Abstract
The explosive growth in our understanding of the molecular underpinnings of glioblastomas has served as an instructive paradigm for other cancers. However, the exact nature by which many of the pathogenic drivers connect is less well known, and elucidation of relationships between critical genetic and signaling alterations may inform the development of therapeutic approaches to the disease. In this issue, Song et al. identify miR-182 induction as a mechanism by which TGF-β stimulation aberrantly activates NF-κB signaling in glioblastoma cells, clarifying a critical point of cross-talk between molecular signaling pathways. Their findings provide a greater understanding of the complex interplay between signaling pathways in cancer that may ultimately prove useful in the development of synergistic targeting approaches.
Glioblastoma (World Health Organization grade IV glioma) is the most prevalent primary brain tumor; these highly lethal cancers are characterized by alterations in multiple critical intracellular signaling networks as well as by inactivation of tumor suppressors (1). Although specific pathways and molecules are frequently hyperactive and appear dominant in glioblastoma, unilateral molecular targeting approaches have been disappointing clinically. For example, most glioblastomas display hyperactive EGFR signaling as a result of increased receptor copy number or oncogenic activating mutations. However, single-agent EGFR targeting has not been successful in clinical trial (2). Because glioblastoma cells display plasticity in signaling networks without addiction to any one oncogene, successful therapy will require multipronged approaches that impede various active pathways for success with molecularly targeted agents (3). In theory, identification of signaling keystones and their interactions within the structurally complex architecture of glioblastoma will inform the development of effective therapeutic approaches to topple the colossus of cancer signaling.
Mapping the signaling axes
The multiple concerted signaling alterations that contribute to the malignant characteristics of glioblastoma have been interrogated by many researchers. NF-κB pathway activation has emerged as one of the critical central signaling axes in glioblastoma cells. NF-κB signaling can be activated by EGFR signaling, which is often a key feature of gliomas (4). Similarly, the constitutively active EGFRvIII mutant often present in glioblastoma activates NF-κB signaling (5). NF-κB is classically activated by inflammatory-related mechanisms, which also may be present and of oncogenic importance (6). In addition, recent work demonstrates that deletion of NF-κB inhibitor–α (NFKBIA, which encodes IκBα) in glioblastomas with nonamplified EGFR is associated with shorter patient survival, which suggests that NF-κB signaling is a central node in oncogenic signaling both in EGFR-amplified and nonamplified glioma (7). It is worth mentioning that signaling via other activated receptor tyrosine kinases (RTKs) can also stimulate NF-κB signaling. Thus, NF-κB is a central pathway mediating the effects of mitogen-activated signaling pathways like PDGFRA, ERBB2, and MET (1).
TGF-β–mediated signaling has paradoxical protumor effects in advanced tumor types, including glioblastoma, despite its tumor-suppressive role in normal tissues and in early-grade tumors (8). Active TGF-β signaling is associated with poor patient prognosis in glioblastoma (9), and multiple cellular alterations are known to be involved in the TGF-β switch from tumor-suppressive to tumor-promoting action in gliomas, including the methylation status of PDGF-B, PI3K/Akt-mediated FOXO1 inactivation, and high MYC activity (10). Additionally, although TGF-β typically represses NF-κB signaling in normal cells, recent evidence suggests an aberrant ability of TGF-β signaling to activate NF-κB signaling in several different cancers (11, 12) as a means of promoting malignant tumor cell phenotypes. However, the exact nature of the link between NF-κB and TGF-β signaling in glioma has to this point been relatively unclear.
A microRNA bridges two pathways
In the study reported in this issue of the JCI, Song et al. demonstrate a novel mechanism by which TGF-β stimulation may sustain NF-κB activity in glioma cells (13). They elucidate a connection between TGF-β stimulation and increased expression of the microRNA miR-182. Furthermore, they demonstrate miR-182–mediated suppression of the CYLD deubiquitinase, which typically functions as a negative regulator of NF-κB signaling via deubiquitinylation and resultant deactivation of multiple NF-κB intermediaries. The tumor-suppressive role of CYLD was verified by demonstrating that restoration of CYLD expression inhibited tumorigenesis in xenografted human gliomas, and the relevance of this finding was extended further when the authors found that human glioma patient survival directly correlated with CYLD expression. Derepression of NF-κB via miR-182 targeting of CYLD resulted in increased IKK activity, while also contributing to the aggressiveness of glioma cells in vitro and in vivo.
The authors found that glioma cell expression of miR-182 results in increased polyubiquitinylation of RIP, NEMO, and IκBα (ostensibly via repression of CYLD deubiquitinase), while miR-182 inhibition resulted in decreased polyubiquitinylation and decreased IKK kinase activity (13). However, still to be determined is the relative role of CYLD in deubiquitinylation of each of these targets and how modulation of each of them hyperactivates NF-κB signaling in glioma. This determination could be particularly relevant in the development of treatment approaches for glioma, in light of the fact that recent findings suggest that a subset of gliomas harbor heterozygosity for NFKBIA deletions (7). One could imagine that if miR-182–mediated CYLD repression preferentially activates NF-κB signaling via hyperubiquitinylation or inhibition of IκBα, anti–miR-182/TGF-β approaches may be more effective in completely inactivating NF-κB activity in tumors that harbor heterozygous NFKBIA deletions (Figure 1). Alternatively, if miR-182 repression of CYLD preferentially acts on NF-κB signaling via NEMO or RIP, the efficacy of anti–miR-182 approaches could potentially be less synergistic with alternative therapies in tumors heterozygous for NFKBIA.
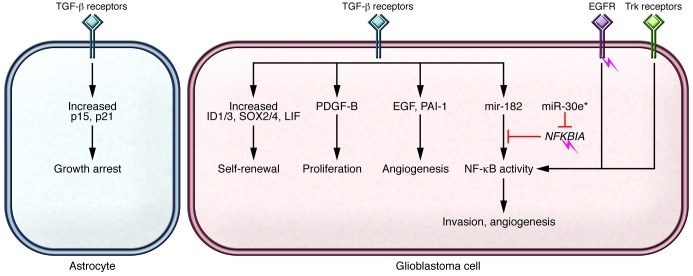
Figure 1. Effects of TGF-β signaling in glioblastoma.
TGF-β signaling is dysregulated on several levels in cancers, including glioblastoma, and mediates many systemic (immunosuppressive and angiogenic) and cellular effects. In contrast to other cancers, the canonical TGF-β pathway (ligand receptor/Smad mediators) is not commonly mutated in glioblastomas. However, modifiers of TGF-β signaling (e.g., FOXO, FOXG1, BF1, and USP15) and interaction with other pathways lead to a shift from primarily tumor-suppressive effects (e.g., antiproliferation) to oncogenesis. Targeting the interactions between TGF-β and NF-κB signaling may offer therapeutic options. Amplification of EGFR, an NF-κB activator, and mutation of NFKBIA (a NF-κB inhibitor) are mutually exclusive and may inform the effects of two miRs that regulate NF-κB activity, miR-182 and miR-30e*. The role of TGF-β and its relationship to other signaling pathways in gliomas contradicts the growth-suppressive effects and alternative signaling effects of TGF-β in noncancerous cells, such as astrocytes.
Caveat experimentor
Song et al. investigated the role of this TGF-β/miR-182/CYLD/NF-κB signaling network largely in serum-cultured cell lines, although the authors did provide some validation of certain key findings using patient-derived glioma cells (13). These cells were cultured using stem cell–permissive conditions, which more appropriately maintain tumor cell populations that reflect in vivo parental tumors than does passage in serum-based cell line culture conditions (14). However, this study did not explicitly address the role of this signaling in the various tumor cell subpopulations that contribute to the behavior of gliomas. In particular, one question that warrants further analysis beyond the scope of the current paper involves the relative contribution of this signaling axis in the highly tumorigenic cancer stem cell subpopulation of glioma versus the non-stem neoplastic population. In truth, the role for NF-κB signaling in glioma stem cells remains to be fully characterized. NF-κB signaling has been reported to be involved in the survival and tumorigenicity of glioma stem cells (15), while alternate evidence suggests that expression of A20, an inhibitor of TNF-α–mediated NF-κB signaling, actually supports the malignant phenotypes and tumorigenicity of glioma stem cells (16). How does derepression of NF-κB signaling via TGF-β–mediated miR-182 expression affect this critical tumor subpopulation? Song et al. provide evidence that this pathway exists in some types of glioma cells, but the relative contribution in stem and non-stem cancer cells remains to be examined and likely will be of critical importance in determining the therapeutic relevance of this group’s findings.
Moving forward
One holy grail sought by cancer researchers is the ability to design personalized (precise) treatment approaches using molecular characteristics of the individual patient tumors. Song and coworkers describe a specific subset of glioblastomas that display derepression of NF-κB signaling via TGF-β–mediated miR-182 expression (13), but this is not the dominant cross-talk in all tumors, as the same group recently described another subset of tumors in which miR-30e* expression dominates (17). Thus, the efficacy of treatments targeting NF-κB and/or TGF-β or other pathways could be affected whether a particular tumor exhibits dependence on miR-182, miR-30e*, or other factors. Furthermore, the complementary genetic alterations within various tumors (e.g., EGFR amplification/mutation, retinoblastoma mutation, MGMT methylation, and NFKBIA heterozygosity) could further determine the critical interweaving pathways that would be optimally targeted for a given tumor. Specifically relevant here, these factors may help determine the relevance of NF-κB derepression via TGF-β–mediated miR-182 expression described by Song et al. Finally, it is intriguing to consider the possibility that many pathways also play a role in resistance to gold-standard therapies such as radiation and chemotherapy. Relevant to this current study, there is evidence that miR-182 may decrease DNA repair (and thus sensitize tumor cells to radiation) via downregulation of BRCA1 (18). Perhaps identification of miR-182 expression levels in tumor tissue could aid in identification of tumors with precipitous genetic instability that could be taken advantage of via DNA-damaging treatments.
The sixth-century Chinese military strategist Sun Tzu wrote, “If the enemy leaves the door open, you must rush in” (19). As our understanding of the complex interplay between glioblastoma signaling pathways and genetic characteristics improves, more doors of therapeutic opportunity will open. In this way, the findings of this study not only highlight a novel signaling pathway within glioblastoma, but also identify a potential chink in the armor of glioblastoma.
Acknowledgments
We thank the sources of our funding, including NIH grants CA154130 and CA129958.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(10):3473–3475. doi:10.1172/JCI66058.
See the related article beginning on page 3563.
References
- 1.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin Cancer Res. 2008;14(4):957–960. doi: 10.1158/1078-0432.CCR-07-1810. [DOI] [PubMed] [Google Scholar]
- 3.Stommel JM, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 4.Habib AA, Chatterjee S, Park SK, Ratan RR, Lefebvre S, Vartanian T. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome. J Biol Chem. 2001;276(12):8865–8874. doi: 10.1074/jbc.M008458200. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor GS, Zhan Y, Johnson GR, O’Rourke DM. Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells. Mol Cell Biol. 2004;24(2):823–836. doi: 10.1128/MCB.24.2.823-836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 7.Bredel M, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364(7):627–637. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rich JN. The role of transforming growth factor-beta in primary brain tumors. Front Biosci. 2003;8:e245–e260. doi: 10.2741/992. [DOI] [PubMed] [Google Scholar]
- 9.Bruna A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11(2):147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis. 2006;27(11):2148–2156. doi: 10.1093/carcin/bgl068. [DOI] [PubMed] [Google Scholar]
- 11.Neil JR, Schiemann WP. Altered TAB1:I kappaB kinase interaction promotes transforming growth factor beta-mediated nuclear factor-kappaB activation during breast cancer progression. Cancer Res. 2008;68(5):1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JI, et al. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene. 2003;22(28):4314–4332. doi: 10.1038/sj.onc.1206478. [DOI] [PubMed] [Google Scholar]
- 13.Song L, et al. TGF-β induces miR-182 to sustain NF-κB activation in glioma subsets. J Clin Invest. 2012;122(10):3563–3578. doi: 10.1172/JCI62339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Nogueira L, et al. Blockade of the NFkappaB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene. 2011;30(32):3537–3548. doi: 10.1038/onc.2011.74. [DOI] [PubMed] [Google Scholar]
- 16.Hjelmeland AB, et al. Targeting A20 decreases glioma stem cell survival and tumor growth. PLoS Biol. 2010;8(2):e1000319. doi: 10.1371/journal.pbio.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang L, et al. MicroRNA-30e* promotes human glioma cell invasiveness in an orthotopic xenotransplantation model by disrupting the NF-kappaB/IkappaBalpha negative feedback loop. J Clin Invest. 2012;122(1):33–47. doi: 10.1172/JCI58849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskwa P, et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell. 2011;41(2):210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun T.The Art of War . Lionel Giles, translator. London, United Kingdom: Project Gutenberg EBook; 1994. http://www.gutenberg.org/files/132/132.txt . Accessed August 22, 2012.