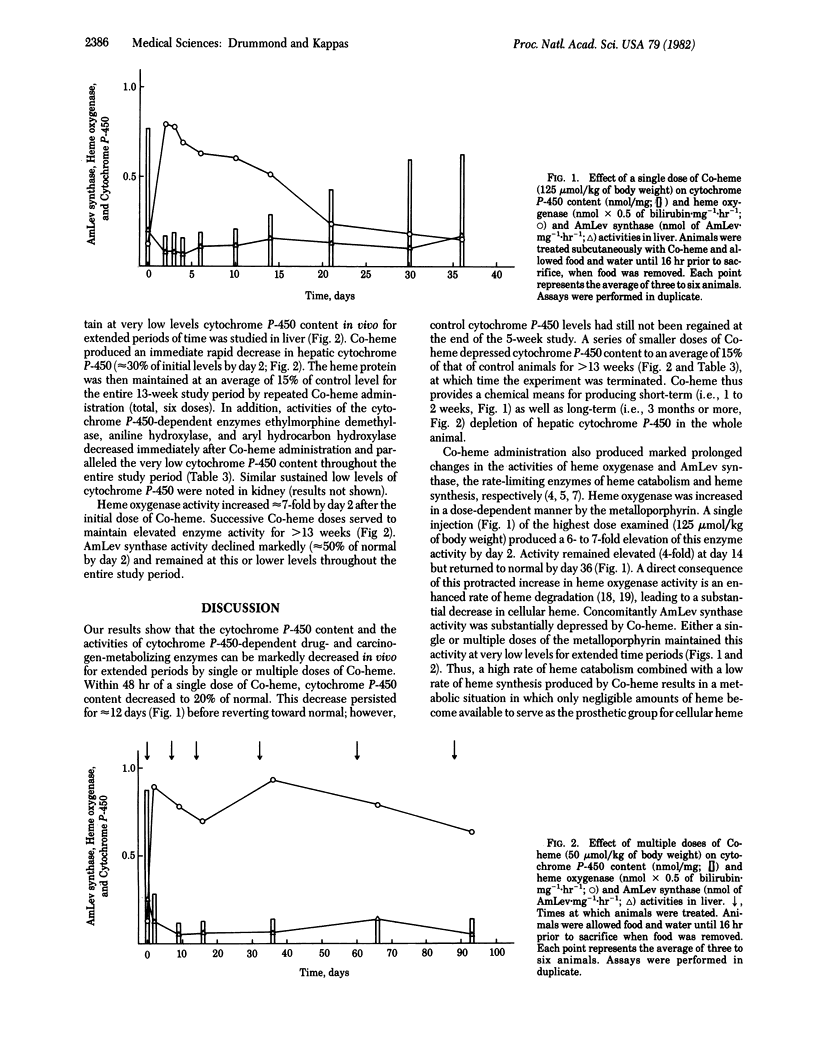
Abstract
An experimental method is described to deplete markedly in vivo the cytochrome P-450 content of liver for prolonged periods of time. The method uses the synthetic metalloporphyrin cobalt-heme (cobalt protoporphyrin IX), which possesses the dual biological properties of repressing delta-aminolevulinate synthase, the rate-limiting enzyme of heme biosynthesis, and of potently inducing microsomal heme oxygenase, the rate-limiting enzyme of heme catabolism. A single dose of cobalt-heme (125 mumol/kg of body weight) decreased within 48 hr hepatic cytochrome P-450 to approximately 20% of normal, at which level it remained for 10 days; normal levels were not achieved by 36 days. Periodic administration (total, six injections) of a smaller dose of cobalt-heme (50 mumol/kg of body weight) maintained the cytochrome P-450 content at levels approximately 15% of normal for greater than 90 days with concurrent profound impairment of mono-oxygenase reactions catalyzed by this heme protein. The ability of cobalt-heme to produce profound and prolonged depletion of cytochrome P-450 in vivo provides a valuable model for examining the role of cytochrome P-450-dependent metabolism in the biology of endogenous and exogenous chemicals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. E., Drummond G. S., Freddara U., Sardana M. K., Sassa S. Porphyrogenic effects and induction of heme oxygenase in vivo by delta-aminolevulinic acid. Biochim Biophys Acta. 1981 Sep 4;676(3):289–299. doi: 10.1016/0304-4165(81)90162-8. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Unseld A. Increased liver haem degradation caused by foreign chemicals: a comparison of the effects of 2-allyl-2-isopropylacetamide and cobaltous chloride. Biochem Soc Trans. 1976;4(2):205–209. doi: 10.1042/bst0040205. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Swarm R. L., Becker J. Ultrastructural study of rat liver and liver neoplasms after long-term treatment with phenobarbital. Cancer Res. 1981 Jun;41(6):2151–2162. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Imai Y., Ito A., Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966 Oct;60(4):417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Welch R. M., Conney A. H. Increased liver microsomal androgen metabolism by phenobarbital: correlation with decreased androgen action on the seminal vesicles of the rat. J Pharmacol Exp Ther. 1974 Feb;188(2):287–292. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res. 1976;8 (Suppl 17):39–46. [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Sassa S., Kappas A., Bernstein S. E., Alvares A. P. Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. Heme oxygenase induction as an adaptive response for maintaining cytochrome P-450 in chronic hemolysis. J Biol Chem. 1979 Feb 10;254(3):729–735. [PubMed] [Google Scholar]
- Sultatos L. G., Vesell E. S., Hepner G. W. Heterogeneous response of hepatic mixed function oxidases to chronic phenobarbital administration. Biochem Pharmacol. 1979 Mar 15;28(6):849–857. doi: 10.1016/0006-2952(79)90368-x. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. C., Gunsalus I. C., Wang M. Y., Hoffman B. M. Cobalt-substituted cytochrome P-450cam. J Biol Chem. 1981 Jun 25;256(12):6266–6273. [PubMed] [Google Scholar]
- Wassom J. S., Huff J. E., Loprieno N. A review of the genetic toxicology of chlorinated dibenzo-p-dioxins. Mutat Res. 1977;47(3-4):141–160. doi: 10.1016/0165-1110(77)90001-x. [DOI] [PubMed] [Google Scholar]



