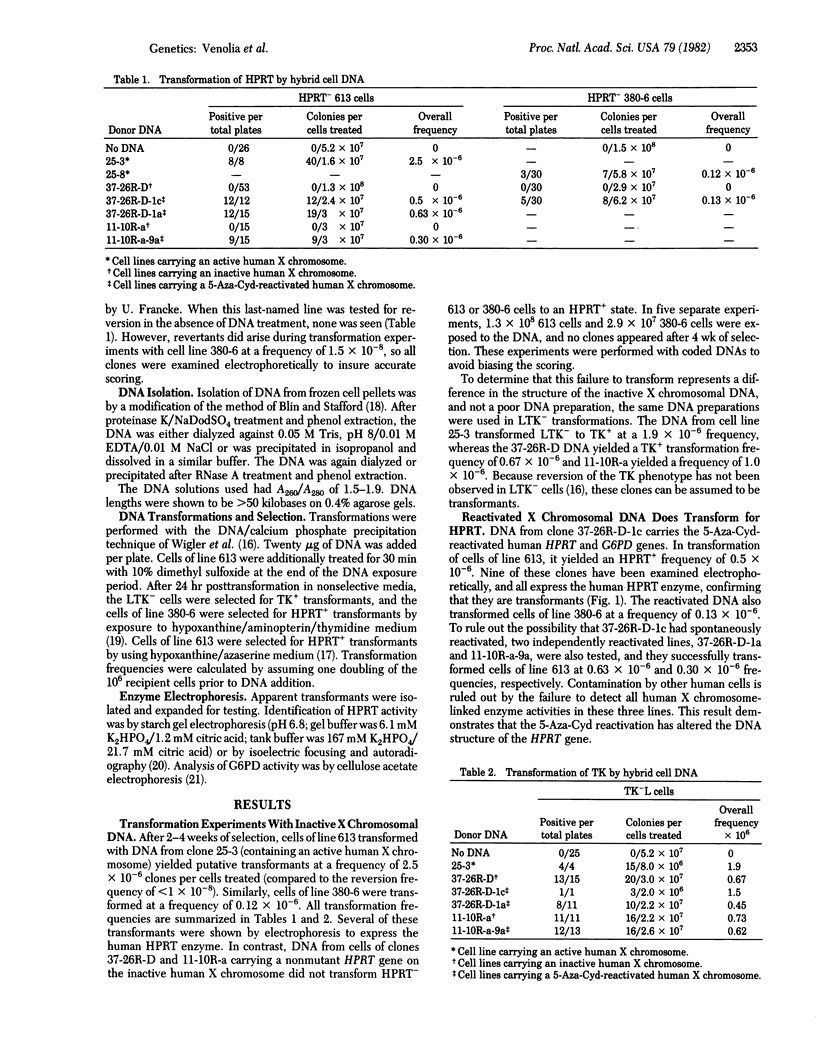
It has been shown that 5-azacytidine (5-Aza-Cyd) can reactivate genes on the inactive human X chromosome. It is assumed that the 5-Aza-Cyd acts by causing demethylation of the DNA at specific sites, but this cannot be demonstrated directly without a cloned probe. Instead, we have utilized the technique of DNA-mediated transformation to show that the 5-Aza-Cyd-induced reactivation occurs at the DNA level. DNAs from various mouse-human or hamster-human hybrid cell lines, deficient for mouse or hamster hypoxanthine phosphoribosyltransferase (HPRT, EC 2.4.2.8) and varying in whether they contained either an active or inactive human X chromosome, were used in transformation of HPRT- cells. DNA from the active human X chromosome-containing cell lines yielded HPRT+ transformants, whereas DNA from the inactive X chromosome-containing cells lines did not. The inactive X chromosomal DNA was able to transform thymidine kinase-deficient mouse cells, indicating that the DNA solution was normal. These results confirm that inactivation of the X chromosome involves a DNA modification. Furthermore, DNAs from three cell lines with a 5-Aza-Cyd-reactivated X chromosome also transform HPRT- cells, demonstrating that the 5-Aza-Cyd has altered the DNA structure and supporting the idea that methylation plays a role in X chromosome inactivation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Andina R. J. Mammalian X-chromosome inactivation. Adv Hum Genet. 1976;7:99–140. doi: 10.1007/978-1-4757-0659-8_3. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Johnson G. G., Eisenberg L. R., Migeon B. R. Human and mouse hypoxanthine-guanine phosphoribosyltransferase: dimers and tetramers. Science. 1979 Jan 12;203(4376):174–176. doi: 10.1126/science.569362. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester S. C., LeVan S. K., Steglich C., DeMars R. Expression of human genes for adenine phosphoribosyltransferase and hypoxanthine-guanine phosphoribosyltransferase after genetic transformation of mouse cells with purified human DNA. Somatic Cell Genet. 1980 Mar;6(2):241–259. doi: 10.1007/BF01538799. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Shapiro L. J., Sparkes R. S., Sparkes M. C. Regional assignment of the steroid sulfatase-X-linked ichthyosis locus: implications for a noninactivated region on the short arm of human X chromosome. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5779–5783. doi: 10.1073/pnas.76.11.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Hellkuhl B., Grzeschik K. H., Shapiro L. J. Expression of an X-linked gene from an inactive human X chromosome in mouse-human hybrid cells: further evidence for the noninactivation of the steroid sulfatase locus in man. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6759–6763. doi: 10.1073/pnas.77.11.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Sparkes M. C., Shulkin J. D., Toomey K. E., Funderburk S. J. Regional localization of human gene loci on chromosome 9: studies of somatic cell hybrids containing human translocations. Am J Hum Genet. 1979 Sep;31(5):586–600. [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Shapiro L. J., Mohandas T., Weiss R., Romeo G. Non-inactivation of an x-chromosome locus in man. Science. 1979 Jun 15;204(4398):1224–1226. doi: 10.1126/science.156396. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Baluda M. C., Townsend D. E. Cellulose acetate electrophoresis of human glucose-6-phosphate dehydrogenase. J Lab Clin Med. 1969 Mar;73(3):531–534. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]