Abstract
Cells of the primitive endoderm (PrE) and the pluripotent epiblast (EPI), the two lineages specified within the inner cell mass (ICM) of the mouse blastocyst stage embryo, are segregated into adjacent tissue layers by the end of the preimplantation period. The PrE layer which emerges as a polarized epithelium adjacent to the blastocoel, with a basement membrane separating it from the EPI, has two derivatives, the visceral and parietal endoderm. In this study we have investigated the localization of two transcriptional regulators of the SOX family, SOX17 and SOX7, within the PrE and its derivatives. We noted that SOX17 was first detected in a salt-and-pepper distribution within the ICM, subsequently becoming restricted to the nascent PrE epithelium. This dynamic distribution of SOX17 resembled the localization of GATA6 and GATA4, two other PrE lineage-specific transcription factors. By contrast, SOX7 was only detected in PrE cells positioned in contact with the blastocoel, raising the possibility that these cells are molecularly distinct. Our observations support a model of sequential GATA6 > SOX17 > GATA4 > SOX7 transcription factor activation within the PrE lineage, perhaps correlating with the consecutive periods of cell lineage ‘naïvete’, commitment and sorting. Furthermore our data suggest that co-expression of SOX17 and SOX7 within sorted PrE cells could account for the absence of a detectable phenotype of Sox17 mutant blastocysts. However, analysis of implantation-delayed blastocysts, revealed a role for SOX17 in the maintenance of PrE epithelial integrity, with the absence of SOX17 leading to premature delamination and migration of parietal endoderm.
Keywords: SOX17, SOX7, Mouse embryo, Cell lineage, Blastocyst, Pluripotent, Epiblast, Primitive endoderm, Implantation delay, Confocal imaging
Introduction
Mammalian development involves the formation of two extraembryonic tissues, the trophectoderm (TE) and the primitive endoderm (PrE), which are essential for embryo patterning, as well as providing the maternal–fetal interface required to sustain embryonic development. These extraembryonic tissues are the first two cell lineages specified in the early embryo, and both exhibit characteristic epithelial morphology. By the time of implantation into the maternal uterus, the TE and PrE tissue layers are segregated from, but encapsulate, the pluripotent epiblast (EPI).
The first cell fate decision arises at the time of embryo compaction when polarized blastomeres undergo asymmetric divisions generating polar outside cells and apolar inside cells (Johnson and Ziomek, 1981). Outside cells will form the TE, which will generate the fetal portion of the placenta, while inside cells will form the inner cell mass (ICM), the cells of which will commit to an EPI or PrE fate. The EPI will give rise to most of the cells of the embryo-proper, while the PrE will eventually differentiate into the parietal endoderm (PE) and visceral endoderm (VE) of the yolk sac.
Up until the 64-cell stage markers of nascent PrE and EPI are co-expressed by individual ‘naïve’ or uncommitted ICM cells (Guo et al., 2010; Plusa et al., 2008). However after the 64-cell stage, a mutually exclusive salt-and-pepper distribution of cells expressing either PrE or EPI markers is established within the ICM, suggesting that lineage commitment occurs around this time (Chazaud et al., 2006; Kurimoto et al., 2006; Plusa et al., 2008). In support of this salt-and-pepper distribution of lineage-committed cells, fate mapping of single ICM cells in E3.5 embryos has revealed their predominant restriction to PrE or EPI lineages (Chazaud et al., 2006; Rossant et al., 2003). By the late (E4.5) blastocyst stage, cells of the PrE and EPI lineages are spatially segregated into two distinct compartments (Gardner and Rossant, 1979). Cells of the PrE eventually congregate on the surface of the ICM. There the PrE matures to form a morphologically-distinct polarized epithelial layer positioned at the interface between the ICM and the blastocoel. In this arrangement, the pluripotent EPI compartment is encapsulated by two epithelia: the PrE on the blastocoelic side, and the polar TE on the maternal uterine side.
The process of PrE vs. EPI spatial segregation, in which cells transition from an initially scattered to a segregated distribution is often referred to as cell sorting. Cell sorting is likely achieved through multiple cell behaviours including actin-dependent active cell movements, retention of position by PrE committed cells initially in contact with the blastocoel cavity, as well as the downregulation of gene expression and selective apoptosis of cells which fail to sort (Meilhac et al., 2009; Plusa et al., 2008). However, the molecular mechanisms underlying the process of cell sorting are still poorly understood. It is presumed however that this results from selective differences in adhesive properties of PrE (vs. EPI) cells that are acquired concomitant with their epithelialization. Embryos lacking the polarity protein DAB2 (Yang et al., 2002) or the extracellular matrix protein LAMC1 (Smyth et al., 1999) correctly specify PrE cells within the ICM, but these cells either fail to correctly sort, or be maintained in contact with the blastocoel cavity. Integrins α5 and β1 have been shown to play a role in extraembryonic endoderm (ExEn) formation in embryoid bodies (EBs) (Liu et al., 2009), and Itgb1 mutant embryos die shortly after implantation with defects in the specification or differentiation of the PrE (Fassler and Meyer, 1995; Stephens et al., 1995).
The specification and differentiation of the PrE relies on the expression of key transcriptional regulators primarily those belonging to GATA, SOX, and HNF protein families. SOX17 is a member of SOX (SRY-related high mobility group box) transcription factor family, members of which act in various developmental processes (Bowles et al., 2000; Pevny and Lovell-Badge, 1997; Wegner, 1999). SOX17, together with SOX7 and SOX18, belongs to the SOX group F subfamily (Bowles et al., 2000). Previous studies have revealed its role in the regulation of fetal hematopoiesis (Kim et al., 2007) and vasculogenesis (Matsui et al., 2006; Sakamoto et al., 2007). SOX17 has also has been proposed to function as a key regulator of endoderm formation and differentiation, a function that is conserved across vertebrates (Alexander and Stainier, 1999; Clements and Woodland, 2000; Hudson et al., 1997). In the mouse, genetic inactivation of Sox17 leads to severe defects in the formation of the definitive endoderm (Kanai-Azuma et al., 2002).
Intriguingly, Sox17 expression has been reported before embryo implantation at the morula/early blastocyst stage where it is localized to the nascent PrE (Niakan et al., 2010; Morris et al., 2010). However, despite this PrE-specific localization, Sox17 mutant embryos develop a PrE layer and can be recovered up to midgestation when they exhibit gut endoderm defects (Kanai-Azuma et al., 2002; M. Viotti and AKH unpublished observations).
However, overexpression of Sox17 in single blastomeres of 8-cell stage embryos biased their commitment towards PrE, while down-regulation biased their commitment towards EPI (Morris et al., 2010). Moreover, several lines of evidence, primarily from ex vivo models of extraembryonic endoderm (ExEn) formation, support a role for SOX17 in the PrE lineage. Extraembryonic Endoderm (XEN) cell lines cannot be established from Sox17 mutant embryos (Niakan et al., 2010), while downregulation of Sox17 by RNA interference impairs XEN cell maintenance (Lim et al., 2008). In addition, there are conflicting findings from studies of Sox17 mutant ES cells. In one study, embryoid bodies (EB) derived from Sox17 mutant mouse ES cells failed to correctly specify an outer ExEn layer (Niakan et al., 2010). By contrast, another study reported normal differentiation of Sox17 mutant ES cells into PrE, but impaired differentiation towards PE and VE fates (Shimoda et al., 2007). It is also not clear whether SOX17 overexpression is sufficient to drive endoderm formation of mouse undifferentiated ES cells or whether it facilitates ExEn formation when ES cells are directed to differentiate (Niakan et al., 2010; Qu et al., 2008; Shimoda et al., 2007). Importantly, by contrast to mouse ES cells, sustained overexpression of SOX17 in human ES cells is sufficient to direct their differentiation into definitive endoderm, but not ExEn, progenitors, supporting the non-equivalence of human and mouse ES cell and their respective differentiation potentials (Seguin et al., 2008).
Prompted by these apparently disparate observations, we performed an in depth analysis of PrE formation both in wild-type and Sox17 mutant mouse embryos to further elucidate the role of Sox17 in ExEn formation and maintenance. We first reasoned that the lack of an apparent phenotype in absence of Sox17 could be compensated by another closely related protein. We therefore investigated the related protein SOX7 and noted that its localization was unaltered in Sox17 mutants, suggesting that SOX7 might compensate for the absence of SOX17.
Moreover, by performing a detailed analysis of the localization of SOX7, we noted it as the first marker of sorted PrE cells, representing the emergent PrE layer, positioned on the surface of the ICM in contact with the blastocoel and co-expressing Sox17. Thus, since SOX7 is localized to a subset of SOX17-positive cells, our observations also suggest that Sox7 expression is not regulated by SOX17. Importantly, these observations reveal that sorted PrE cells are molecularly distinct from unsorted cells, and that a signature transcription factor code may operate within the PrE lineage. Intriguingly such a precisely coordinated sequence of transcription factor activation might be used to orchestrate the successive steps lineage differentiation.
Next, to rule out the possibility that maternal stores of mRNA or protein might mask a phenotype in mutant embryos we generated mouse embryos with a maternal–zygotic depletion of Sox17. In these embryos we noted a moderate reduction of the number of PrE cells in absence of Sox17. However, since we also noted that maternal deletion of Sox17 affected early embryonic development, such that ICMs were smaller, these experiments did not formally corroborate a role for Sox17 in PrE specification. We propose that this maternal effect may be due to a moderate cytotoxic effect of CRE protein activity and/or genetic background.
Since these experiments did not allow us to determine a role for SOX17 in PrE lineage specification, we went on to test whether SOX17 might function in PrE lineage maintenance, expansion or differentiation. To do so, we analyzed the effect of artificially delaying embryo implantation, an approach that preserves the topology of the three tissue layers of the blastocyst but promotes their expansion (Nichols et al., 2001; Artus et al., 2010). Surprisingly, implantation-delayed Sox17−/− blastocysts exhibited defects in the PrE layer leading to premature differentiation and PE cell migration along the mural TE. These observations suggest a role for SOX17 not in PrE lineage specification per se, but within the emergent and polarized PrE layer for the maintenance of its epithelial integrity and regulation of its differentiation.
Based on these observations, we propose a model whereby SOX17 is involved in the maintenance of the epithelial integrity of the PrE tissue layer. Moreover, we suggest that the lack of a PrE defect in implanting Sox17 mutant blastocysts is likely not due to maternal stores of Sox17, but may be compensated for by the presence of SOX7, a related transcription factor specifically expressed in sorted PrE cells.
Materials and methods
Mouse husbandry
Mouse strains used in this study were: Sox17cKO/cKO (Kim et al., 2007); Sox2::Cre (Hayashi et al., 2002); Zp3::Cre (Lewandoski et al., 1997); PdgfraH2B–GFP/+ (Hamilton et al., 2003) and wild-type ICR (Taconic). Sox17KO/+ animals were generated by crossing of Sox17cKO/cKO to a Sox2:: Cre strain of mice. Embryos with maternal and zygotic ablation of Sox17 were obtained by breeding Sox17KO/CKO; Zp3::CreTg/+ females with Sox17KO/+ males.
Induction of diapause
Diapause was induced following intraperitoneal injection of 10 μg Tamoxifen (Sigma) and 2–3 mg progesterone (Abraxis) at 2.5 days post coitum. Embryos were recovered 3 days later (referred to as E2.5+3-day implantation delay).
Embryo recovery and processing
Mice were maintained under a 12-hour light cycle. Preimplantation embryos were flushed from uteri or oviducts in M2 (Millipore). The zona pellucida was removed using acid Tyrode’s solution (Sigma). Embryos were fixed in 4% paraformaldehyde (PFA) in PBS with 0.1% Tween-20 (Sigma) and 0.01% Triton X-100 (Sigma) for 10 min at room temperature or overnight at 4 °C. Postimplantation embryos were dissected in DMEM-F12 containing 5% fetal calf serum, fixed in 4% PFA in PBS for 1 h to overnight at 4 °C and processed for cryostat sectioning.
Immunostaining
Immunostaining was carried out as previously described (Plusa et al., 2008; Artus et al., 2010). In brief, embryos were permeabilised in 0.55% Triton X-100 in PBS for 20 min and blocked in 10% fetal bovine serum in PBS for 1 h. Embryos were incubated in primary antibody solution overnight at 4 °C, or for 1 h at room temperature. Following primary antibodies were used at dilution of 1/100: anti-DAB2 (BD Transduction Laboratories), anti-GATA4 (Santa Cruz), anti-GATA6 (R&D Systems), anti-GFP (Invitrogen), anti-Laminin (Sigma), anti-SOX7 (R&D Systems) and anti-SOX17 (R&D Systems); anti-OCT4 (Santa Cruz) at 1/200; anti-NANOG (CosmoBio) at 1/500; anti-CDX2 (BioGenex) undiluted. Secondary Alexa Fluor (Invitrogen)-conjugated antibodies were used at a dilution of 1/500. DNA was visualized using Hoechst 33342 (5 μg/mL, Molecular Probes).
Image data acquisition, processing and quantitation
Laser scanning confocal images of immunostained embryos were acquired on a Zeiss LSM 510 META. Embryos were mounted in Vectashield (Vector Laboratories). Fluorescence was excited with a 405-nm laser diode (Hoechst), a 488-nm Argon laser (GFP, Alexa Fluor 488), a 543-nm HeNe laser (Alexa Fluor 543, 555) and a 633-nm HeNe laser (Alexa Fluor 633 and 647). Images were acquired using a Plan-apochromat 20×/NA 0.75 objective, with optical section thickness of 1–1.2 μm. Raw data were processed using Zeiss AIM software (Carl Zeiss Microsystems) or IMARIS 6.4.2 software (Bitplane AG).
For cell counting, the number of PrE and EPI cells was established from the number of nuclei positive for GATA4/6 and NANOG respectively. TE cells were defined as all cells located at the surface of the embryo and negative for GATA4/6 and NANOG.
For quantification, z-stacks of laser scanning confocal images of diapause embryos were 3D-reconstructed using IMARIS 6.4.2 software. Embryonic/abembryonic axis was defined as the axis orthogonal to the PrE/EPI interface. PrE cell nuclei were then identified using the “measurement point” option, and their distance from the embryonic region was calculated. To view the distribution of PrE cells from the embryonic pole, the mural TE was removed from 3D-reconstructed embryos using the “clipping plane” option.
Genotyping
Embryos were subsequently lyzed into 10 μL lysis buffer (10 mM Tris pH 8.5, 50 mM KCl, 0.01% gelatin, 100 μg/mL Proteinase K) for 50 min at 50 °C. Proteinase K was then inactivated 10 min at 90 °C. 2 μL of lyzed embryo was used as PCR template. PCR was performed using Advantage 2 polymerase (Clontech) on a Mastercycler PCR machine (Eppendorf) using the following program: 1 cycle: 94 °C 3 min; 42 cycles: 94 °C 30 s, 61 °C 1 min, 72 °C 1 min; 1 cycle: 72 °C 10 min. Primer sequences: Sox17For: 5′-TTGCCGAACACACAAAAG-GAG-3′, Sox17RevFlox: 5′-TGGAGGTGCTGCTCACTGTAAC-3′ and Sox17RevKO: 5′-GGACTGGAAAATGAGAGAATAGCG-3′ (Kim et al., 2007).
Results and discussion
Localization of SOX17 correlates with the emergence of the PrE lineage in the preimplantation mouse embryo
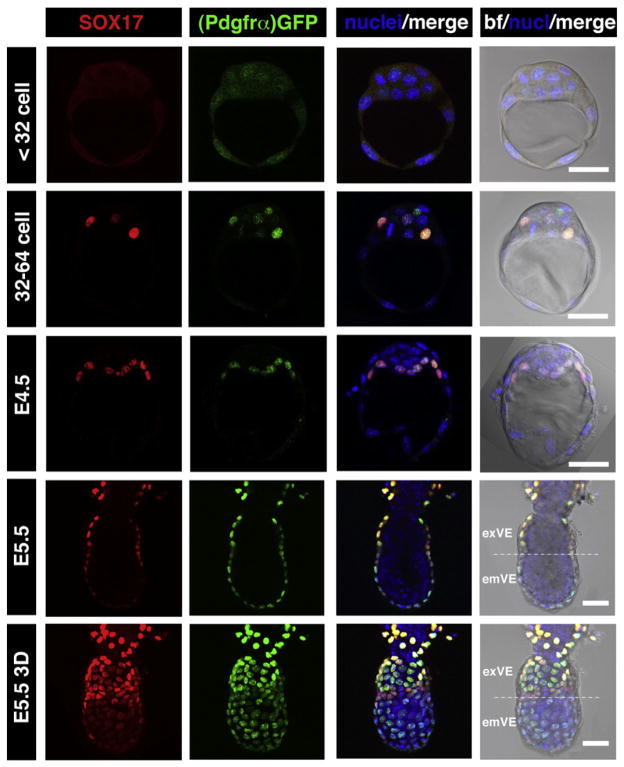
We noted that SOX17 was first detected in preimplantation embryos starting at E3.5, as previously reported (Niakan et al., 2010; Morris et al., 2010). Since it has recently emerged that many lineage-specific factors exhibit a dynamic and stereotypical localization (Dietrich and Hiiragi, 2007; Plusa et al., 2008), we analyzed in detail and quantified the distribution of SOX17 protein at pre- and periimplantation stages in relation to other lineage-specific markers. SOX17 was first detected in the nuclei of a subset (1–3) of ICM cells at the 32–64 cell stage (Fig. 1), corresponding to the stage at which overlapping expression of the PrE (GATA6) and EPI (NANOG) lineage-specific trans-factors is observed. From its onset, SOX17 was detected only in a subset of Pdgfrα-GFP-positive cells (Fig. 1). We have previously described PDGFRα as one of the earliest PrE markers, and a PdgfraH2B–GFP knock-in allele as one of the earliest reporters of the PrE lineage (Plusa et al., 2008; Artus et al., 2010). At the stage corresponding to salt-and-pepper distribution of PrE and EPI markers (>64-cell embryo) the number of SOX17-positive cells had increased and exhibited complete co-expression with Pdgfrα-GFP (data not shown). By the late blastocyst stage (E4.5), SOX17 was exclusively detected in PrE cells lining the blastocoel. Since it has been established that the emergence of GATA4 expression coincides with the appearance of a salt-and-pepper distribution of cells within the ICM corresponding to lineage commitment (Plusa et al., 2008), this would suggest a sequence of activation of GATA6 > PDGFRα > SOX17 > GATA4 within the PrE lineage of the ICM.
Fig. 1.
SOX17 is a marker of the primitive endoderm and its derivatives, the visceral and parietal endoderm of the mouse embryo. SOX17 is first detected in E3.5 embryos at 32–64 cell stage, localizing to GFP expressing cells in PdgfraH2B–GFP/+ embryos, and at E4.5 is found exclusively in the PrE layer. After implantation (E5.5) SOX17 is expressed only in the two PrE derivatives, PE, which exhibits the highest levels of expression, and VE, which exhibits reduced levels of expression towards distal end of the embryo overlying the epiblast. Each horizontal row represents one embryo. All panels depict single optical sections, the lowest panel depicts a 3D-reconstructions of a confocal z-stack. em: embryonic region; ex: extraembryonic region. Pdgfrα-GFP, green; SOX17, red; Hoechst, blue. Scale bar: 20 μm.
We next investigated the localization of SOX17 in the two PrE derivatives, the visceral endoderm (VE) and parietal endoderm (PE), at early postimplantation stages (E5.25–5.75). Initially, at E5.25, nuclear-localized immunofluorescence was detected in all VE cells (data not shown). However, later on after migration of the anterior visceral endoderm (AVE), SOX17 appeared to be downregulated in the distal portion of the VE, often referred to as the emVE (Mesnard et al., 2006), overlying the EPI, while being maintained at high levels in VE overlying extraembryonic ectoderm, which is often referred to as the exVE (Figs. 1 and 4a–d). We noted that throughout this early postimplantation period SOX17 was maintained at uniformly high levels throughout the PE, and that at all stages examined, the level of fluorescence in the PE appeared to be higher than that in the VE (Fig. 4a).
Fig. 4.
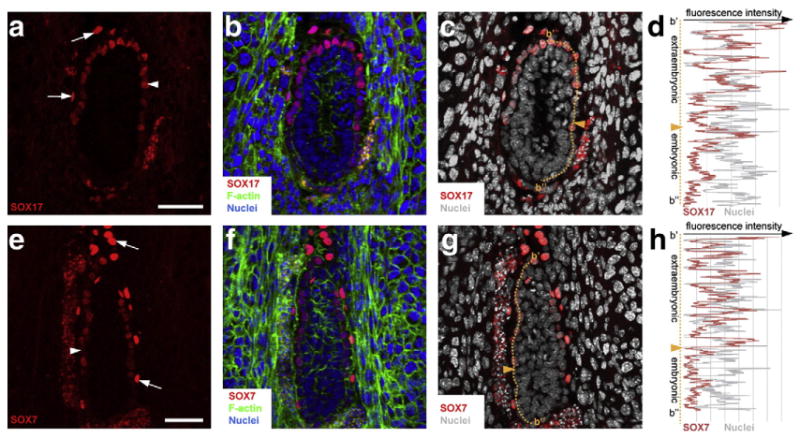
SOX7 and SOX17 are detected in PrE derivatives at E5.5 postimplantation stage. Immunodetection of SOX17 (a–d) and SOX7 (e–h) on cryosections of decidua containing E5.5 wild-type embryos. SOX17 and SOX7 were detected both in PE (arrows) and VE (arrowheads). (d, h) Measurement of fluorescence intensity of SOX17 (d) and SOX7 (h) along the proximal–distal axis. Orange dashed lines indicate where the measurements were done and orange arrowheads indicate the boundary between embryonic and abembryonic regions. SOX7, SOX17, red; nuclei, blue and grey; F-actin, green. Scale bar: 50 μm.
Sox7 is localized to PrE cells positioned on the blastocoel cavity
Phylogenetic analyses place SOX17 in the F group of the SOX protein family along with its close relatives SOX7 and SOX18 (Bowles et al., 2000). Previous studies have reported that Sox7 is expressed in the PE at E7.5 (Murakami et al., 2004), and that it is involved in PE fate induction in F9 embryonal carcinoma cells (Futaki et al., 2004). However any earlier expression of Sox7, specifically at preimplantation stages, has not been previously reported. Given the mild phenotype observed in Sox17 mutants, coupled with the extensive homology between SOX7 and SOX17, we sought to determine the localization of SOX7 at periimplantation stages.
Our analyses revealed that SOX7 was expressed specifically within the PrE lineage at periimplantation stages (Fig. 2a). SOX7 was first detected only in embryos of >64-cells in a subset of Pdgfrα-GFP-positive cells (Fig. 2a). Initially, SOX7 was detected in 1–3 cells per embryo of 65–89 cells (2.3 cells in average per embryo, N=7), comprising 3.2% of the total cell number (Fig. 3). However, since only a subset of Pdgfrα-GFP-positive cells were SOX7-positive (15%, N=5), we noted that SOX7 was observed only in PrE cells that were on the surface of the ICM in contact with the blastocoel (Figs. 2a, 3). Neither Pdgfrα-GFP-positive cells localized in deeper layers of the ICM, nor NANOG-positive cells located on the cavity roof expressed SOX7 (Figs. 2a, 3). SOX7 was expressed later than other PrE lineage-specific markers, appearing only in a small subset of cells in embryos of >64-cells, when other markers including GATA6, GATA4 and PDGFRα, as well as SOX17, were expressed in all PrE-committed cells. These data suggest that SOX7 is a marker of cells committed to the PrE lineage that are in their final position within the future PrE layer. Therefore SOX7 is the first transcription factor reported to be restricted to this sorted PrE population, and in doing so defines it as molecularly distinct.
Fig. 2.
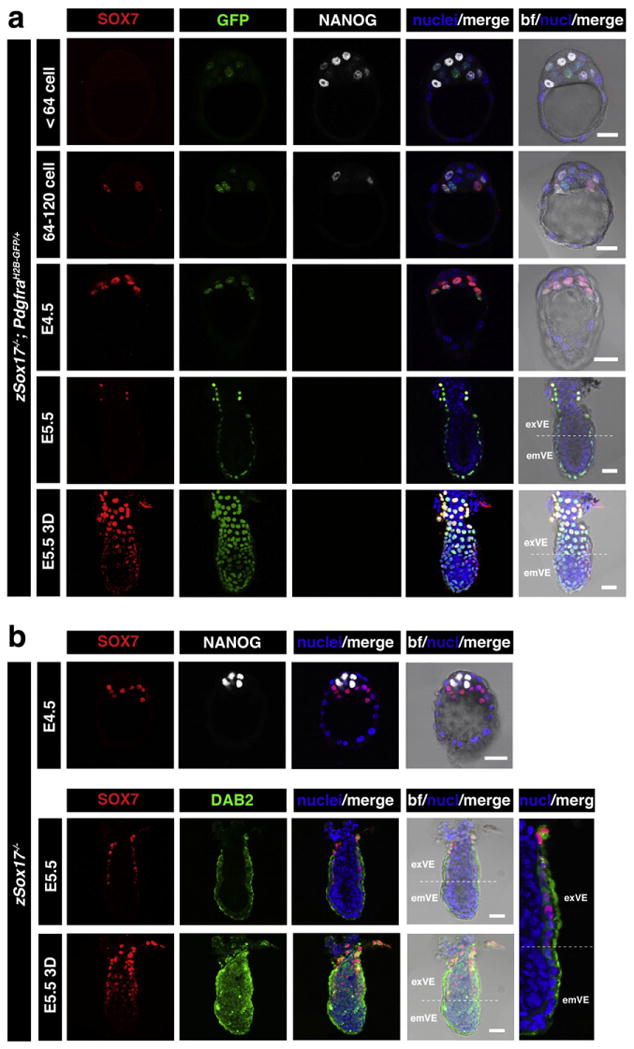
SOX7 marks PrE cells positioned adjacent to the blastocyst cavity and is expressed in the absence of Sox17. Immunodetection of SOX7 in periimplantation mouse embryos. (a) SOX7 is first detected in E3.5 embryos after 64-cell stage, localizing only to cells in contact with the blastocoel cavity, and in PdgfraH2B–GFP/+ embryos to GFP-positive cells (PrE committed). At E4.5 its expression is restricted to PrE. After implantation (E5.5) SOX7 is detected weakly in exVE. (b) In Sox17−/− embryos, Sox7 expression is not affected at E4.5 and E5.5. Each row represents one embryo. All panels show single optical sections, the lowest panel shows 3D-reconstruction of confocal image. em: embryonic region; ex: extraembryonic region. Pdgfrα-GFP, green (a); DAB2, green (b); SOX7, red; NANOG, white; Hoechst, blue. Scale bar: 20 μm.
Fig. 3.
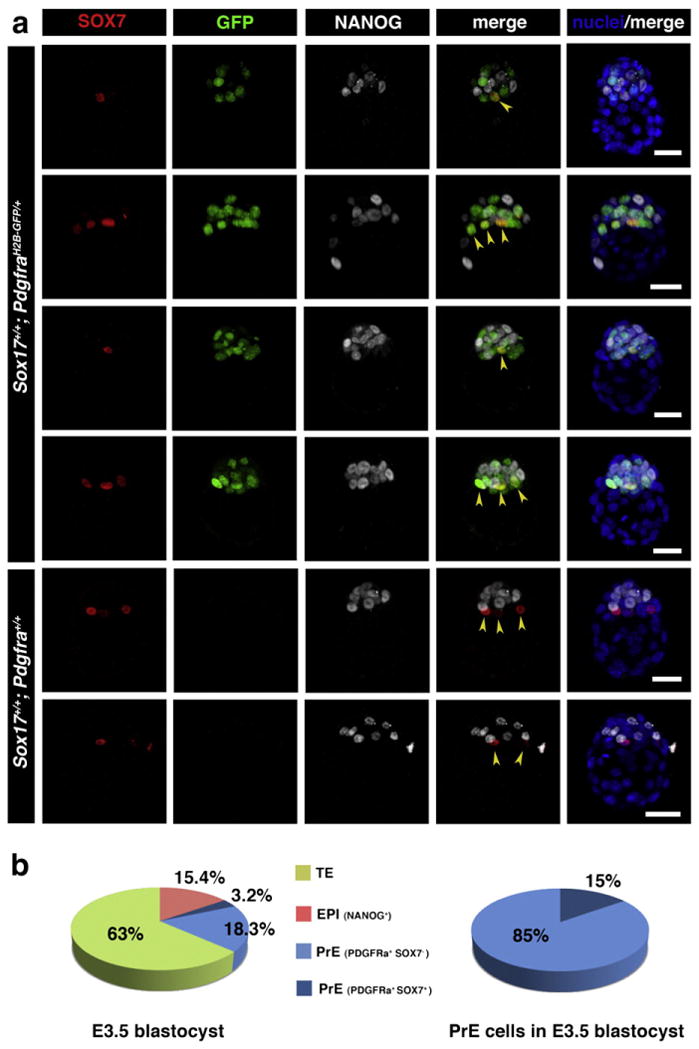
SOX7 is selectively localized to the PrE cells positioned adjacent to the blastocyst cavity prior to lineage segregation. (a) Immunodetection of SOX7 in preimplantation mouse embryos at E3.5 (65–80 cells). SOX7 is first detected in E3.5 embryos after 64-cell stage, localizing only to cells in contact with the blastocoel cavity (arrowheads). In PdgfraH2B–GFP/+ embryos it is co-localized with GFP in cells adjacent to the cavity. Each row represents one embryo, all panels show 3D-reconstruction of confocal images. Pdgfrα-GFP, green; SOX7, red; NANOG, white; Hoechst, blue. Scale bar: 20 μm. (b) Distribution of TE (green), EPI (red) and PrE (SOX7-negative — light blue, and SOX7-positive — dark blue) cells in embryos at E3.5.
As cell sorting into respective layers proceeded in embryos of >64-cells, the number of SOX7-positive cells increased until all PrE cells became SOX7-positive, and came to be positioned on the cavity roof. At this time all PrE cells were positive for GATA6, GATA4 and PDGFRα, as well as SOX17. These observations support a model of sequential GATA6 > PDGFRα > SOX17 > GATA4 > SOX7 marker activation within cells of the PrE lineage, perhaps correlating with the successive periods of cell lineage ‘naïvete’, commitment, scattered distribution and congregation within the ICM.
Positioning of PrE cells in their final location at the cavity roof is likely driven by expression of lineage-specific transcriptional regulators acting in combination with positional induction (Plusa et al., 2008; Meilhac et al., 2009). However the mechanism(s) coupling these contributing factors remains unknown. In E3.75 mouse blastocysts DAB2 and LRP2 are already localized apically in some ICM cells adjacent to the blastocoel cavity (Gerbe et al., 2008), perhaps reflecting this early positioning process. Given its lineage and positional-specific localization, it is intriguing to speculate that SOX7 may play a role in regulating or reinforcing positional signals in cells fated to the PrE lineage (reviewed in Yamanaka et al., 2006).
After early postimplantation stages (E5.5), SOX7 was detected in both VE and PE derivatives of the PrE (Figs. 2a and 4e–h). While strongly expressed in the PE, we noted that Sox7 was also expressed in the exVE and downregulated in the emVE similarly to Sox17 expression (Figs. 4a–d).
SOX7 is expressed in Sox17-deficient embryos
Having determined that SOX7 is expressed in SOX17 expressing cells as they congregate on the cavity roof, we investigated the expression of SOX7 in Sox17 mutant embryos. We noted that Sox7 was present and its localization was unperturbed in Sox17-deficient embryos, both at preimplantation and early postimplantation stages (Fig. 2b). The presence of SOX7 in PrE cells on the cavity roof of Sox17 mutants would suggest that Sox7 expression is not regulated by SOX17, and that SOX7 might compensate for loss of SOX17.
In support of a possible redundancy between these two closely related proteins, previous reports have noted that Sox7 transcripts levels were elevated at later (E8.0) stages in Sox17 deficient extraembryonic tissues (Shimoda et al., 2007). In addition SOX7 and SOX17 are able to bind enhancers of Fn1 (Shirai et al., 2005) and Lama1 (Niimi et al., 2004) genes encoding constituents of the basement membrane. It is therefore likely that a similar redundancy may be in effect in the PrE, and this may account for the modest defects observed in Sox17 mutants. Future studies analyzing double Sox7; Sox17 mutants will therefore be required, and may help uncover a precise role for these SOX factors within the PrE lineage.
PrE endoderm specification is unaffected in embryos deficient in Sox17
To address the role of Sox17 in PrE formation, we quantified cell lineage commitment in Sox17−/− mutants compared to stage-matched Sox17+/− heterozygous and Sox17+/+ wild-type embryos (Figs. 5a, d). Absence of SOX17 protein confirmed the identity of mutant embryos, which were otherwise morphologically indistinguishable from heterozygous or wild-type embryos. AtE4.5 Sox17 mutant embryos expressed both PrE (GATA4) and EPI (NANOG) specific transcription factors, and both these lineages were fully sorted such that the PrE layer was in contact with the blastocoel (Fig. 5a). By analyzing the distribution of cells within each of the three lineages of the blastocyst, a quantitative measure of lineage specification can be obtained (Fig. 5d). We failed to detect a statistically significant difference in the number of TE or EPI cells in mutant embryos as compared to stage-matched wild-type or heterozygous embryos. Similarly, the number of PrE cells did not deviate in mutants (25.9±9.7 PrE cells) when compared to wild-type (33.9±7.6 PrE cells) or heterozygous (28.0±10.0 PrE cells) embryos. From these observations, we conclude that the allocation of cells within the first three lineages is not affected by the absence of Sox17. In addition, by early postimplantation stages, E5.5 Sox17 mutant embryos were recovered at the expected Mendelian ratios and, at least by gross morphology, were indistinguishable from heterozygous and wild-type littermates (data not shown).
Fig. 5.
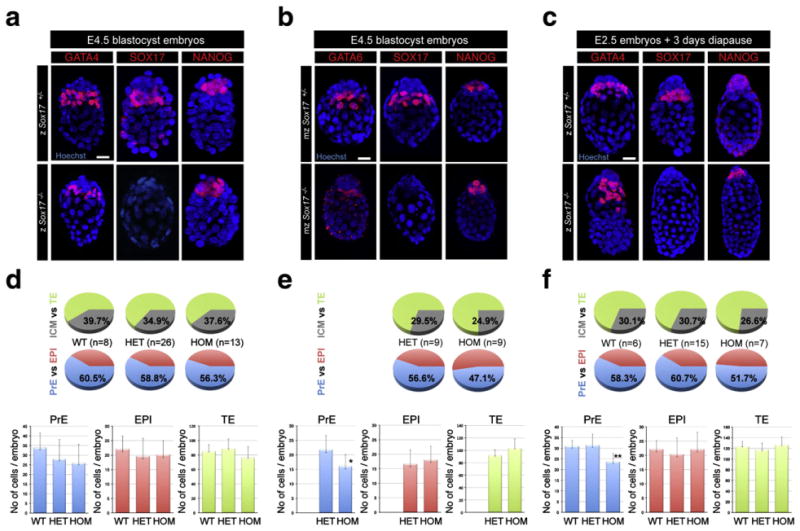
Mouse blastocysts lacking Sox17 correctly specify and segregate embryonic and extraembryonic lineages. Zygotically zSox17 +/− (upper panels) and zSox17−/− embryos (lower panels) at E4.5 (a) and 3 days after tamoxifen injection (c). Maternally and zygotically ablated mzSox17 +/− (upper panels) and mzSox17−/− embryos (lower panels) at E4.5 (b), Blue, nuclei; red, GATA4, GATA6, SOX17 and NANOG. Scale bar: 20 μm. (d–f) Distribution of PrE, EPI and TE cells in Sox17+/+, Sox17+/−, Sox17−/− embryos at E4.5 (d), 3 days after tamoxifen injection (f) and in maternal/zygotic mutant embryos at E4.5 and (e). Blue, PrE; red, EPI; green, TE; grey, ICM. Statistical T-tests are indicated when significant (*:p<0.02; **:p<0.003). Error bars indicate s.d.
To determine if the lack of a discernable PrE-specific phenotype was due to a maternal contribution of Sox17 we analyzed embryos in which maternal, as well as zygotic, Sox17 transcripts were removed using the oocyte-specific Cre recombinase expressing strain Zp3::cre (Lewandoski et al., 1997). At E4.5, maternal/zygotic (mz) Sox17 mutant embryos expressed PrE and EPI markers in cells correctly sorted to their appropriate tissue layers (Fig. 5b), and the total cell number was comparable between mzSox17+/− (130.2±10.6 cells) and mzSox17−/− (137.6±9.5 cells) embryos (Fig. 5e). However, we first noted a significant reduction in overall ICM cell number between mzSox17+/− (38.4±8.4 ICM cells) and zygotic (z) Sox17+/− embryos (52.3±7.6 ICM cells) and between mzSox17−/−(34.2±8.0 ICM cells) and zSox17−/− embryos (43.1±10.7 ICM cells). The TE lineage was also significantly expanded in the maternal/zygotic compared to zygotic Sox17−/− embryos (103.3±14.8 mzSox17−/− TE cells vs. 68.3±24.2 zSox17−/− TE cells) but not in Sox17+/− embryos (91.8± 9.0 mzSox17+/− TE cells vs. 86.8±12.2 zSox17+/− TE cells). Thus, the presence of a Zp3::Cre transgene and/or removal of maternal Sox17 transcripts/protein affected the size of the ICM independently of the genotype of the embryo (Sox17+/− or Sox17−/−). This might suggest that accumulation of CRE protein from the Zp3::Cre transgene during oogenesis may influence early cell fate decisions biasing cells against an ICM fate or affecting ICM cell lineage expansion, an effect that has not been observed to date (Stephenson et al., 2010). Interestingly, several studies reported the adverse effect of high CRE activity on cell proliferation (Loonstra et al., 2001; Pfeifer et al., 2001; Silver and Livingston, 2001; Naiche and Papaioannou, 2007; Schmidt-Supprian and Rajewsky, 2007). Alternatively, we cannot formally exclude a possible contribution of the genetic background in the reduction of the size of the ICM. We therefore analyzed whether the distribution of PrE and EPI cells was affected in maternal and zygotic mutant embryos. While the number of EPI cells was comparable between mzSox17−/− (18.1±4.6 EPI cells) and mzSox17+/− (16.7±4.8 EPI cells), there was a statistically significant reduction in the number of PrE cells (16.1±4.0 Sox17−/− PrE cells vs. 21.8±4.9 Sox17+/− PrE cells). We therefore conclude that maternal deletion of Sox17 provided a modest exacerbation of the phenotype resulting from the absence of zygotic Sox17, and in doing so suggested a possible role for this transcription factor in the PrE lineage. However, we cannot rule out any additive effects on the observed phenotype resulting from the Cre transgene, as well as well as the genetic background.
Moderate reduction of PrE cell number in Sox17-deficient implantation-delayed embryos
Artificially delaying implantation and inducing a period of diapause by blocking estrogen at the morula stage can be used to investigate gene function in contexts where routine analyses of blastocyst stage embryos fail to reveal a readily detectable defect. Prompted by the fact that LIF/gp130 signalling is required for maintaining mouse ES cells in an undifferentiated state, analysis of implantation-delayed blastocyst stage embryos revealed a requirement for LIF/gp130 signalling in the maintenance of the EPI lineage (Li et al., 1995; Nichols et al., 2001; Ware et al., 1995). In addition, we recently used implantation-delayed embryos to reveal a role for the PDGF signalling pathway in PrE lineage expansion (Artus et al., 2010).
Prompted by the failure to establish XEN cells from Sox17 mutants, which supported a critical role for SOX17 in the PrE (Niakan et al., 2010), we generated implantation-delayed Sox17 mutant embryos. We analyzed the localization of lineage-specific transcription factors. Similar to heterozygous embryos, Sox17-deficient blastocysts comprised a TE layer encapsulating the ICM in which the GATA4-positive PrE cells were localized between the blastocoel cavity and a cohort of NANOG-positive cells representing the EPI lineage (Fig. 5c). These data suggested that lineage specification proceeded normally in implantation-delayed Sox17 mutant embryos.
To gain further insight we quantified the respective composition of the TE, EPI and PrE lineages in 3 day implantation-delayed embryos. zSox17−/− mutant embryos contained the same mean number of TE cells (126.3±14.9 zSox17−/− TE cells, as compared to zSox17+/− 117.0±13.0, or zSox17+/+ 123.7±8.8) and EPI cells (22.1±5.9 zSox17−/− EPI cells, as compared to zSox17+/− 20.4±5.8, or zSox17+/+ 22.2±3.1). However, they exhibited a significant reduction of the number of PrE cells (23.7±3.7 zSox17−/− PrE cells as compared to zSox17+/− 31.5±5.1, or zSox17+/+ 31.0±2.6) (Fig. 5f). Thus delaying implantation revealed a novel role for SOX17 in PrE cell survival and/or proliferation.
Altered morphology and premature migration of PrE cells along the mural trophectoderm in implantation delayed Sox17 mutant blastocysts
While investigating the respective contributions of the first lineages in implantation-delayed Sox17-deficient blastocysts, we noted that whereas the majority of PrE cells are maintained in the vicinity of the EPI compartment within the ICM of wild-type and heterozygous embryos (Fig. 6a and Movie 1), a subset of PrE cells were located at a distance from the ICM in Sox17 mutant embryos (Fig. 6d and Movie 2). To better visualize the morphology of the PrE layer and formulate a metric for the distribution of PrE cells in embryos, we generated 3D reconstructions of our confocal imaging data. Specifically, we sought to quantify the distances between PrE cells and the embryonic pole. To do so, we noted the relative position of PrE cells along the long (embryonic–abembryonic) axis of the embryo, by measuring the position of individual PrE cell nuclei from the embryonic pole. The distribution of cells within the PrE layer was visualized using either a lateral view showing the embryonic/abembryonic axis (Figs. 6b, e), or a polar view from the embryonic pole overlying the polar TE (Figs. 6c, f). In zSox17 heterozygotes, PrE cells primarily congregated at the embryonic pole, in close proximity to the EPI (Fig. 6b). PrE cells were in close proximity as revealed by the density of nuclei and small inter-nuclear distances (Fig. 6c). By contrast, in zSox17 mutants, a subset of PrE cells were not associated with the EPI, but instead were associated with the TE, such that some nuclei were distant to the embryonic pole (Fig. 6e, cells are numbered 1 to 10). Cell density appeared reduced and inter-nuclear distances were increased in zSox17 mutants such that the PrE layer appeared more dispersed when viewed from the embryonic pole (Fig. 6f).
Fig. 6.
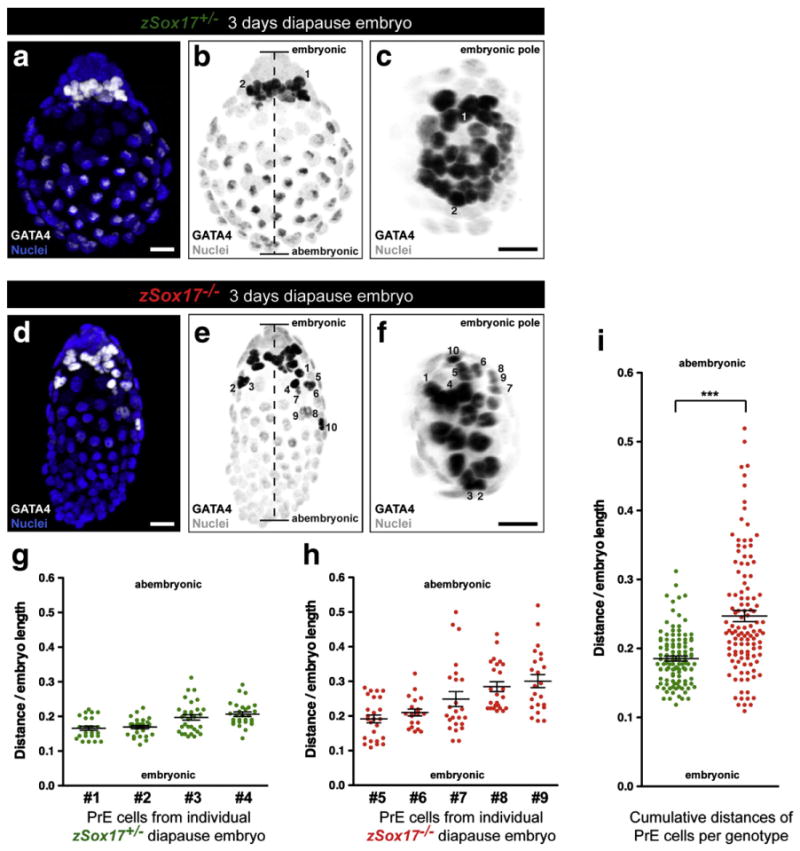
Premature migration of PrE cells along mural trophectoderm in implantation delayed Sox17 mutant blastocysts. (a, d) Immunodetection of GATA4 (white) in 3 day implantation-delayed Sox17+/− (a) and Sox17−/− embryos (d). Nuclei were counterstained with Hoechst (blue). (b, e) Greyscale conversion of (a) and (d) respectively. Dashed line defines the length between the embryonic and abembryonic poles. (c, f) View from the embryonic pole of (b) and (e) respectively. GATA4-positive nuclei from PrE cells (dark, number) can be tracked and located according to these two views. (a–f) Scale bars: 20 μm. (g) Average and relative distances to the proximal embryonic region of the PrE cells in 4 individual Sox17+/− (green) embryos. (h) Average and relative distances to the proximal embryonic region of the PrE cells in 5 individual Sox17−/− (red) embryos. (i) Combined average and relative distances of PrE cells in all analyzed Sox17+/− (green) and Sox17−/− (red) embryos. (***: significant statistical t-test with a p<0.0001). (g–i) Error bars indicate s.e.m.
We next measured the relative distribution of PrE cells in zSox17 heterozygous and zSox17 mutant embryos as the distance from the embryonic pole divided by the length of the embryonic/abembryonic axis (Figs. 6b, e). This would take into account the size variability routinely observed between embryos, and therefore allow for direct comparison between embryos. Independent measurements of the relative distance of PrE cells from the embryonic pole in individual Sox17 heterozygous blastocysts revealed a relative homogeneity with an average varying from 0.17 to 0.21 (Fig. 6g). This indicated that most of PrE cells were positioned in close proximity to the EPI. By contrast, the distribution of PrE cells in Sox17 mutant embryos was more variable within any single embryo, and also between embryos, such that in some embryos PrE cells were located close to the EPI (Fig. 6h, embryos #5 and #6), whereas in others, PrE cells were dispersed along the length of the embryonic/abembryonic axis (Fig. 6h, embryos #7, #8 and #9). Combined distances from Sox17 heterozygote and Sox17 mutant implantation-delayed embryos demonstrated that, on average, PrE cells in heterozygous embryos were located closer (0.18±0.04) to the embryonic pole than in Sox17 mutants (0.25± 0.09) (Fig. 6i). The higher standard deviation associated with measurements in Sox17 mutants is an indication of the dispersal of PrE cells along the embryonic/abembryonic axis. In total, we calculated that 94% (106 out of 113 cells) of Sox17+/− PrE cells were located in the first fourth of the embryo (embryonic pole). By contrast, only 61% (71 out of 117 cells) of Sox17−/− PrE cells were located in the first fourth. These data suggest that while not necessary for the specification or expansion of the PrE, SOX17 plays a role in its epithelial integrity and/or differentiation into PE.
Sox17-deficient PrE cells establish and retain apicobasal polarity
The nascent PrE layer progressively matures to form a polarized epithelium so that PrE cells acquire an apical side adjacent to the cavity which is characterized by the regional localization of proteins involved in adhesion or trafficking, including DAB2, LRP2, AMN and CUBN (Gerbe et al., 2008). The basal surface lies at the interface with the EPI tissue layer and is enriched in extracellular matrix (ECM) components including collagen IV (Adamson and Ayers, 1979) and laminin (Dziadek and Timpl, 1985). Interestingly, SOX17 has been reported to bind the promoter of several genes encoding ECM proteins including Col4a1, Col4a2, Fn1, Lama1, Lamb1-1, and Lamc1 (Niakan et al., 2010; Niimi et al., 2004; Shirai et al., 2005) suggesting that SOX17 may regulate the composition of the basement membrane, which could subsequently affect the integrity of PrE epithelium. We therefore performed a detailed examination of the localization of DAB2 and Laminin proteins in implantation-delayed embryos. In Sox17+/− embryos, DAB2 was apically enriched in both TE and PrE cells (Fig. 7a, a′, a″ and Movies 3 and 4). Conversely, Laminin was detected basally (Fig. 7b, b′, b″). In Sox17−/− mutant embryos, regions exhibiting an enrichment of DAB2 persisted in PrE cells associated with the TE, suggesting they maintained some aspect of polarity (Fig. 7d, d′, f″ and Movies 5 and 6). By contrast, Laminin appeared more homogenously distributed around PrE cells (Fig. 6e, e′, e″) and was not detected in areas in direct contact with the EPI (Fig. 6e, f). These data suggest that PrE cells retain some properties of apicobasal polarity, but that the basement membrane is affected in absence of Sox17.
Fig. 7.
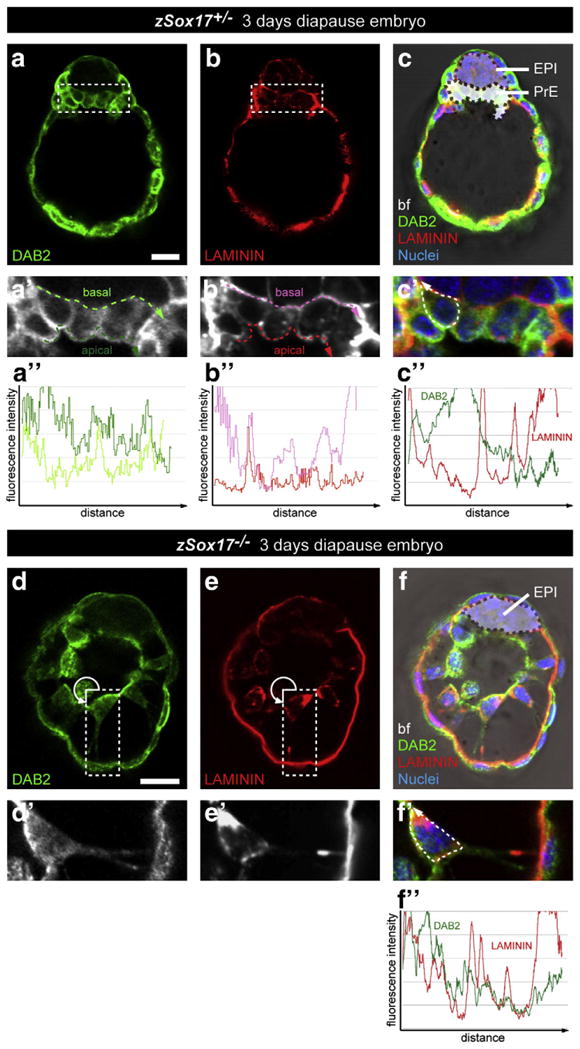
Disrupted organization of primitive endoderm correlates with basement membrane deposition in the absence of Sox17. Immunodetection of DAB2 and Laminin in Sox17+/− (a–c, a′–c′, a″–c″) and Sox17−/− (d–f, d′–f′, f″) 3 day diapause embryos. (a′–f′) magnified views of (a–f) respectively. (a″,b″) Measurements of the fluorescence intensity of DAB2 (a″) and Laminin (b″) staining along the apical (green and red dashed lines) and basal (light green and pink dashed lines) of the PrE layer. (c″,f″) Measurements of the fluorescence intensity of Laminin (red) and DAB2 (green) throughout the cell membrane (dashed line). All panels show single optical section. DAB2, green; Laminin, red; nuclei, blue; bf, bright field. Scale bar: 20 μm.
Conclusions
In this study we analyzed the dynamic localization of SOX17 and SOX7, two closely related members of the SOX transcription factor family noting that they are specific to the PrE and its derivatives (Fig. 8a). SOX17 was localized to prospective PrE cells. While the localization of SOX7 revealed that sorted PrE cells, located on the surface of the ICM, are molecularly distinct and exhibit a unique transcription factor signature (GATA6+, SOX17+, GATA4+, SOX7+), compared to unsorted (GATA6+, SOX17+, GATA4+, SOX7−) PrE cells. Our data therefore lend further support to a model of sequential activation of transcription factors within the PrE lineage, which we propose might correlate with the consecutive periods of ICM cell lineage naïvete’, commitment and segregation.
Fig. 8.
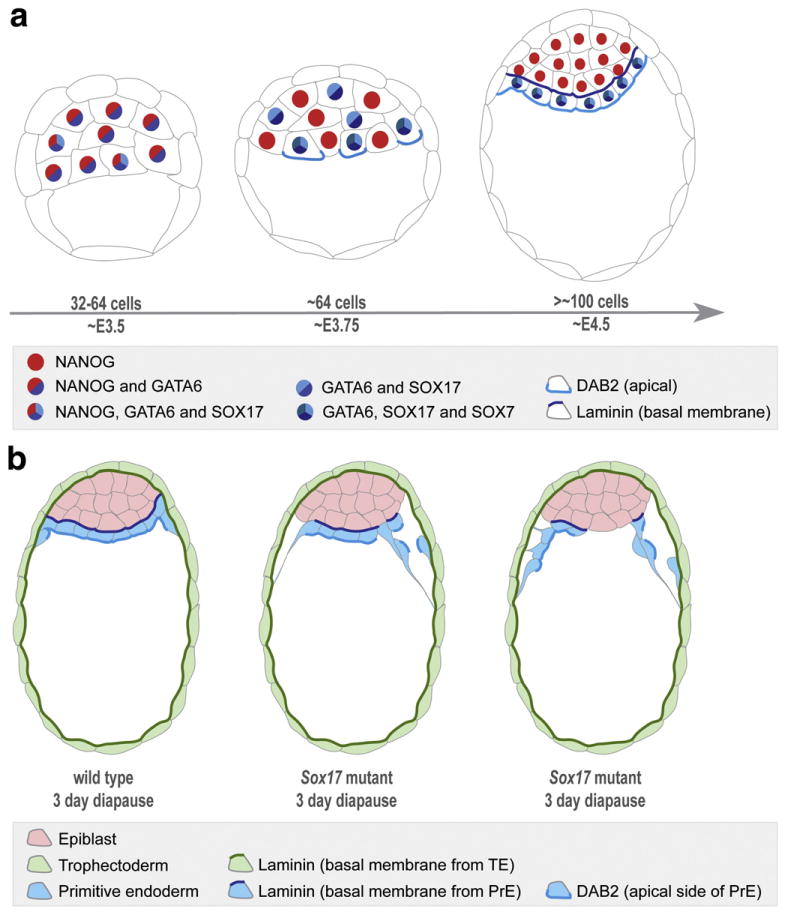
Schematic representations of sequential activation of transcription factors in the PrE cell lineage and the phenotype resulting from inactivation of Sox17 in implantation-delayed blastocysts. (a) At E3.5, in embryos of 32–64 cells, GATA6 is expressed in all cells of the ICM, and SOX17 can be detected in some cells of the ICM. At around the 64-cell stage, coincident with the emergence of salt-and-pepper distribution of EPI/PrE cells, GATA6 becomes downregulated in presumptive EPI cells, and SOX17 can be detected in most of these presumptive PrE cells, such that GATA6 and SOX17 co-localize. SOX7 is localized to GATA6/SOX17-positive cells that are positioned on the cavity roof. These are the cells that will, or are just beginning to, accumulate DAB2 apically (Gerbe et al., 2008). At the time of implantation (E4.5), the PrE is apparent as a morphologically-distinct epithelial cell layer in contact to the cavity with cells being GATA6/SOX17/SOX7-positive. (b) Inactivation of Sox17 in implantation-delayed blastocyst stage embryos results in disruption of the organization of the PrE epithelium and premature parietal endoderm differentiation. Premature disattachment and migration of nascent parietal endoderm cells over the TE layer are observed. Wild-type embryo is depicted on the left, middle panel represents a Sox17 mutant exhibiting a mild defect, whereas the right panel depicts a mutant exhibiting a more severe defect.
Since SOX7 localization was comparable in wild-type and Sox17 mutant embryos, it suggested a lack of cross-regulation, but possible compensation between these two SOX family transcription factors. Genetic removal of both maternal and zygotic Sox17 contribution revealed a phenotype in mutant embryos at preimplantation stages possibly due to removal of maternal protein or mRNA stores, presence of the Zp3::Cre transgene and/or genetic background. Unfortunately the cumulative effect of these factors could not be rigorously experimentally tested. However, Sox17 mutant embryos were readily distinguishable from wild-type and heterozygotes when implantation was artificially delayed. In this situation, the PrE epithelium was abnormal with PE cells prematurely delaminating from the epithelium and migrating along the mural TE. Taken together, these data lead us to suggest that even if not necessary for the specification or expansion of the PrE, SOX17 plays a role in maintaining the epithelial integrity of this tissue layer and/or its differentiation into PE (Fig. 8b). Finally, the additive effects of the related SOX17 and SOX7 proteins, as well as any functional redundancy, within the PrE lineage will need to be addressed by the analysis of Sox17; Sox7 double mutants.
Supplementary Material
Acknowledgments
We thank Sean Morrison for the Sox17 conditional mutant strain; Zhirong Bao for discussions on image data quantitation; Ann Foley, Minjung Kang and Manuel Viotti for comments on the manuscript. AKH would especially like to acknowledge the hospitality of Arthur Soriano, and Jenny Nichols for providing an introduction to the merits of diapause. Work in AKH’s lab is supported by the NIH (RO1-HD052115 and RO1-DK084391), NYSTEM and The Starr Foundation.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.ydbio.2010.12.007.
References
- Adamson ED, Ayers SE. The localization and synthesis of some collagen types in developing mouse embryos. Cell. 1979;16:953–965. doi: 10.1016/0092-8674(79)90110-7. [DOI] [PubMed] [Google Scholar]
- Alexander J, Stainier DY. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. doi: 10.1016/S0960-9822(00)80016-0. [DOI] [PubMed] [Google Scholar]
- Artus J, Panthier JJ, Hadjantonakis AK. A role for PDGF signaling in expansion of the extra-embryonic endoderm lineage of the mouse blastocyst. Development. 2010;137:3361–3372. doi: 10.1242/dev.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2–MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Clements D, Woodland HR. Changes in embryonic cell fate produced by expression of an endodermal transcription factor, Xsox17. Mech Dev. 2000;99:65–70. doi: 10.1016/s0925-4773(00)00476-7. [DOI] [PubMed] [Google Scholar]
- Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- Dziadek M, Timpl R. Expression of nidogen and laminin in basement membranes during mouse embryogenesis and in teratocarcinoma cells. Dev Biol. 1985;111:372–382. doi: 10.1016/0012-1606(85)90491-9. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Futaki S, Hayashi Y, Emoto T, Weber CN, Sekiguchi K. Sox7 plays crucial roles in parietal endoderm differentiation in F9 embryonal carcinoma cells through regulating Gata-4 and Gata-6 expression. Mol Cell Biol. 2004;24:10492–10503. doi: 10.1128/MCB.24.23.10492-10503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL, Rossant J. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol. 1979;52:141–152. [PubMed] [Google Scholar]
- Gerbe F, Cox B, Rossant J, Chazaud C. Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Dev Biol. 2008;313:594–602. doi: 10.1016/j.ydbio.2007.10.048. [DOI] [PubMed] [Google Scholar]
- Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119 (Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto K, Yabuta Y, Ohinata Y, Ono Y, Uno KD, Yamada RG, Ueda HR, Saitou M. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 2006;34:e42. doi: 10.1093/nar/gkl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- Li M, Sendtner M, Smith A. Essential function of LIF receptor in motor neurons. Nature. 1995;378:724–727. doi: 10.1038/378724a0. [DOI] [PubMed] [Google Scholar]
- Lim CY, Tam WL, Zhang J, Ang HS, Jia H, Lipovich L, Ng HH, Wei CL, Sung WK, Robson P, Yang H, Lim B. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3:543–554. doi: 10.1016/j.stem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Liu J, He X, Corbett SA, Lowry SF, Graham AM, Fassler R, Li S. Integrins are required for the differentiation of visceral endoderm. J Cell Sci. 2009;122:233–242. doi: 10.1242/jcs.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, Berns A, Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci USA. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Adams RJ, Morris SA, Danckaert A, Le Garrec JF, Zernicka-Goetz M. Active cell movements coupled to positional induction are involved in lineage segregation in the mouse blastocyst. Dev Biol. 2009;331:210–221. doi: 10.1016/j.ydbio.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnard D, Guzman-Ayala M, Constam DB. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–2505. doi: 10.1242/dev.02413. [DOI] [PubMed] [Google Scholar]
- Morris SA, Teo RT, Li H, Robson P, Glover DM, Zernicka-Goetz M. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc Natl Acad Sci USA. 2010;107:6364–6369. doi: 10.1073/pnas.0915063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A, Shen H, Ishida S, Dickson C. SOX7 and GATA-4 are competitive activators of Fgf-3 transcription. J Biol Chem. 2004;279:28564–28573. doi: 10.1074/jbc.M313814200. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–775. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- Niakan KK, Ji H, Maehr R, Vokes SA, Rodolfa KT, Sherwood RI, Yamaki M, Dimos JT, Chen AE, Melton DA, McMahon AP, Eggan K. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010;24:312–326. doi: 10.1101/gad.1833510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339. doi: 10.1242/dev.128.12.2333. [DOI] [PubMed] [Google Scholar]
- Niimi T, Hayashi Y, Futaki S, Sekiguchi K. SOX7 and SOX17 regulate the parietal endoderm-specific enhancer activity of mouse laminin alpha1 gene. J Biol Chem. 2004;279:38055–38061. doi: 10.1074/jbc.M403724200. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7:338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci USA. 2001;98:11450–11455. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu XB, Pan J, Zhang C, Huang SY. Sox17 facilitates the differentiation of mouse embryonic stem cells into primitive and definitive endoderm in vitro. Dev Growth Differ. 2008;50:585–593. doi: 10.1111/j.1440-169x.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Rossant J, Chazaud C, Yamanaka Y. Lineage allocation and asymmetries in the early mouse embryo. Philos Trans R Soc Lond B Biol Sci. 2003;358:1341–1348. doi: 10.1098/rstb.2003.1329. discussion 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Seguin CA, Draper JS, Nagy A, Rossant J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. doi: 10.1016/j.stem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Shimoda M, Kanai-Azuma M, Hara K, Miyazaki S, Kanai Y, Monden M, Miyazaki J. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci. 2007;120:3859–3869. doi: 10.1242/jcs.007856. [DOI] [PubMed] [Google Scholar]
- Shirai T, Miyagi S, Horiuchi D, Okuda-Katayanagi T, Nishimoto M, Muramatsu M, Sakamoto Y, Nagata M, Hagiwara K, Okuda A. Identification of an enhancer that controls up-regulation of fibronectin during differentiation of embryonic stem cells into extraembryonic endoderm. J Biol Chem. 2005;280:7244–7252. doi: 10.1074/jbc.M410731200. [DOI] [PubMed] [Google Scholar]
- Silver DP, Livingston DM. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Stephenson RO, Yamanaka Y, Rossant J. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development. 2010;137:3383–3391. doi: 10.1242/dev.050195. [DOI] [PubMed] [Google Scholar]
- Ware CB, Horowitz MC, Renshaw BR, Hunt JS, Liggitt D, Koblar SA, Gliniak BC, McKenna HJ, Papayannopoulou T, Thoma B, et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Ralston A, Stephenson RO, Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006;235:2301–2314. doi: 10.1002/dvdy.20844. [DOI] [PubMed] [Google Scholar]
- Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD, Xu XX. Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol. 2002;251:27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.