Abstract
Hyperprolactinemia is the most common cause of hypogonadotropic anovulation and is one of the leading causes of infertility in women aged 25–34. Hyperprolactinemia has been proposed to block ovulation through inhibition of GnRH release. Kisspeptin neurons, which express prolactin receptors, were recently identified as major regulators of GnRH neurons. To mimic the human pathology of anovulation, we continuously infused female mice with prolactin. Our studies demonstrated that hyperprolactinemia in mice induced anovulation, reduced GnRH and gonadotropin secretion, and diminished kisspeptin expression. Kisspeptin administration restored gonadotropin secretion and ovarian cyclicity, suggesting that kisspeptin neurons play a major role in hyperprolactinemic anovulation. Our studies indicate that administration of kisspeptin may serve as an alternative therapeutic approach to restore the fertility of hyperprolactinemic women who are resistant or intolerant to dopamine agonists.
Introduction
Hyperprolactinemia is the most common cause of hypogonadotropic anovulation (WHO Group I) and represents a major etiology of infertility, with highest incidence in women aged 25–34 years (1). In men, hyperprolactinemia is also frequently associated with hypogonadotropic hypogonadism. This gonadotropic deficiency has been proposed to result from direct suppression of prolactin (PRL) on gonadotrophin-releasing hormone (GnRH) release, but evidence supporting this mechanism has never been provided. PRL is synthesized and secreted by the lactotrope cells of the pituitary, and high levels of circulating PRL are mainly caused by lactotroph adenomas, which account for approximately 40% of all pituitary tumors. Pulsatile GnRH replacement can reverse hypogonadotropic hypogonadism and infertility induced by hyperprolactinemia in women as well as men (2, 3), suggesting that PRL excess in humans affects hypothalamic release of GnRH rather than directly affecting pituitary or gonad function. However, very few GnRH neurons in mice express PRL receptors (PRLRs) (4), suggesting that PRL exerts its actions on upstream neurons regulating the GnRH neuron. Because GnRH neurons are stimulated by kisspeptin (Kp) neurons (5, 6), which unequivocally express PRLR (7), we hypothesized that GnRH deficiency resulting from hyperprolactinemia is caused by reduced Kp input, which is now considered to be a primary gatekeeper governing reproduction (8, 9). Here, we show that hyperprolactinemia in mice induces hypogonadotropic anovulation and diminished Kp expression and that peripheral Kp administration restores GnRH and gonadotropin secretion and ovarian cyclicity. Therefore, we suggest that hyperprolactinemic women resistant or intolerant to dopamine agonists could take advantage of this therapeutic approach as a treatment for their infertility.
Results and Discussion
To address the mechanism of hyperprolactinemic anovulation, we developed a hyperprolactinemic mouse model simulating the human pathology by inserting micropumps releasing PRL over a period of 28 days. Control animals had normal estrous cycles every 5 days, while PRL-treated mice were acyclic or had irregular cycles following their first estrous cycle (Figure 1A). Another group of mice receiving PRL for only the first 14 days showed a resumption of normal estrous cycles after cessation of PRL delivery (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI63937DS1).
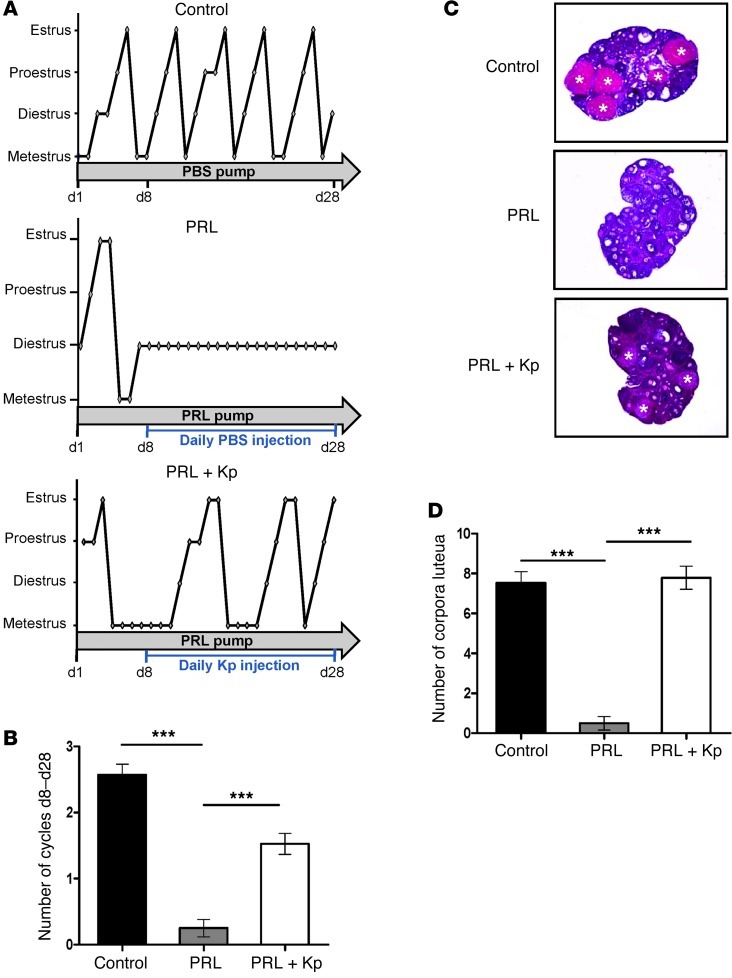
Figure 1. Effects of PRL infusion with daily injection of PBS or Kp on 6-week-old adult female mice.
(A) Estrous cycle profiles of a single representative mouse from control, PRL, and PRL+Kp groups over 28 days. Each stage of the estrous cycle was determined by daily vaginal smears. Lines under each diagram represent daily intraperitoneal injections of Kp or PBS as control in mice treated with PRL. (B) Mean cycle numbers during 21 days. PRL mice (n = 12) had significantly fewer cycles than control (n = 21) and Kp-treated mice (n = 19). ***P < 0.0001. (C) Representative ovarian histological sections from each group. Asterisks indicate corpora lutea, reflecting ovulation rate. (D) Mean number of corpora lutea in both ovaries at the end of the experiment. Analysis revealed a marked decrease in corpora lutea in the PRL group (n = 6) versus the control (n = 8) and PRL+Kp groups (n = 19). ***P < 0.0001.
Consistent with our hypothesis, daily Kp injections commencing on day 8 of PRL treatment and continuing to day 28 restored cyclicity (Figure 1B). Histological sections of both ovaries from each female were analyzed for the number of corpora lutea, a reflection of ovulation rate (Figure 1C). PRL-treated females exhibited few or no corpora lutea (0.50 ± 0.34, n = 6) as compared with control animals (7.5 ± 0.6, n = 8). These results indicate a clear impairment of ovulation by elevated PRL. Remarkably, daily injections of Kp were able to restore the ovulation rate (7.8 ± 0.6, n = 19) to that found in control females (Figure 1D). We have recently shown that Kp administration can restore luteinizing hormone (LH) pulsatility in neurokinin B–deficient patients, suggesting that the GnRH neuron sets up its own pulsatility when stimulated with Kp (10). This may explain the restoration by once-daily Kp in the hyperprolactinemic mice.
A set of mating experiments was performed to confirm that estrus took place. After introducing a fertile stud male mouse into the cage at the end of treatment, we recovered plugs in 3 of 4 PRL plus Kp–treated mice (data not shown), which is an index of successful mating that leads to pregnancy (11). The female mouse copulates only during estrus, when ova are ready for fertilization.
The anovulation in PRL-treated mice was accompanied by a significant decrease in pituitary Lhb and follicle-stimulating hormone β (Fshb) transcripts (Figure 2A) and serum LH and FSH levels (Supplemental Figure 2), indicating a gonadotropin deficiency, and this was reversed by Kp administration.
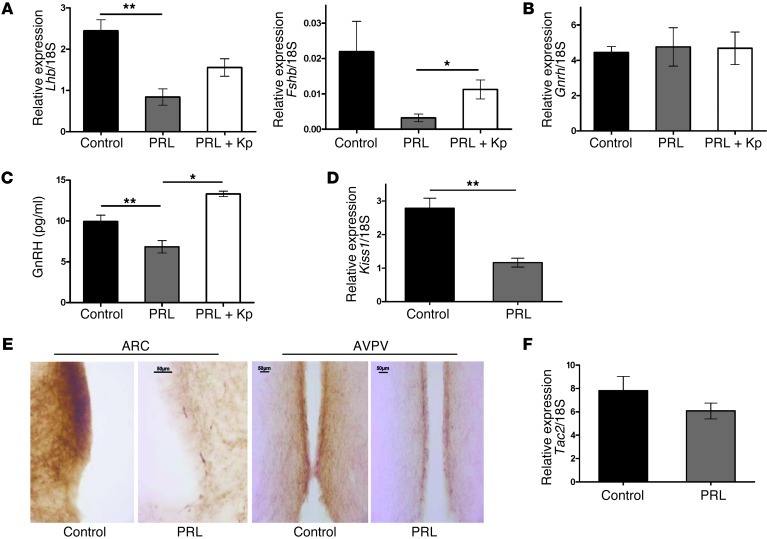
Figure 2. Effect of PRL on gene expression and Kp immunostaining and GnRH release, and effect of Kp on PRL inhibition of GnRH release.
(A) Gene expression of pituitary Lhb and Fshb transcripts is decreased in PRL mice (n = 4) compared with control mice (n = 19) and increased in PRL+Kp mice (n = 8). **P < 0.01, *P < 0.05. (B) Gene expression of hypothalamic Gnrh transcripts is not altered among all 3 groups: control (n = 27), PRL (n = 10), and PRL+Kp mice (n = 10). (C) PRL inhibition of GnRH release from hypothalamic explants is reversed by 500 nM Kp. n = 10 for each group. **P < 0.01, *P < 0.05; see Methods for details. (D) Gene expression of Kiss1 transcripts is significantly decreased in PRL-treated (n = 10) compared with control (n = 26) mice. **P < 0.01. (E) Representative of 6 independent experiments. Kiss neurons staining in ARC and AVPV sections from control and PRL-treated mice. Scale bars: 50 μm. (F) Gene expression of hypothalamic Tac2 transcripts is not altered in the PRL-treated group of mice (n = 8) compared with the control group (n = 18).
This suggests a deficiency in Gnrh gene expression and/or biosynthesis and/or GnRH peptide release. However, hypothalamic Gnrh gene expression was not altered in the 3 groups of mice (Figure 2B), as was reported in Kiss1-null mice (12, 13). To investigate whether GnRH release was impaired after PRL treatment and to test whether Kp could overcome this, we conducted a series of in vitro experiments using hypothalamic explants. We challenged medial basal hypothalamus (MBH) explants, including the median eminence, the arcuate nucleus, as well as the ventromedial nucleus, with PRL followed by Kp. GnRH released into the culture medium was significantly inhibited during the second hour of exposure to PRL (Figure 2C). The addition of Kp (500 nM) during the second hour of PRL treatment was able to restore GnRH secretion (Figure 2C). Our experiments therefore demonstrate the ability of PRL to inhibit the secretion of GnRH, and this seems to be mediated through decreased Kp secretion. These experiments aimed to avoid desensitization of the Kp receptor to continuous Kp exposure by limiting the periods of incubation (14). Overall, these data suggest that reduced Kp might mediate PRL inhibition of cycles but cannot completely exclude the possible involvement of other GnRH stimulators or inhibitors, or upstream regulators of Kp.
Since Kp reversed the inhibition of GnRH release and anovulation in PRL-treated female mice, we hypothesized that anovulation is due to hypothalamic Kp decline. In support of this, we demonstrated a significant decrease of hypothalamic Kiss1 mRNA in PRL-treated mice (Figure 2D). The emerging view of Kp signaling is that it is responsible for two modes of GnRH secretion: the estrogen-induced ovulatory surge of GnRH/LH and basal pulsatile GnRH/LH release. In rodents, there are two populations of Kp neurons, one in the anteroventral-periventricular nucleus (AVPV) involved in positive feedback of sex steroids on the gonadotrope axis and the second one in the arcuate nucleus (ARC) involved in negative feedback (15). Here, we have evidence of a decrease in immunoreactive Kp in both the ARC and AVPV, suggesting the involvement of these two nuclei in hyperprolactinemia-induced anovulation (Figure 2E). This contrasts with the opposing effects of sex steroids in the two regions (15). Recent studies suggest that the regulation of GnRH in ARC Kp neurons is more complex, as this population of Kp neurons has been found to coexpress another neuromodulator implicated in the regulation of gonadotrope axis, neurokinin B (16, 17). Thus, we also measured Tac2 gene and protein (NKB) expression, which was not altered after PRL administration (Figure 2F and Supplemental Figure 3). The presence of PRL receptors on Kp neurons (5) together with the suppression of Kp mRNA and peptide expression and restoration of ovarian cyclicity by Kp administration in our study are supportive of a direct inhibitory action of PRL on Kp neurons as the most parsimonious explanation. However, other GnRH stimulators or inhibitors, or upstream regulators of Kp, might be involved in PRL inhibition of cycles.
The data presented here reveal that treatment with PRL alone is sufficient to decrease Kiss1 mRNA. Thus, the decrease in Kiss1 mRNA levels found in the ARC and AVPV in rats during lactation (18, 19) is likely due to the increased PRL secretion during lactation. This had been uncertain in these previous studies, as there are many changes associated with lactation other than increased PRL. These include metabolic changes that have been shown to affect Kp and GnRH (15).
Together, these data are consistent with the hypothesis that the anovulation of hyperprolactinemia is mediated through PRL inhibition of Kiss1 gene expression and downstream diminution of GnRH and gonadotropin secretion. Kp neurons appear, therefore, to be the possible missing link between hyperprolactinemia and GnRH deficiency in mammals. Our findings suggest the possibility of therapeutic administration of Kp to hyperprolactinemic women who are resistant to dopamine agonists as a treatment for infertility.
Methods
Mice and treatment.
Six-week-old female mice were anesthetized with isoflurane inhalation. ALZET minipumps (model 2004) containing 200 μg PRL (National Hormone and Peptide Program [NHPP] AFP10692C, provided by A. Parlow, NIDDK, Harbor-UCLA Medical Center, Torrance, California, USA) or PBS (control group) were implanted s.c. between the scapulae as previously described (20). Minipumps delivered PRL at a rate of 7 μg/24 hours over 28 days. Pilot experiments established that the serum concentration of PRL was 260 ng/ml. Thirty-one mice were treated with PRL over 28 days, and 4 received PRL for only 14 days and were then observed for recovery of cycles. An additional group received PRL and daily intraperitoneal injections of 100 ng Kp (kisspeptin-10, synthesized by Bachem Distribution Service GmBH) commencing after 8 days of PRL treatment. Mice were sacrificed and tissues removed, weighed, and stored for subsequent gene expression and histological analyses.
Analysis of estrous cyclicity.
Estrous cycles were monitored by vaginal smears taken at the same time daily over 28 days and analyzed for the predominance of either lymphocytes, or nucleated epithelial cells or keratinocytes. One drop of PBS from a Pasteur pipette was expelled into the vagina, aspired, and then transferred to a microscope slide.
Histological studies.
Ovaries were dissected and fixed for 5 hours in 4% PFA, washed, paraffin-embedded, and sectioned at 3–4 μm. Tissue sections were analyzed after hematoxylin and eosin staining.
Immunohistochemistry.
Ten mice were anesthetized with intraperitoneal injection of pentobarbital and perfused through the heart first with saline (0.9% NaCl) and then with 15 ml 4% PFA in PBS over 9 minutes. The brains were removed, and pituitaries, hypothalami, and ovaries were dissected out and immersed in 15% aqueous sucrose solution (2% PFA, 15% sucrose in PBS) overnight. The tissues were stored in 30% sucrose in PBS, 0.02% sodium azide. Coronal brain sections (40 μm thickness) were cut on a freezing microtome and collected from the diagonal band of Broca (DGB) to the mammillary bodies. Free-floating sections were permeabilized in PBS/Triton X-100 (0.3%), followed by 10 minutes of heat-induced epitope retrieval in a microwave using 10 mM citrate buffer (pH6). Endogenous peroxidases were quenched using 0.3% H2O2 in PBS (25 minutes), and sections were blocked for 1 hour at room temperature using 10% normal goat serum, PBS, and 5% BSA. Sections were then incubated overnight at 4°C using a highly specific Kp antibody (1:15,000) (21). Immunoreactive sites were visualized using an ABC kit (Vector Elite Kit PK6101, Vector Laboratories) and DAB chromogen (ImmPACT DAB, SK-4105, Vector Laboratories). Kp-immunoreactive neuron counts in both the AVPV and ARC were performed in a blinded manner.
Gene expression analyses.
Total RNA was extracted from pituitaries and hypothalami using TRIzol reagent (Invitrogen). Quantitative real-time PCR was performed as described previously (22). After DNAse I treatment (Invitrogen), RNA was reverse transcribed and used for quantitative RT-PCR (qRT-PCR) using the Power SYBR Green PCR Master Mix (Applied Biosystems). Final primer concentrations were 300 nM for each primer (Supplemental Table 1 shows primer sequences). Reaction parameters were carried out on a StepOne Real-Time PCR System (Applied Biosystems). Reaction parameters were as follows: 95°C for 20 seconds, then 40 cycles at 95°C for 1 second and 60°C for 20 seconds. Controls without reverse transcriptase and without template were included to verify that fluorescence was not overestimated by residual genomic DNA amplification or from primer dimer formation. Moreover RT-PCR products were analyzed in a post-amplification melting curve to ensure that a single amplicon was obtained. Quantification was performed by the standard curve method. For preparation of standards, amplicons were purified from agarose gel and subcloned into a pGEM-T Easy plasmid (Promega), then sequenced to verify the identity of each fragment. Standard curves were generated by serial dilutions, spanning 6 orders of magnitude, yielding a correlation coefficient of at least 0.98 in all experiments. For all experiments, PCR efficiency was close to 1, indicating a doubling of DNA at each PCR cycle, as expected. Ribosomal 18S was used as reference gene for data normalization. Relative expression of a given gene is expressed as the ratio of attomoles of specific gene to femtomoles of rRNA 18S. Results are mean ± SEM and represent the relative expression compared with that obtained with controls.
Hormone ELISA.
For the LH ELISA, mouse LH (AFP5306A, NIDDK) was used as standard (range, 0.78–25 ng/ml), with anti–bovine LH chain mAb (2 μg/ml; 518B7; gift from J. Roser, UCLA, Los Angeles, California, USA) used as capture antibody. The second antibody was biotinylated anti–human LH mAb (1 μg/ml; Medix Biochemica 5303), and Amdex streptavidin-HRP conjugate (1:10,000; GE Healthcare) RPN4401Vq was used for signal detection.
For the FSH ELISA, mouse FSH (NIDDK AFP5308D; provided by A. Parlow, Harbor-UCLA Medical Center, Torrance, California, USA) was used as standard (range, 0.78–50 ng/ml), with anti–human FSH mAb (4 μg/ml; Medix Biochemica 6602, BiosPacific Inc.) used as capture antibody. The second antibody was biotinylated rabbit anti–rat α-subunit IC1 (5 μg/ml; NIDDK AFP66P99860), and anti–rabbit HRP conjugate (1:10,000; Thermo Scientific 31458) was used for signal detection.
Study of GnRH release.
To determine whether hyperprolactinemia acts through Kp on GnRH release, 30 hypothalami were dissected from untreated female mice, incubated individually in wells of a 48-well plate in 250 μl DMEM (21047033, Invitrogen) saturated with 95% O2/5% CO2, and placed at 37°C in a 5% CO2 atmosphere cell culture incubator. Hypothalamic explants from normal 10-week-old female mice were incubated for 1 hour to acclimate them and then exposed to vehicle or 1 μg/μl of PRL for 1 hour. In the third hour, explants were exposed to fresh vehicle or PRL, or PRL plus 500 nM Kp, and the medium was stored at –20°C for GnRH assay. The hypothalamic fragments were then challenged for 1 hour with 60 mM KCl to elicit GnRH release to confirm functional viability of the tissue fragments. GnRH concentrations were determined by a specific RIA as previously described (23) using the BDS 037 antibody. The sensitivity averaged 2 pg/ml (10 assays), and intraassay and interassay coefficients of variation averaged 10% and 13%, respectively.
Statistics.
Data are expressed as mean ± SEM. Mann-Whitney U test was used to determine significant differences between 2 groups. For multiple comparisons, Kruskal-Wallis test followed by Dunn’s post-test was performed with the use of Prism 4 (GraphPad Software). P values less than 0.05 were considered statistically significant.
Study approval.
All procedures were approved by the Ministère de l’Agriculture, France.
Supplementary Material
Acknowledgments
We would like to thank Philippe Zizzari for helpful discussions. C. Sonigo was supported by student fellowships from La Fondation pour la Recherche Médicale (FRM). R. Millar is recipient of a grant from the Medical Research Council (South Africa) and the University of Pretoria.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(10):3791–3795. doi:10.1172/JCI63937.
See the related Commentary beginning on page 3467.
References
- 1.Molitch ME. Pituitary gland: can prolactinomas be cured medically? Nat Rev Endocrinol. 2010;6(4):186–188. doi: 10.1038/nrendo.2009.278. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard P, Lagoguey M, Brailly S, Schaison G. Gonadotropin-releasing hormone pulsatile administration restores luteinizing hormone pulsatility and normal testosterone levels in males with hyperprolactinemia. J Clin Endocrinol Metab. 1985;60(2):258–262. doi: 10.1210/jcem-60-2-258. [DOI] [PubMed] [Google Scholar]
- 3.Lecomte P, Lecomte C, Lansac J, Gallier J, Sonier CB, Simonetta C. Pregnancy after intravenous pulsatile gonadotropin-releasing hormone in a hyperprolactinaemic woman resistant to treatment with dopamine agonists. Eur J Obstet Gynecol Reprod Biol. 1997;74(2):219–221. doi: 10.1016/S0301-2115(97)00091-2. [DOI] [PubMed] [Google Scholar]
- 4.Grattan DR, Jasoni CL, Liu X, Anderson GM, Herbison AE. Prolactin regulation of gonadotropin-releasing hormone neurons to suppress luteinizing hormone secretion in mice. Endocrinology. 2007;148(9):4344–4351. doi: 10.1210/en.2007-0403. [DOI] [PubMed] [Google Scholar]
- 5.d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JT, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 7.Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152(2):526–535. doi: 10.1210/en.2010-0668. [DOI] [PubMed] [Google Scholar]
- 8.Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides. 2009;30(1):57–66. doi: 10.1016/j.peptides.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Topaloglu AK, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 10.Young J, et al. Neuroendocrinology. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications [published online ahead of print February 24, 2012]. doi: 10.1159/000336376 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugh R. The Mouse: Its Reproduction and Development. New York, New York, USA: Oxford University Press; 1994. [Google Scholar]
- 12.d’Anglemont de Tassigny X, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 14.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147(5):2122–2126. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 15.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. doi: 10.1210/en.2011-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Kirigiti MA, Grove KL, Smith MS. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology. 2009;150(9):4231–4240. doi: 10.1210/en.2009-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada S, et al. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148(5):2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Kreye E, Kuo CB, Walker AM. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence and size when du145 human prostate cancer cells were grown in nude mice. Cancer Res. 2001;61(16):6098–6104. [PubMed] [Google Scholar]
- 21.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Viengchareun S, Servel N, Feve B, Freemark M, Lombes M, Binart N. Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2. PLoS One. 2008;3(2):e1535. doi: 10.1371/journal.pone.0001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caraty A, et al. Nature and bioactivity of gonadotropin-releasing hormone (GnRH) secreted during the GnRH surge. Endocrinology. 1995;136(8):3452–3460. doi: 10.1210/en.136.8.3452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.