Abstract
OBJECTIVES
Appropriate antimicrobial dosing maximizes therapeutic benefit while minimizing development of antimicrobial resistance. Common pediatric references recommend vancomycin dosing of 40 mg/kg/day divided every 6 to 8 hours for non-central nervous system infections, while some clinicians report utilizing higher initial doses to optimize efficacy. This study compares vancomycin serum concentrations following traditional dosing of 10 mg/kg/dose every 6 to 8 hours versus 15 to 20 mg/kg/dose every 6 to 8 hours.
STUDY DESIGN
Retrospective database review of vancomycin serum concentrations in pediatric patients.
RESULTS
Three hundred fifty-seven patients were analyzed. The mean peak concentration of the 10 mg/kg groups every 6 and every 8 hours were below 25 mg/L, whereas the mean peak concentrations of the 15 mg/ kg groups every 6 and 8 hours were within the 25–40 mg/L range (p < 0.001). The mean trough concentration of the 10 mg/kg group every 6 hours was within the 5–15 mg/L range while the 10 mg/kg group dosed every 8 hours was below target. However, the mean trough concentrations of the 15 mg/kg group dosed every 6 and 8 hours were both within the 5–15 mg/L range (p < 0.001).
CONCLUSIONS
Vancomycin doses of 15 mg/kg every 6 to 8 hours produce peak and trough serum concentrations within target range more often than 10 mg/kg every 6 to 8 hours.
Keywords: pediatrics, pharmacokinetics, vancomycin dosing, vancomycin serum concentrations
INTRODUCTION
Vancomycin is a glycopeptide antibiotic with a narrow spectrum of activity directed against Gram-positive microorganisms. Vancomycin has become a commonly used agent in hospital settings, where the incidence of resistant Gram-positive organisms is high. This has been especially true in recent years with the increase in skin and soft tissue infections, as well as invasive disease, caused by methicillin-resistant Staphylococcus aureus (MRSA). Vancomycin is now being employed frequently as empiric Gram-positive coverage in both immunocompromised and immunocompetent patients as well as in pediatric patients with indwelling intravascular and prosthetic devices.1
Vancomycin has age-dependent pharmacokinetics. Several studies have evaluated the altered pharmacokinetics in the pediatric population.2–4 Total body clearance of vancomycin in pediatric patients is 2 to 3 times higher than in adults. In addition, the volume of distribution of vancomycin at steady state is similar or smaller than that of adult patients.2 A vancomycin dosing regimen of 40 mg/kg/day was predicated by Spears and Koch in 1959 and has become the most frequently used regimen in pediatric patients.5 A later study by Schaad et al. also concluded that 40 mg/kg/day of vancomycin was necessary to obtain therapeutic concentrations in older infants and children, but that larger doses of 60 mg/kg/ day were indicated for pediatric patients with staphylococcal central nervous system (CNS) disease.6 These authors also found that peak serum concentrations greater than 25 mg/L and trough concentrations less than 12 mg/L were associated with appropriate inhibitory and bactericidal titers. Commonly used pediatric references recommend similar dosing.7–9
The routine monitoring of serum vancomycin concentrations has recently been debated.10–12 While much of the literature documents the target range of vancomycin peak concentrations to be 25 to 40 mg/L and the target range of vancomycin trough concentrations to be 5 to 15 mg/L, there is no proven correlation between clinical efficacy and defined serum concentrations.5,6,11,13 Due to this lack of data and cost associated with monitoring serum concentrations, some clinicians and institutions are limiting the use of monitoring serum peak and trough concentrations of vancomycin and/or are using dosing nomograms. Since the bactericidal activity of vancomycin depends on the duration of time above the minimum inhibitory concentration (MIC), it is desirable to achieve targeted vancomycin concentrations as quickly as possible in a therapeutic course of treatment.
In this study, we examine vancomycin serum concentrations at various doses and frequencies in our pediatric population to determine the dosage(s) of vancomycin that are most likely to achieve serum vancomycin concentrations that are within the defined target range.
MATERIALS AND METHODS
Patients
This retrospective study reviewed data from patients admitted to The Children's Hospital of Alabama who received intravenous vancomycin and had both a peak and a trough serum concentration obtained from January to July of 2004. The database used in these assessments had been developed in-house as a tool for pharmacists to perform pharmacokinetic calculations and to track resulting changes in dosing. Entries in the database are performed by a clinical pharmacist as part of the assessment of a patient receiving vancomycin who has serum concentrations obtained. Patients were included if they were between the ages of 1 month to 18 years, had normal renal function as defined by age-adjusted creatinine clearance,14,15 had both a peak and trough vancomycin serum concentration, and had received a vancomycin dose within a predetermined range (see Methods for further details). Our institution accepts all types of pediatric patients with varying diseases including, but not limited to, general pediatric patients, oncology, stem cell transplantation, critically ill, and cystic fibrosis patients, or those with congenital heart defects; no patient types or diseases were excluded from review. Patient data were entered into the database as an intervention. Each time a new peak and trough value was obtained for a patient, there was a new intervention.
Methods
This study was approved by institutional review board committees at Samford University and the University of Alabama at Birmingham. Patients were stratified into 8 groups according to dose and dosing interval: 1) 10 mg/kg/dose every 6 hours; 2) 10 mg/kg/dose every 8 hours; 3) 15 mg/kg/dose every 6 hours; 4) 15 mg/kg/ dose every 8 hours; 5) 15 mg/kg/dose every 12 hours; 6) 20 mg/kg/dose every 6 hours; 7) 20 mg/kg/dose every 8 hours; and 8) 20 mg/kg/ dose every 12 hours. Patients who received a dose or treatment interval that fell out of these predetermined stratifications by more than 10% were excluded. Patient weight, age, peak concentration, trough concentration, dose, and dosing interval were obtained. The defined target range used at this institution for vancomycin peaks is 25–40 mg/L and troughs is 5–15 mg/L, based on previously published ranges.11,16,17 All peak and trough concentrations were adjusted to reflect peak concentrations at one hour after a one hour infusion of the dose and trough concentrations immediately prior to the dose utilizing one-compartment model calculations. These adjustments were based on the actual time the concentration was obtained in relation to the dose. At our institution peak and trough concentrations are typically collected at steady-state (i.e., around the third dose) and the majority of levels evaluated reflected this practice. The vancomycin dose the concentrations were drawn around is not routinely documented in our database; therefore, these data are not known for these study patients.
STATISTICAL ANALYSIS
Descriptive statistics were used to summarize all continuous and categorical variables. Normally distributed data are presented with mean and standard deviation while non-normally distributed data are presented with median and interquartile range (IQR). Comparisons of peak and trough values between dosing groups were performed using analysis of variance. Because the data showed homogeneity, post hoc analysis was performed using Student-Newman-Keuls method. Nominal data were compared using a Chi-square test. All statistical tests were two-sided and were performed with a p value < 0.05 indicating statistical significance using SPSS 11.5 statistical software package (SPSS, Chicago, IL).
RESULTS
Data from 951 patients who received vancomycin were extracted from the database. Of these, 394 patients failed to meet inclusion criteria, 4 were duplicate entries, and 196 patients were excluded due to a dose or a dosing interval that was outside of the predefined ranges. The remaining 357 patients were stratified into dosing groups (Figure 1).
Figure 1.
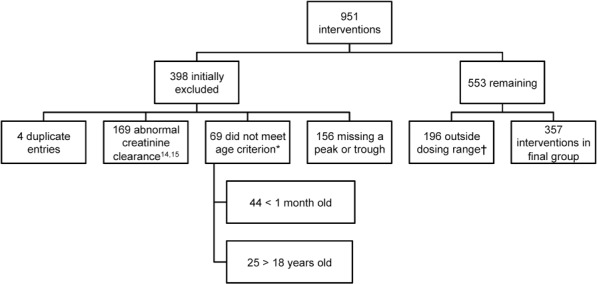
Flow Diagram of Inclusion and Exclusion Criteria
* age criterion was > one month or < 18 years of age
† dosing groups: 10 mg/kg q 6 h;10 mg/kg q 8 h;15 mg/kg every 6 h;15 mg/kg q 8 h;15 mg/kg q 12 h; 20 mg/kg q 6 h; 20 mg/kg q 8 h; 20 mg/kg q 12 h
The median weight was 12.9 kg (IQR 7.45, 20.35), the median age was 66 months (IQR 9, 76) and 59% (n = 210) were male. The mean vancomycin dose was 15.33 ± 3.29 mg/kg/dose, with a mean dosing interval of 7.53 ± 1.75 hours. The mean peak serum concentration was 29.45 ± 11.26 mg/L and the mean trough serum concentration was 8.66 ± 5.93 mg/L. Group demographics and mean peak and trough serum concentrations for each group are shown in the Table.
Table.
Group Demographics and Mean Peak and Trough Serum Concentrations
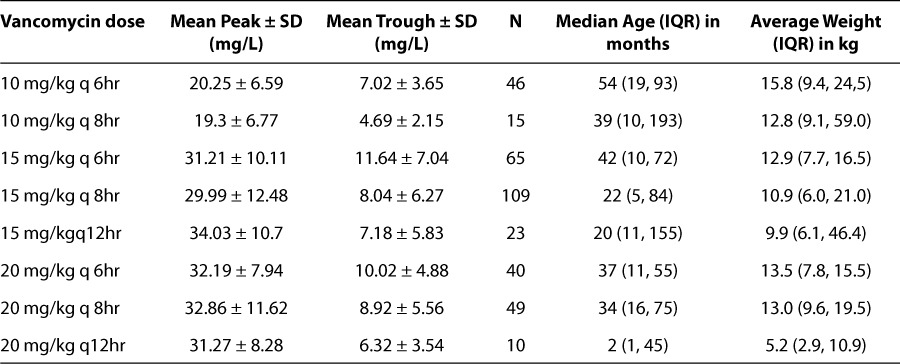
The mean peak serum concentrations for the 10 mg/kg/dose groups dosed every 6 and 8 hours were below the target range (≥ 25 mg/L). The group dosed 10 mg/kg/dose every 6 hours had a peak that was below target 85% of the time while the group administered every 8 hours was below target 87% of the time. No patient in either 10 mg/ kg/dose group had a peak concentration that was greater than 40 mg/L. Conversely, the mean peak concentrations of the 15 mg/kg/dose and 20 mg/ kg/dose groups dosed every 6, 8 or 12 hours were within the target range (25–40 mg/L) (p < 0.001).
The mean trough concentration for the 10 mg/ kg group dosed every 8 hours was just below the target range (< 5 mg/L), whereas the mean trough concentrations in the 15 mg/kg/dose and 20 mg/kg/dose groups dosed every 6, 8 or 12 hours were within the target range (5 to 15 mg/L) (p < 0.001). Figure 2 show the distribution of peaks and troughs in each group in relation to below the target (peaks of < 25–40 mg/L, troughs of <5 mg/L), within the target (peaks 25–40 mg/L, troughs of 5–15 mg/L) and above the target (peaks of >40 mg/L, troughs of >15 mg/L).
Figure 2.
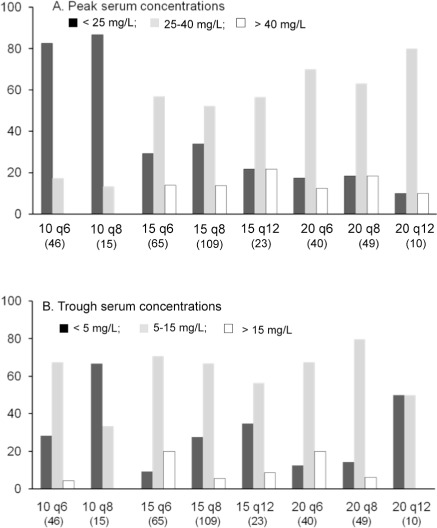
Summary of Peak and Trough Serum Vancomycin Concentrations Resulting from 8 Dosing Regimens (number of patients in each dosing group)
Among all patients, trough concentrations exceeded 15 mg/L in only 34 patients (9%). Most of these patients (n = 23) were in the groups dosed every 6 hours; the group dosed at 15 mg/kg/ dose every 6 hours had the most patients (n = 13) with troughs higher than 15 mg/L. Twenty-three percent (n = 84) of all patients had below target troughs. Of note, 67% of the patients in the 10 mg/kg/dose group dosed every 8 hours had below target troughs and the group dosed at 20 mg/kg/dose every 12 hours had below target troughs 50% of the time.
Overall, patients receiving 15–20 mg/kg/dose of vancomycin had a peak and trough within the target range more often than patients in the 10 mg/kg/dose group. All patients in the groups dosed every 8 hours with an above target trough also had an above target peak, whereas only 43% of patients in the groups dosed every 6 hours who had an above target trough also had an above target peak.
DISCUSSION
The purpose of this retrospective database review was to determine how often different dosing regimens of vancomycin produced peak and trough serum concentrations within a defined target range in a pediatric patient population. Common pediatric references recommend vancomycin dosing of 40 mg/kg/day divided every 6 to 8 hours for non-central nervous system infections, and 60 mg/kg/day divided every 6 hours for CNS infections.7–9 Anecdotally, we have found that the published dosing recommendations for vancomycin often produce suboptimal serum concentrations. Our data suggest that in the pediatric population, the dose of vancomycin initially employed should be 15–20 mg/kg/ dose administered intravenously every 6 to 8 hours to produce serum concentrations within the target range.
Early studies of vancomycin dosing in the pediatric population had flaws in their research design. In 1959, Spears and Koch studied vancomycin in 53 patients one month to 18 years of age. Thirty patients received only a single intravenous (n = 16) or intramuscular (n = 14) dose of vancomycin, with subsequent serum concentration measurements. Only 23 patients received multiple doses of vancomycin; however, no serum concentrations were measured in this group. Based upon these data, the authors concluded that vancomycin doses of 40 mg/kg/day IM or IV provided adequate serum concentrations.5
A second small study by Schaad et al. looked at 55 patients including premature and full term neonates, infants, and children.6 They administered vancomycin 10 to 15 mg/kg and measured serial vancomycin concentrations after each dose. They concluded that 40 mg/kg/day of vancomycin was necessary to obtain “therapeutic” concentrations in older infants and children, and that up to 60 mg/kg/day was indicated in patients with staphylococcal central nervous system disease. They also found that peaks above 25 mg/L and troughs less than 12 mg/L were associated with appropriate inhibitory and bactericidal titers. In addition, 3 references that are commonly used by pediatricians also recommend initial vancomycin doses of 40 mg/kg/day divided every 6 to 8 hours for children greater than one month of age.7–9
More recent studies have refuted the 40 mg/ kg/day recommendation, particularly in certain populations. Chang et al. reported that mean vancomycin dosages of 75 mg/kg/day were required in pediatric oncology patients to obtain mean peak serum concentrations of 23.1 mg/L and mean trough concentrations of 6.2 mg/L.18 Glover et al. studied 135 pediatric intensive care unit patients with normal renal function and found that vancomycin dosages of 60 mg/kg/ day divided every 8 hours were necessary to attain vancomycin peak concentrations above 20 mg/L and troughs above 5 mg/L.19
Our data concur with these more recent studies. Of our patients who received 10 mg/kg of vancomycin every 6 to 8 hours, 41% had a trough value below the target. This is of particular concern with the rising incidence of resistant Gram-positive organisms, given that the bactericidal activity of vancomycin depends on the duration of time above the MIC. Knowledge of the pathogen's MIC is important, as MIC breakpoints determine if an organism is susceptible to a particular antimicrobial. It has been suggested that maintaining a trough concentration that is 4 to 5 times the MIC of the organism is desired for eradication and clinical success.20 Considering that the protein binding of vancomycin approximates 50%, a target vancomycin trough of 15–20 mg/L would be appropriate.20 Indeed, recent literature has recommended that even higher trough concentrations of vancomycin should be attained in the management of Gram-positive infections. In 2005, the American Thoracic Society and the Infectious Diseases Society of America published recommendations for treating adult health care associated pneumonia with troughs of 15–20 mg/L21; however, this was not based upon clinical evidence. When treating MRSA specifically, troughs in the 10–15 mg/L range are associated with a shorter duration of therapy and less treatment failures.16,22 With the newer concerns surrounding the prevention of the heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) strain, some clinicians have suggested target vancomycin trough concentrations of 10–20 mg/L.23 One plausible explanation for the recommendation for higher serum concentrations may be due to increasing reports of elevated vancomycin MIC's to MRSA and associated clinical failures.20,24,25 This has prompted the Clinical Laboratory Standards Institute to change the vancomycin susceptibility breakpoint to ≤ 2 mg/L.26
Vancomycin continuous infusion is another therapeutic option that addresses the pharmaco-dynamic need for time above the MIC. Certain studies have found no difference in bactericidal activity between intermittent and continuous dosing of vancomycin.27,28 Klepser et al. reported similar findings.29 Although the authors noted that infusing vancomycin continuously can have advantages such as expected serum concentrations and easy administration, they discourage the use of continuous infusion of vancomycin due to its long half-life and potential improved tolerability with intermittent injections.29 Due to this controversy, our institution does not currently use this method and none of the patients in this study received continuous infusion vancomycin.
The most appropriate target serum trough concentration that achieves maximum killing continues to be debated, while higher trough concentrations have been associated with toxicity. An early study reported a 5% incidence of nephrotoxicity when vancomycin was given alone and a 35% incidence when vancomycin was given with an aminoglycoside.30 In this study of 98 adult patients, 3 were found to have nephrotoxicity, all of whom had vancomycin trough levels from 30–65 mg/L.30 A more recent study of 742 adult oncology patients reported no relationship between elevated vancomycin peak concentrations and nephrotoxicity but found an 8% incidence of nephrotoxicity with vancomycin alone. A significant finding was that patients whose trough concentrations exceeded 10 mg/L and had received a long duration of vancomycin therapy were more likely to develop nephrotoxicity.31 This is similar to what was seen in the previous studies.30,32 Animal models confirm a synergistic nephrotoxic effect of vancomycin and aminoglycosides. Still, the overall incidence of vancomycin-induced nephrotoxicity (as a single agent) is low, and even fewer reports are available in more recent literature. In our study, only 34 patients (9%) had serum trough concentrations that exceeded 15 mg/L; 23 of these patients were dosed every 6 hours.
In our study group of patients receiving 10 mg/kg/dose, 84% had peak values below our target range. Since vancomycin does not exert concentration-dependent killing, peak concentrations may have little to no role in efficacy. However, it seems prudent to maintain a peak concentration within a defined target range, particularly to help this large molecule to penetrate certain sites, such as the cerebrospinal fluid, endocardial tissue or bone.23,33 Subsequently, because vancomycin's central nervous system penetration into the meninges is suboptimal, guidelines for pneumococcal infections from the American Academy of Pediatrics recommend serum trough concentrations of 10 to 15 mg/L and peak concentrations of 35 to 40 mg/L when treating meningitis.34
When recommending higher than traditionally published doses (i.e., 40 mg/kg/day), there may be concern surrounding toxicity associated with higher than target peak concentrations. There were early reports of ototoxicity associated with elevated vancomycin peak concentrations, possibly due to the contaminants (such as pyrogens and impurities that may have produced other adverse effects, namely nephrotoxicity) in the manufacturing process; however, manufacturing of vancomycin has since included a purification process.31 Farber et al. reported no cases of auditory toxicity in the report mentioned previously, although one patient developed vertigo.30 A review of vancomycin-induced hearing loss found no reports of ototoxicity in animals but found multiple reports of hearing loss in humans. All of the patients with hearing loss had received an aminoglycoside in addition to the vancomycin.35 Only 44 patients (12%) in our study had peak concentrations greater than 40 mg/L; most of these patients were receiving 15 mg/kg every 8 hours. Considering that a higher dose usually results in a higher peak, a similar number of patients who had peaks over 40 mg/L were in the groups receiving 20 mg/kg/dose. With the minimal concern of vancomycin-induced ototoxicity related to higher peaks, it is unlikely that utilizing 15 to 20 mg/kg/dose will result in an increased incidence of ototoxicity. We did not assess the occurrence of ototoxicity or nephrotoxicity in our patient population due to the retrospective nature of the investigation.
Our study is limited in that smaller patient numbers in 3 of the groups (the 10 mg/kg every 8 hours, 15 mg/kg every 12 hours, and 20 mg/ kg every 12 hours groups) limit one's ability to extrapolate these data broadly. In addition, while we graphically separated out those patients with above target levels, our study was not designed to explore the occurrence of toxicity in these patients. Lastly, patients were only included if they had normal renal function as defined by age-adjusted creatinine clearance;14,15 some patients who actually had reduced renal function due to some unknown disease state with a normal serum creatinine may have been included in our study population.
CONCLUSIONS
The data presented herein suggest that the recommended vancomycin intravenous dose of 10 mg/kg administered every 6 hours often produces below target peak and trough serum concentrations, and that vancomycin doses of 15 to 20 mg/kg administered every 6 to 8 hours more consistently produce targeted peak and trough serum concentrations. In addition, these doses infrequently exceed the recommended peak or trough ranges, decreasing the likelihood of toxicity. Vancomycin dosing in pediatric patients should be further evaluated in prospective studies to define the precise dosing regimen appropriate for the pediatric population.
ACKNOWLEDGMENTS
The authors would like to acknowledge Marc May, RPh for his technical assistance with the database that was used in this review. A portion of this work was presented at the Pediatric Pharmacy Advocacy Group meeting in Chicago, IL in October 2005.
ABBREVIATIONS
- CNS
central nervous system
- hVISA
heterogenous vancomycin-intermediate Staphylococcus aureus
- IQR
interquartile range
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
Footnotes
DISCLOSURE Dr. Benner is on the speakers bureau for Pfizer and Elan Pharmaceuticals. All of the other authors have nothing to disclose.
see Editorial page 64
REFERENCES
- 1.Keyserling HL, Sinkowitz-Cochran RL, Harris JM. Vancomycin use in hospitalized pediatric patients. Pediatrics. 2003;112:104–111. doi: 10.1542/peds.112.2.e104. et al. [DOI] [PubMed] [Google Scholar]
- 2.Rodvold KA, Everett JA, Pryka RD, Kraus DM. Pharmacokinetics and administration regimens of vancomycin in neonates, infants and children. Clin Pharmacokinet. 1997;33:32–51. doi: 10.2165/00003088-199733010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Capparelli EV, Lane JR, Romanowski GL. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol. 2001;41:927–934. doi: 10.1177/00912700122010898. et al. [DOI] [PubMed] [Google Scholar]
- 4.Lisby-Sutch SM, Nahata MC. Dosage guidelines for the use of vancomycin based on its pharmacokinetics in infants. Eur J Clin Pharmacol. 1988;35:637–642. doi: 10.1007/BF00637600. [DOI] [PubMed] [Google Scholar]
- 5.Spears RL, Koch R. The use of vancomycin in pediatrics. Antibiot Annu. 1959;1960:798–803. [PubMed] [Google Scholar]
- 6.Schaad UB, McCracken GH, Nelson JD. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980;96:119–126. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- 7.Taketomo CK, Hodding JH, Kraus DM, editors. Lexi-Comp's Pediatric Dosage Handbook. 14th ed. Hudson: Lexi-Comp; 2007. p. 1597. [Google Scholar]
- 8.Robertson J, Shilkofski N. The Harriet Lane Handbook. 17th ed. Philadelphia: Elsevier Mosby; 2005. p. 996. [Google Scholar]
- 9.American Academy of Pediatrics. Tables of Antibacterial Drug Dosages. In: Pickering LK, Baker CJ, Long SS, editors. Red Book: 2006 Report of Committee on Infectious Diseases. 27th ed. Elk Grove Village: American Academy of Pediatrics; 2006. p. 765. et al. [Google Scholar]
- 10.Saunders NJ. Why monitor peak vancomycin concentrations? Lancet. 1994;344:1748–1750. doi: 10.1016/s0140-6736(94)92890-8. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MP, Steele RW. Monitoring serum vancomycin concentrations in children: is it necessary? Pediatr Infect Dis J. 1998;17:351–353. doi: 10.1097/00006454-199804000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Cantu TG, Yamanaka-Yuen NA, Lietman PS. Serum vancomycin concentrations: reappraisal of their clinical value. Clin Infect Dis. 1994;18:533–543. [PubMed] [Google Scholar]
- 13.Schaad UB, Nelson JD, McCracken GH., Jr. Pharmacology and efficacy of vancomycin for staphylococcal infections in children. Rev Infect Dis. 1981;3(Suppl):S282–288. [PubMed] [Google Scholar]
- 14.Traub SL, Johnson CE. Comparison of methods of estimating creatinine clearance in children. Am J Hosp Pharm. 1980;37:195–200. [PubMed] [Google Scholar]
- 15.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Nephrol. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto T, Kagawa Y, Kojima M. Clinical efficacy of therapeutic drug monitoring in patients receiving vancomycin. Biol Pharm Bull. 2003;26:876–879. doi: 10.1248/bpb.26.876. [DOI] [PubMed] [Google Scholar]
- 17.Lee KR, Phelps SJ. Implementation of vancomycin monitoring criteria in a pediatric hospital. J Pediatr Pharmacol Ther. 2004;9:179–186. [Google Scholar]
- 18.Chang D, Liem L, Malogolowkin M. A prospective study of vancomycin pharmacokinetics and dosage requirements in pediatric cancer patients. Pediatr Infect Dis J. 1994;13:969–974. doi: 10.1097/00006454-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Glover ML, Cole E, Wolfsdorf J. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care. 2000;15:1–4. doi: 10.1053/jcrc.2000.0150001. [DOI] [PubMed] [Google Scholar]
- 20.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections. Arch Intern Med. 2006;166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 22.Wootton M, Walsh TR, MacGowan AP. Evidence for reduction in breakpoints used to determine vancomycin susceptibility in Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:3982–3983. doi: 10.1128/AAC.49.9.3982-3983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermsen ED, Toss GH, Rotschafter JC. Glycopeptide pharmacodynamics. In: Nightingale CH, Ambrose PG, Drusano GL, Murakawa T, editors. Antimicrobial pharmacodynamics in theory and clinical practice. 2nd ed. New York, NY: Informa Healthcare USA, Inc.; 2007. pp. 189–215. [Google Scholar]
- 24.Soriano A, Marco F, Martinez JA. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. et al. [DOI] [PubMed] [Google Scholar]
- 25.Sakoulas G, Moise-Broder PA, Schentag J. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenover FC, Moellering RC., Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 27.James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother. 1996;40:696–700. doi: 10.1128/aac.40.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houlihan HH, Mercier R, Rybak MJ. Pharmacodynamics of vancomycin alone and in combination with gentamicin at various dosing intervals against methicillin-resistant Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob Agents Chemother. 1997;41:2497–2501. doi: 10.1128/aac.41.11.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klepser ME, Patel KB, Nicolau DP. Comparison of bactericidal activities of intermittent and continuous infusion dosing of vancomycin against methicilllin-resistant Staphylococcus aureus and Enterococcus faecalis. Pharmacotherapy. 1998;18:1069–1074. [PubMed] [Google Scholar]
- 30.Farber BF, Moellering RC. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983;23:138–141. doi: 10.1128/aac.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elting LS, Rubenstein EB, Kurtin D. Mississippi mud in the 1990's: risks and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer. 1998;83:2597–2607. doi: 10.1002/(sici)1097-0142(19981215)83:12<2597::aid-cncr27>3.0.co;2-l. et al. [DOI] [PubMed] [Google Scholar]
- 32.Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25:679–687. doi: 10.1093/jac/25.4.679. [DOI] [PubMed] [Google Scholar]
- 33.Vuagnat A, Stern R, Lotthe A. High dose vancomycin for osteomyelitis: continuous vs. intermittent infusion. J Clin Pharm Ther. 2004;29:351–357. doi: 10.1111/j.1365-2710.2004.00572.x. et al. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Pediatrics, Committee on Infectious Diseases. Therapy for children with invasive pneumococcal infections. Pediatrics. 1997;99:289–299. [PubMed] [Google Scholar]
- 35.Brummett RE, Fox KE. Vancomycin and erythromycin-induced hearing loss in humans. Antimicrob Agents Chemother. 1989;33:791–796. doi: 10.1128/aac.33.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]


