Abstract
Src kinase is elevated in breast tumors that are ER/PR negative and do not overexpress HER2 but clinical trials with Src inhibitors have demonstrated little activity. The present study evaluated preclinical efficacy of a novel peptidomimetic compound, KX-01 (KX2-391), that exhibits dual action as a Src and pretubulin inhibitor. KX-01 was evaluated as a single agent and in combination with paclitaxel in MDA-MB-231, MDA-MB-157, and MDA-MB-468 human ER/PR/HER2-negative breast cancer cells. Treatments were evaluated by growth/apoptosis, isobologram analysis, migration/invasion assays, tumor xenograft volume, metastasis, and measurement of Src, FAK, microtubules, Ki67, and microvessel density. KX-01 inhibited cell growth in vitro and in combination with paclitaxel resulted in synergistic growth inhibition. KX-01 resulted in a dose dependent inhibition of MDA-MB-231 and MDA-MB-157 tumor xenografts (1 and 5 mg/kg, BID). KX-01 inhibited activity of Src and downstream mediator FAK in tumors that was coincident with reduced proliferation and angiogenesis, and increased apoptosis. KX01 also resulted in microtubule disruption in tumors. Combination of KX-01 with paclitaxel resulted in significant regression of MDA-MB-231 tumors and reduced metastasis to mouse lung and liver. KX-01 is a potently active Src/pretubulin inhibitor that inhibits breast tumor growth and metastasis. As ER/PR/HER2-negative patients are candidates for paclitaxel therapy, combination with KX-01 may potentiate antitumor efficacy in management of this aggressive breast cancer subtype.
Keywords: Breast cancer, KX-01 (KX2-391), paclitaxel, microtubule, src, preclinical
Introduction
Patients with ER/PR/HER2-negative breast cancer subtype have been difficult to treat due to tumor heterogeneity and lack of definitive targets for targeted therapeutics (1). Chemotherapy remains the standard of care for patients as hormonal therapies and HER2-targeting agents are not indicated (2). Paclitaxel is a microtubule stabilizing agent widely used in breast cancer (3). Although ER/PR/HER2-negative breast cancer responds to these and other conventional agents, patients become resistant to treatment and relapse more frequently than patients with other breast cancer subtypes (4).
c-Src is an oncogenic non-receptor tyrosine kinase that is up-regulated in approximately half of all breast cancers (5). Src kinase has been associated with breast cancer proliferation, angiogenesis, cell motility, migration/invasion and metastasis (6, 7). The role of Src in proliferation, migration and invasion, coupled with the elevated Src expression in breast cancer make Src a promising target for development of therapeutics. Moreover, Src inhibition has been identified as a therapeutic target for ER/PR/HER2-negative breast cancer (8). However as a single agent, the broad specificity Src/tyrosine kinase inhibitor dasatinib (Sprycel) resulted in only a modest response rate of 5% in a phase II trial of 43 patients with advanced ER/PR/HER2-negative breast cancer (4, 9). Another Src inhibitor, Sarcatinib failed as monotherapy in hormone receptor negative breast cancer patients (10). It is unknown why these compounds failed in these early trials or whether combinations with other drugs may have resulted in better response.
Peptidomimetics represent a novel class of drugs that interact with the peptide substrate sites of proteins. KX-01 (clinical-reference, KX2-391) was developed as a ‘first in class’ peptidomimetic Src kinase inhibitor that binds to the peptide substrate site and inhibits Src kinase activity and downstream targets (11, 12). Orally bioavailable KX-01 completed phase 1 clinical testing (13) and is currently in phase 2 trials for prostate cancer (14) as well as phase 1b trial for Acute Myeloid Leukemia (15). In addition to Src inhibition, KX-01 has a second mechanism of action (MOA) through binding to novel sites on alpha-beta tubulin heterodimer that results in inhibition of microtubule polymerization (16). The dual activity of KX-01 to both inhibit Src and to disrupt microtubules may provide KX-01 with additional antitumor activity for ER/PR/HER2-negative breast cancer in comparison to compounds that exhibit Src inhibition alone.
A previous study from this laboratory demonstrated that KX-01 combined with tamoxifen resulted in synergistic growth inhibition in ER positive xenograft tumors, in part, through reduced phosphorylation and transcriptional activity of ERα (11). Given the elevated expression of Src in ER/PR/HER2-negative breast cancer (17), the present study evaluated the preclinical activity of peptidomimetic Src inhibitor KX-01 alone, and in combination with chemotherapeutic drug paclitaxel. Since Src has been implicated in invasion and metastasis, this study also examined the anti-invasive and anti-metastatic potential of KX-01 both in vitro and in vivo.
Materials and Methods
Cell lines, culture conditions and reagents
The human breast cancer cell lines MDA-MB-231, MDA-MB-157, and MDA-MB-468 were obtained from the American Type Culture Collection. These cell lines lack expression of ERα, and PR and do not exhibit amplification/overexpression of HER2 (18) . The cells were cultured in DMEM medium (Invitrogen, Gibco) supplemented with 10% FBS, 1% penicillin/streptomycin in a humidified incubator at 37°C containing 5% CO2. No further authentication was performed for cell lines. KX-01was provided by Kinex Pharmaceuticals (Buffalo, NY) in powder form [KX-01:MSA salt (MSA-Methanesulfonic acid)] that was water soluble. Paclitaxel was purchased from Hospira Inc. and doxorubicin from Bedford laboratories.
MTT and Apoptosis assay
MTT and apoptosis assays (ELISA, Roche) were performed as described (11). Cell growth was measured in MDA-MB-231, MDA-MB-157, MDA-MB-468 cells after incubation with vehicle and varying concentrations of KX-01, paclitaxel, doxorubicin or dasatinib (5, 10, 25, 50, 100, 250nmol/L) for 48h. Cell growth was expressed as percent of vehicle. Apoptosis was evaluated 24h after drug incubation.
Invasion assay
Invasion assays were performed as described (19). Cells were incubated with KX-01 (10, 25, 50nmol/L) for 24h. Invaded cells were counted (Image J software) and photomicrographed. The correction for growth inhibition by KX-01 is described in ‘Supplementary Methods’. The number of cells invaded was counted and percent invasion was calculated by setting vehicle as 100%. The invasion was normalized to cell number as shown below,
3D-on top assay
This assay was performed as described (20) and is described in ‘Supplementary Methods’. KX-01 was added to the medium (10, 25, 50nmol/L) at the time of cell plating. The Matrigel-medium mixture and KX-01 was replaced every 2days for the duration of the 4days and photomicrographed.
Scratch assay
Scratch assay was performed as described (19). Cells were incubated with vehicle or KX-01 (5, 10 and 25nmol/L) for 24h after scratch and wound closure was photographed at 40× magnification. Wound closure was evaluated by measuring wound width by Image J. Percent gap closure was calculated as:
Animals
Female athymic NUDE mice (BALB/c) aged between 4-5 weeks obtained from Charles River (Indianapolis, IN) were housed in sterile cages and maintained in pathogen-free aseptic rooms with 12h/12h light/dark schedule. Mice were fed with autoclaved food pellets and water ad libitum. All experiments were performed in accordance with approved IACUC protocol #2941R2 from Tulane University.
Tumor xenograft studies
Xenograft procedures and KX-01 oral dosing were as described (11). Briefly, mammary fat pad tumors were established by injecting 5×106 MDA-MB-231 cells in 150μl of PBS-Matrigel mixture (1:2) orthotopically and bilaterally into the mammary fat pads of female NUDE mice (two tumors/mouse). Treatments were started when tumors reached ∼80-100mm3. The first study used MDA-MB-231 xenografts and was performed using vehicle (ultra-pure water) and two doses of KX-01 (1, 5mg/kg) administered twice/day (BID) by oral gavage (using metal 22g feeding needle) for 28 days. A similar experiment was performed with MDA-MB-157 xenografts (another ER/PR/HER2 negative model) to assess KX-01 response. A second study was performed to test combination of KX-01 with paclitaxel on tumor growth. MDA-MD-231 tumor xenograft bearing mice were treated with vehicle or KX-01 (5mg/kg) BID, paclitaxel by intraperitoneal injection (IP) once/week, or combination of KX-0+paclitaxel. Treatments were for 40 days for all groups. A third study used MDA-MB-157 xenografts with the same combination treatment. A fourth study tested the effect of KX-01 or combination with paclitaxel for 24 days on larger MDA-MB-231 tumors (∼300mm3). Tumors were allowed to reach ∼300mm3 before beginning treatments. In this experiment mice were treated with KX-01 at a higher dose of 15mg/kg, and mice were treated once/day instead of twice/day. Paclitaxel was used at a dose of 20mg/kg IP once/week. In all experiments, tumor caliper measurements were taken twice/week and tumor volume was by calculated by the formula: 0.523×LM2 (where L-large diameter, M-small diameter). At the end the experiments animals were sacrificed and tumors and mouse organs removed. Tissues were either stored in 10% neutral buffered formalin for paraffin embedding, or snap frozen for measurement of chromosome-17 by real-time PCR, and embedded for frozen sectioning for CD-31 staining. Immunohistochemistry (IHC) was performed as described (11) on paraffin-embedded tumor tissues. The detailed IHC procedure is described in ‘Supplementary Methods’.
Immunofluorescence staining of alpha tubulin in MDA-MB-231 cells and tumors
For in vitro experiments, MDA-MB-231 cells were incubated with 200nmol/L KX-01 for 24h, fixed and immunostained with alpha-tubulin antibody conjugated with Alexafluor-488 (Invitrogen #32-2588). Fluorescence images were captured with a BD Pathway 855 Confocal fluorescence microscope at 60× using Attovision software. Immunofluorescence for alpha-tubulin was performed in MDA-MB-231 tumors as described in ‘Supplementary Methods’. All fluorescent images were captured using an identical exposure time to permit comparison of staining intensity between treatment groups.
Quantification of micrometastases
Human DNA within the mouse organs was measured using quantitative real time RT-PCR to detect human chromosome-17. The detection is based on a human-specific α-satellite DNA sequence of the centromere region of human chromosome 17 (21). After necropsy (n=5, animals/group), mouse organs were removed, homogenized, and DNA extracted and quantified using a NanoDrop Spectrophotometer (ThermoScientific). Quantitative real-time PCR procedure is described in ‘Supplementary Methods’. The CT value obtained for human chromosome -17 was normalized to housekeeping gene GAPDH (CT value) that detected both mouse and human GAPDH as a measure of total DNA for the samples (GAPDH served as a loading control). Since the extracted DNA could have both mouse and human DNA, we used primers that detected both mouse and human GAPDH. ΔCT=CT value of human chromosome-17 gene minus CT value of mouse/human GAPDH. Standard curves (SC) for number of human cells within a mouse organ were generated by serial additions of MCF-7 human breast cancer cells [106, 105, 104, 103, 102, 0 cells] to mouse whole lung, whole liver or bone marrow (from one femur) just prior to homogenization of the tissues and isolation of DNA. Mouse organs were derived from untreated female NUDE mice at the same age as mice from the treatment groups. Human/mouse GAPDH was used as an internal control. Real time RT-PCR for human/mouse GAPDH was performed as described (22). Real-time PCR-assays were performed in triplicate. The SC for lung, liver and femur was used to calculate the absolute number of human cells present in the lung, liver and bone. Non-linear regression analysis was used to interpolate unknowns from the SC (GraphPad Prism5). The detection limit for real-time PCR in this assay was 100 human cells per mouse lung, liver or femur.
Statistical Analysis
Data were expressed as mean ± SD. P<0.05 was considered significant. The mean and S.D. were calculated using Microsoft Excel or GraphPad Prism 5 software (La Jolla, CA). Statistical significance was determined by two-sample student t-tests (P<0.05) (two-tailed) and one-way ANOVA followed by Newman-Keuls multiple comparison test. Combination indices (CI) for combination drug treatments were performed using Calcusyn software (Biosoft, Cambridge, UK). The extent of cooperation between KX-01 with paclitaxel was determined from the CI as follows: CI<1.0 indicated synergism; CI = 1.0 indicated additivity; CI>1.0 indicated antagonism (23).
Results
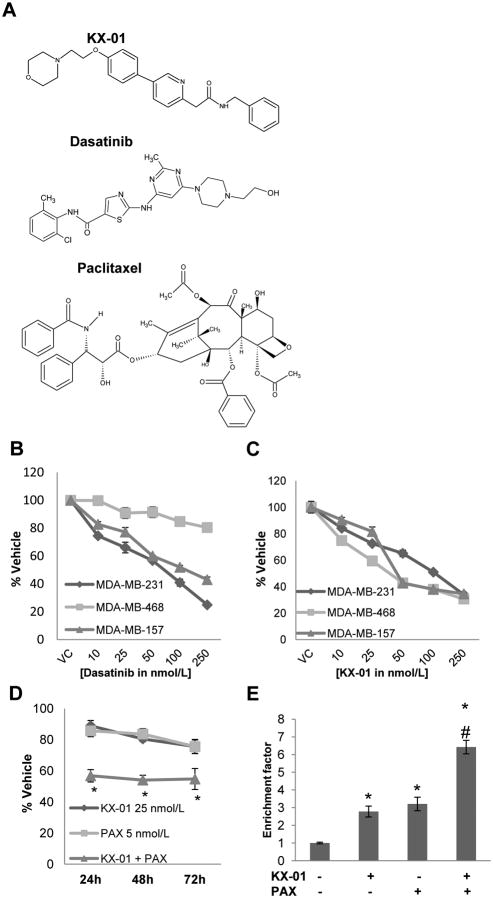
Chemical structures of KX-01, Dasatinib and Paclitaxel were shown in Fig.1A.
Figure 1.
KX-01 induced growth inhibition and apoptosis in ER/PR/HER2-negative breast cancer cells and synergized with paclitaxel in vitro. A) Chemical structures of KX-01, dasatinib and paclitaxel. B,C) Growth inhibitory effects of the broad specificity Src inhibitor dasatinib (B) and KX-01 (C) at varying concentrations (10, 25, 50, 100, 250nmol/L) on ER/PR/HER2-negative MDA-MB-231, MDA-MB-157, and MDA-MB-468 cells. Cells were incubated with drugs for 48h and growth inhibition was assessed by MTT assay. Results presented as % vehicle ± SD (n=3). D) KX-01 induced synergistic growth inhibition in MDA-MB-231 cells when combined with paclitaxel (PAX). Cell growth was measured by MTT assay. Cells were incubated with KX-01 (25nmol/L), PAX (5nmol/L), or combination for 24, 48, 72h. Results presented as % vehicle ± SD (n=3). E) KX-01 in combination with PAX resulted in enhanced apoptosis in MDA-MB-231 cells. Apoptosis assay was performed following 24h incubation with vehicle KX-01 (25nmol/L), PAX (5nmol/L) or KX-01+PAX. *, P<0.05 (Student's t-test), significantly different compared to vehicle, #, P<0.05 (Student's t-test), statistical evidence of enhanced apoptosis in combination treatment compared to either drug alone. Data are representative of three independent experiments.
Effect of KX-01 and dasatinib on ERα negative breast cancer cells
The ATP-analogue Src-inhibitor dasatinib was demonstrated to inhibit growth of MDA-MB-231 breast cancer cells, but not ERα/PR/HER2- negative MDA-MB-468 cells (18) (Fig.1B). KX-01 at varying concentrations (10-250nmol/L) inhibited in vitro growth of both MDA-MB-231 and MDA-MB-468 cells as well as another ERα/PR/HER2-negative cell line, MDA-MB-157 (Fig.1C). For dasatinib-resistant MDA-MB-468 cells, KX-01 inhibited growth by ∼75.0% at the same concentration range where dasatinib inhibited growth by only ∼20.0% (Fig.1C). Moreover KX-01 induced significant apoptosis in MDA-MB-468 cells at 25nmol/L, whereas dasatinib did not induce apoptosis at a 10× higher concentration (250nmol/L) (Suppl.Fig.1).
Combination of KX-01 with paclitaxel resulted in synergistic growth inhibition of breast cancer cells in vitro
A previous study from this laboratory demonstrated that KX-01 inhibited growth of a panel of breast cancer cell lines with different ERα status [ER-positive MCF-7 cells, and ER-negative MDA-MB-231, MDA-MB-157, MDA-MB-468, and BT-549 cells], but did not affect growth of non-tumorigenic breast epithelial MCF-10A cells (11). After 24h incubation, KX-01 (25nmol/L) alone or paclitaxel (5nmol/L) alone at sub-optimal growth inhibitory concentrations produced modest growth inhibition of 11.2% and 14.1%, respectively (Fig.1D). Co-incubation of KX-01 with paclitaxel resulted in synergistic 43.2% growth inhibition of MDA-MB-231 cells compared to vehicle treated cells (Fig.1D). A dose response for growth inhibition was determined over concentration ranges of 5-100 nmol/L for KX-01 alone and 1-10 nmol/L for paclitaxel [PAX] (data not shown). From these data combination index (CI) values were calculated (as described (23)) for KX-01+PAX over a concentration range (Suppl.Table1A). Similar patterns of synergistic growth inhibition of KX-01 with paclitaxel at various concentrations were observed in MDA-MB-157 and MDA-MB-468 cells (Suppl.Table1A).
Combination of KX-01 with paclitaxel induced apoptosis in MDA-MB-231 breast cancer cells
To determine whether synergistic growth inhibition by combination of paclitaxel+KX-01 resulted from increased apoptosis, an ELISA-based DNA fragmentation assay was used. KX-01 or paclitaxel at the concentrations used in Figure 1D resulted in modest apoptosis in MDA-MB-231 cells (Fig.1E). Combination of KX-01 with paclitaxel enhanced apoptosis two-fold compared to single drug treatments (Fig.1E).
Oral administration of KX-01 reduced growth of ER/PR/HER2-negative breast tumor xenografts in mice
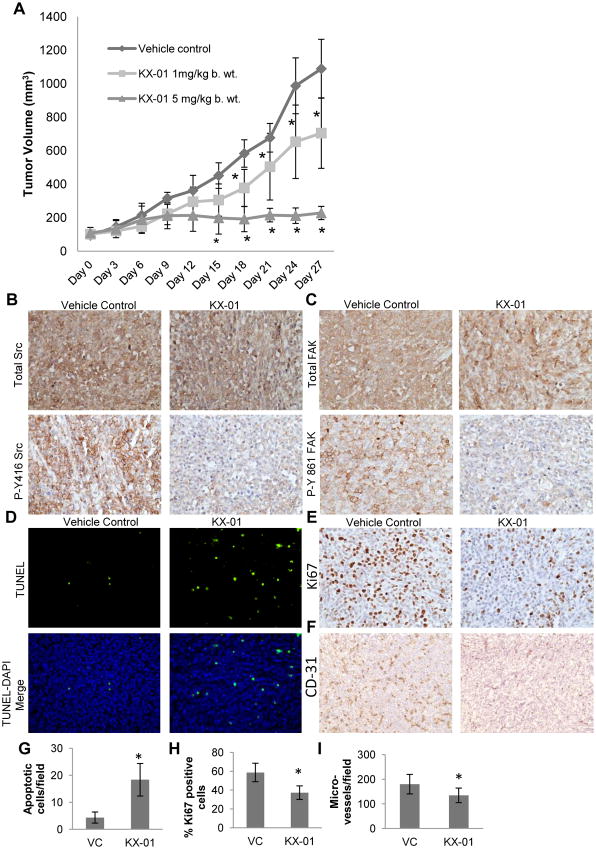
MDA-MB-231 and MDA-MB-157 xenograft tumors (80-100 mm3) in NUDE mice were established to determine the effect of KX-01 on tumor growth in vivo. Mice were treated with vehicle (ultra-pure water), KX-01 at 1mg/kg and KX-01 at 5mg/kg for 28 days, BID. KX-01 resulted in marked tumor growth inhibition in both tumor models (Fig.2A and Suppl.Fig.2A). Mice receiving KX-01 at 1mg/kg and 5mg/kg showed reduced MDA-MB-231 tumor volume compared to vehicle from days 18 and 15, respectively (P<0.05;Fig.2A). KX-01 at 1mg/kg and 5mg/kg reduced MDA-MB-231 tumor volume 35.3% and 79.0%, respectively (Fig.2A), and reduced MDA-MB-157 tumor volume 23.5% and 42.0%, respectively (Suppl.Fig.2A).
Figure 2.
KX-01 inhibited growth of MDA-MB-231 xenografts. A) Athymic NUDE mice bearing MDA-MB-231 tumors (∼100mm3) were separated into three treatment groups randomly (n=10 tumors/group). The vehicle group received ultra-pure water and the other groups were treated with two different doses of KX-01 (1, 5mg/kg) for 28 days and tumor volumes were recorded as mm3 ± SD. *, P<0.05; (One-way ANOVA followed by Newman-keuls multiple comparison test). B,C) KX-01 inhibited phosphorylation of Src and downstream mediator FAK in MDA-MB-231 xenografts. IHC was performed as described in ‘Supplementary Methods’ for total Src, phospho-Src-Y416 (P-Y416-Src), FAK and phospho-FAK-Y861 (P-Y861-FAK). Representative photomicrographs are presented (200×). D-F) KX-01 induced apoptosis, and reduced proliferation and angiogenesis in MDA-MB-231 xenografts. Paraffin embedded tumor sections from vehicle and KX-01 (5mg/kg) groups were stained for TUNEL (D) and Ki67 (E). Fluorescent (TUNEL) and bright field (Ki67) photomicrographs were taken and representative images are presented (200×). F) Frozen tumor sections were stained for mouse CD31. Bright field microscope images (40×) were taken and representative photomicrographs are presented.
KX-01 decreased activity of Src and downstream target FAK in MDA-MB-231 tumor xenografts
To determine whether KX-01 effectively inhibited activation of Src in primary tumors, tumors were prepared for IHC using antibodies to the active forms of Src kinase (P-Y416-Src) and its downstream target FAK (P-Y861-FAK). KX-01 significantly inhibited Src phosphorylation (Fig.2B) and FAK phosphorylation (Fig.2C) with no significant effect on expression of total Src or FAK.
KX-01 reduced proliferation and angiogenesis and increased apoptosis in MDA-MB-231 tumor xenografts
TdT-mediated dUTP nick-end labeling (TUNEL) was used to measure apoptotic cells. KX-01 significantly enhanced the number of apoptotic cells 3-4 fold compared to vehicle treated tumors (P<0.001) (Fig.2D,G). KX-01 reduced the percentage of tumor cells positive for proliferation marker Ki67 positive tumor cells to 37.3% compared to vehicle (58.7 %) (P<0.001) (Fig.2E,H). To evaluate the effect of KX-01 on angiogenesis, microvessel density (MVD) was assessed by counting CD31 positive microvessels. KX-01 significantly reduced MVD (P<0.05) compared to vehicle (Fig.2F,I). In summary, these data demonstrate that KX-01 significantly reduced growth of MDA-MB-231 tumors that was attributed, in part, to reduced tumor cell proliferation, increased apoptosis and decreased blood vessel density (modestly) that was coincident with reduced Src kinase activity in the tumors.
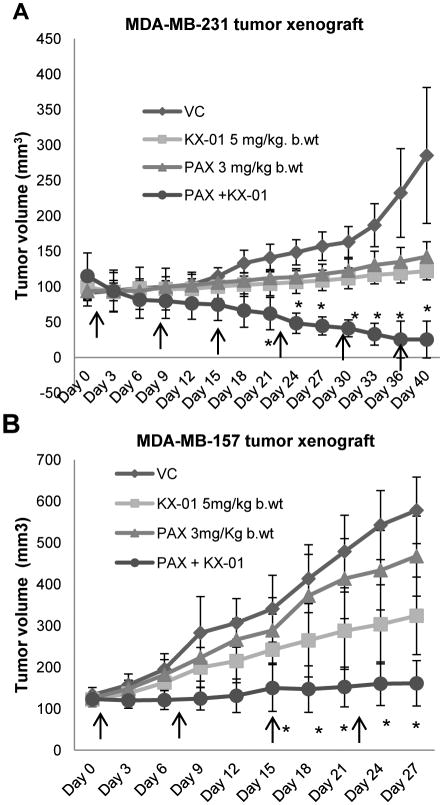
Combination of KX-01 with paclitaxel resulted in regression of ER/PR/HER2-negative tumor xenografts
The in vitro observations of synergistic growth inhibition of breast cancer cell lines co-incubated with KX-01 and paclitaxel were evaluated in vivo using MDA-MB-231 and MDA-MB-157 tumor xenografts. KX-01 administered alone (5mg/kg, BID) decreased MDA-MB-231 tumor volume by 61.1%, and treatment with paclitaxel alone (3mg/kg, once/week) decreased tumor volume by 33.4% compared to vehicle (P<0.01, Fig.3A). Combination of KX-01 with paclitaxel reduced tumor volume by 93.0% (Fig.3A). Most significantly, combination of KX-01 with paclitaxel resulted in complete regression of tumors in two of five mice. In MDA-MB-157 xenografts, the same single agent doses of KX-01 and paclitaxel reduced tumor volumes by 42.0% and 16.3%, respectively (Fig.3B). Combination of KX-01+paclitaxel reduced MDA-MB-157 tumor volumes by 71.1% compared to vehicle (P<0.001; Fig.3B). No noticeable toxicity or significant body weights reduction was observed in either drug alone or combinational treatment groups (Suppl.Fig.3A). Hematoxylin and eosin (H&E) staining of MDA-MB-231 tumor sections revealed more acellular, necrotic regions in tumors from the combination treatment groups compared to single drug treatments (Suppl.Fig.3B, arrows).
Figure 3.
KX-01 combined with paclitaxel resulted in regression of MDA-MB-231 and MDA-MB-157 xenografts. Mice bearing MDA-MB-231 or MDA-MB-157 xenografts (∼100mm3) were separated into four treatment groups randomly with 5 mice/group (2 tumors/mouse). MDA-MB-231 (A) and MDA-MB-157 (B) xenograft bearing mice were treated with ultra-pure water, KX-01 alone (5mg/kg) BID, paclitaxel (PAX) (3mg/kg) once/week by IP as indicated by the arrows, and KX-01+PAX. Tumors volumes were recorded as mm3±SD. *, P<0.05; (One-Way ANOVA followed by Newman-keuls multiple comparison test).
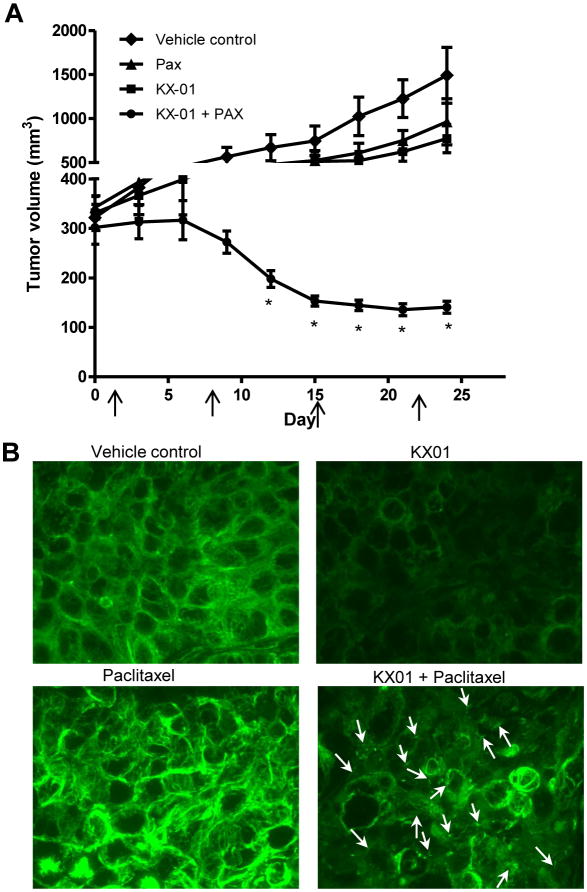
Combination of KX-01 with paclitaxel resulted in regression of larger, established MDA-MB-231 tumors
Xenograft experiments described in Figures 2, 3 and Supplemental Figure 2 began drug treatments when tumors reached a palpable size of ∼80-100 mm3. To determine whether combination of KX-01 with paclitaxel would cause regression of larger and more established tumors, MDA-MB-231 xenografts were allowed to reach ∼300mm3 before treatments began. Animals were treated with KX-01 at a higher dose of 15mg/kg only once/day, which when compared to 5mg/kg KX-01 twice/day showed similar tumor growth inhibition in MDA-MB-231 xenografts (data not shown). Previous studies demonstrated that a higher dose of paclitaxel was needed for efficacy against larger, established MDA-MB-231 tumors as compared to smaller tumors (24, 25). For this reason, paclitaxel was used at a dose of 20 mg/kg, once/week. KX-01 alone resulted in significant tumor growth inhibition but without tumor regression; tumor volume was decreased by 48.2% (P<0.001 versus vehicle; Fig.4A). Paclitaxel alone resulted in significant tumor growth inhibition but without tumor regression; tumor volume was decreased by 35.4% (P<0.001; Fig.4A) with no significant reduction in mouse body weight (Suppl.Fig.3C). Combination of KX-01 (15mg/kg) with paclitaxel (20mg/kg) resulted in 90.6% tumor regression to a volume that was markedly below the starting tumor volume (P<0.001, Fig.4A) with approximately 10% mouse body weight reduction but without noticeable toxicity (initial mean body weight= 19.4g; final mean body weight= 17.6g; Suppl.Fig.3C). By day 12, mice receiving KX-01+paclitaxel showed significant tumor regression compared to single treatments (P<0.001, Fig.4A). H&E staining of MDA-MB-231 tumor sections showed more acellular, necrotic regions in tumors from combination treatments compared to single treatments (Suppl.Fig.3D, arrows).
Figure 4.
Combination of high dose KX-01 with paclitaxel induced regression of established, large MDA-MB-231 tumors. A) MDA-MB-231 tumors were established in NUDE mice (tumor volume ∼300 mm3) before treatments began. Mice (n=5/group) were treated with vehicle, KX-01 (15mg/kg) by oral gavage once/daily, paclitaxel (PAX, 20mg/kg) once/week by IP (arrows), or KX-01+PAX. Tumor volumes were recorded as mm3±SD. *, P<0.05; (One-Way ANOVA followed by Newman-keuls multiple comparison test. B) Paraffin tumor sections from treatment groups were analyzed by immunofluorescence with anti–tubulin antibody conjugated with Alexafluor-488 (green) and imaged with a confocal microscope (60×). White arrows indicate fragmented microtubules in the KX-01+PAX group.
KX-01 disrupted microtubules in MDA-MB-231 cells and tumors
In addition to Src inhibition, KX-01 was demonstrated to inhibit microtubule polymerization in vitro indicating a second MOA (16, 26). KX-01 (200nmol/L, 24 h) markedly inhibited microtubule formation in MDA-MB-231 cells in vitro (Suppl.Fig.4A). To assess the effect of KX-01 on microtubule organization in tumor tissues, paraffin embedded MDA-MB-231 tumor sections were incubated with an anti-tubulin fluorescent antibody. In vehicle treated animals, tumor cells exhibited staining of distinct microtubule fibers demonstrative of an intact microtubule network (Fig.4B). In KX-01 treated mice, tumor cells exhibited a more diffuse staining pattern with markedly reduced fluorescence intensity indicative of a disrupted and reduced microtubule network in the cells. In contrast, paclitaxel resulted in more intensely stained microtubule arrays, consistent with the microtubule stabilizing activity of paclitaxel (Fig.4B). Interestingly, the tumors from mice treated with KX-01+paclitaxel showed a microtubule staining pattern that was markedly different from either single treatment. KX-01+paclitaxel resulted in a diffuse tubulin staining pattern indicating loss of the microtubule network, as well as evidence of fragmented microtubules (arrows). Taken together, these data provide direct evidence that KX01 alone, and in combination with paclitaxel resulted in disruption of the microtubule network in tumors.
KX-01 inhibited breast cancer cell outgrowth, invasion and migration in vitro
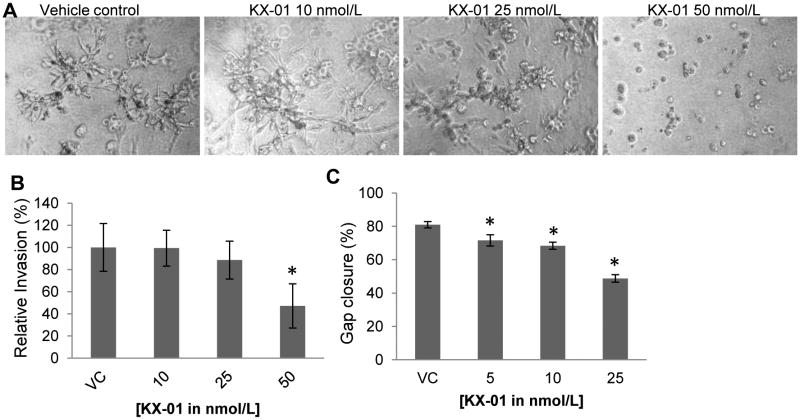
Cell invasiveness was assessed using a 3D invasion assay in which MDA-MB-231 cells were cultured in reconstituted extracellular matrix to mimic an in vivo microenvironment (20). Treatment of MDA-MB-231 cells with 25 and 50nmol/L KX-01 reduced invasive stellate structures that are a hallmark of invasive cells (Fig.5A). There was almost complete inhibition of stellate structures at 50nmol/L KX-01. The effect of KX-01 on MDA-MB-231 invasion in vitro was assessed using the Boyden chamber Matrigel invasion assay. A significant decrease in MDA-MB-231 cell invasion by 52.9% occurred with 50 nmol/L KX-01 (Fig 5B; P<0.05; Suppl.Fig.4B). The anti-invasive effect was independent of the growth inhibitory effect of KX-01 since invasion data were normalized to cell number (see Materials and Methods). Cancer cell migration was assessed by a wound healing/scratch assay. Concentrations of 5, 10, and 25nmol/L KX-01 significantly decreased MDA-MB-231 cell migration by 11.7%, 15.6% and 39.9%, respectively, compared with vehicle (Fig.5C; P<0.05; Suppl.Fig.4C).
Figure 5.
KX-01 inhibited invasive stellate formation, invasion, and migration of MDA-MB-231 cells in vitro. A) Invasive stellate structures of MDA-MB-231 cells in 3D culture following vehicle or KX-01 treatment were photomicrographed day4. B) MDA-MB-231 cells were incubated with vehicle (VC) or KX-01 (10, 25, 50nmol/L) for 24h and the number of cells invaded was quantified and normalized to cell number and presented as % relative invasion ±SD. *, P<0.05 statistically significant compared to VC. C) Monolayer cultures of MDA-MB-231 cells were gently scratched with a pipette tip to produce a wound. Photographs of cultures were taken immediately after the scratch (0h) and after 24h incubation with vehicle or KX-01 (5, 10, 25nmol/L). Migration is presented as % gap closure ±SD, *, p<0.05, significantly different from VC. Data are representative of three independent experiments.
Effect of KX-01 on MDA-MB-231 micrometastasis in vivo
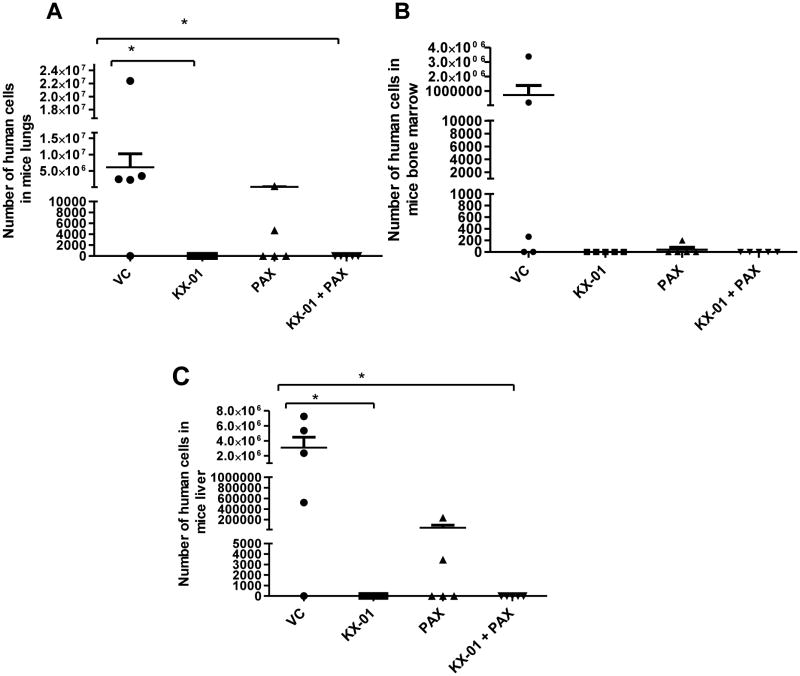
Although the relatively short treatment time (40 days) for the MDA-MB-231 xenograft tumors was not sufficient for tumors to exhibit visual macrometastases to mouse organs, 40 days was sufficient to measure micrometastasis by quantitating human DNA in mouse tissues. At the termination of experiments described in Figure 3A, lung, liver, and the bone marrow from femurs were removed (n=5 mice), and the amount of human DNA in these mouse tissues was measured by quantitative real-time RT-PCR directed towards an α-satellite sequence specific for human chromosome 17. Standard curves (SC) for the number of human cells within control mouse organs were generated by serial additions of human breast cancer cells to whole lung, whole liver and bone marrow (one femur) just prior to homogenization of the tissues and DNA isolation (Suppl.Fig.5A). The incidence of metastasis to the lung in the vehicle group was 4 out of 5 mice (80%). The mean number of MDA-MB-231 cells in the mouse lung for the vehicle group was 6.0×106 cells (number extrapolated from the standard curve, Suppl.Fig.5A). KX-01 alone (5mg/kg) or KX-01+paclitaxel (3mg/kg) significantly reduced metastasis to the lungs (P<0.05, Fig.6A). No human cells were detected in the lungs of mice treated with either KX-01 alone or KX-01+paclitaxel. The incidence of metastasis to the bone marrow was 3/5 mice (60%) and the mean number of MDA-MB-231 cells in the bone marrow for the vehicle group was 7.1×105 cells. Although there were no detectable human cells in the bone marrow from mice treated with KX-01 alone or paclitaxel+KX-01, the differences between these treatment groups and vehicle were not statistically significant (Fig.6B). Additionally, there was no statistical difference between vehicle and paclitaxel alone for metastatic cells in the bone marrow (Fig.6B). The incidence of micrometastasis to liver in the vehicle group was 4/5 mice (80%) and the mean number of MDA-MB-231 cells in the liver for the vehicle group was 3.0×106 cells. As with the lungs and bone marrow, no detectable human cells were found in the livers of mice treated with KX-01 alone or KX-0+paclitaxel (Fig.6C). There was a statistically significant reduction in liver metastasis in the KX-01 alone group and the KX-01+paclitaxel group compared to the vehicle group (P<0.05;Fig.6C). Paclitaxel alone did not significantly alter liver metastasis compared to vehicle. Taken together, these data demonstrate that KX-01 alone and in combination with paclitaxel significantly reduced metastasis of MDA-MB-231 cells to the lung and liver.
Figure 6.
Quantification of micrometastasis of MDA-MB-231 tumors in mouse organs. After necropsy, lung, bone marrow (femur) and liver were collected from treatment groups described in Figure 3 [vehicle control (VC), KX-01, PAX, KX-01+PAX (n=5 mice/group)] and genomic DNA isolated. Micrometastases originating from human primary MDA-MB-231 xenografts were detected by quantitative real-time RT-PCR for an α-satellite DNA sequence in the centromere region of human chromosome-17. Test samples (the number of human cells in each mouse tissue sample) were interpolated from a standard curve as described in Materials and Methods. Graphs represent the number of human cells in intact mouse lung (A), bone marrow from one femur (B) and intact mouse liver (C). *, P<0.05, significantly different compared to VC.
Discussion
Activation of Src kinase has been shown to decrease apoptosis of cancer cells and to promote cell mitosis, cell motility/invasion, and neo-angiogenesis to tumors (6, 7). In the present study, the clinical Src/pretubulin inhibitor KX-01 as a single agent inhibited growth, migration and invasive potential and increased apoptosis of ER/PR/HER2-negative breast cancer cells in vitro. Treatment of mice bearing ER/PR/HER2-negative tumors with KX-01 resulted in a dose dependent decrease in tumor volume with concomitant reduction in proliferation, microvessel density and increased apoptosis. When KX-01 was combined with paclitaxel there was significantly reduced tumor volume and tumor regression compared to either drug alone without noticeable toxicities. KX-01 also exhibited an anti-metastatic effect against MDA-MB-231 tumors. The antitumor and anti-metastatic effects of KX-01 were coincident with marked inhibition of Src activity, and disruption of the microtubule network in the primary tumors.
ER/PR/HER2-negative breast cancers are intrinsically more chemo-resistant than other breast cancer subtypes (27). Given the lack of targeted agents and the rapid onset of chemo-resistance in metastatic ER/PR/HER2-negative breast cancers, there is urgent need for novel therapeutic approaches to target these tumors. Combination of targeted agents with conventional chemotherapeutic agents are currently being investigated and have proven clinically successful in several cancers, including breast cancer (28, 29). The present study presents KX-01 as a first in class clinical Src/pretubulin inhibitor that results in significant tumor regression when combined with standard of care chemotherapy agent paclitaxel demonstrating that this combination could be considered in the clinical setting.
KX-01 efficacy in preclinical tumor models was correlated with strong inhibition of Src and FAK activity suggesting that Src inhibition contributed to the antitumor effect. In addition to Src inhibition, KX-01 also inhibits microtubule polymerization as a second MOA (16, 26). This second MOA likely contributes to the robust apoptosis and antitumor activity in comparison to drugs that exhibit only Src inhibition. In this regard, KX-01 as a single compound may exert similar effects as combination of clinical Src inhibitors with microtubule targeting chemotherapy (30), and may also be effective in Src inhibitor-resistant tumors.
Paclitaxel stabilizes microtubules resulting in mitotic blockade and subsequent cell cycle arrest or apoptosis. Interestingly, KX-01 and paclitaxel disrupt microtubules by different means; inhibition of microtubule assembly and stabilization of microtubules, respectively, as was observed by the markedly different microtubule staining patterns in tumors exposed to these two agents (Fig.4B). The combination of KX-01 with paclitaxel resulted in a reduced microtubule network along with fragmentation of many of the remaining microtubules. It was recently shown that microtubule inhibiting drugs can cause microtubule minus-end detachment from the microtubule organizing centers (MTOC)(31). It is possible that KX-01 through inhibition of tubulin polymerization has a similar effect in causing microtubule detachment from MTOC. However in the KX-01+paclitaxel group, the resulting detached microtubules are protected by paclitaxel from further KX-01-induced depolymerization thus giving rise to fragmented microtubules. It is likely that these two different mechanisms for microtubule disruption contribute to the anti-tumor synergy observed when KX-01 and paclitaxel were combined.
Invading cells produce various membrane protrusions that promote migration and proteolysis of extracellular matrix (ECM) (32). Src has been shown to regulate microtentacles and invadopodia (32). Src is both necessary and sufficient for the formation of invadopodia at sites of integrin-mediated ECM adhesion (33). Cells coordinate focal adhesions and invadopodia to provide traction (34). MDA-MB-231 cells are highly invasive and migratory and are shown to produce microtentacle protrusions and invadopodia (32). Src inhibition by KX-01 significantly reduced formation of invasive stellate structures in MDA-MB-231 cells, and inhibited cell migration and invasion. These findings correlated with KX-01 inhibition of Src and focal adhesion kinase in tumors.
Metastasis is the final step in the progression of many solid tumors with the lungs, liver, and bone as frequent sites for breast cancer metastasis (35, 36). The brief treatment period (40 days) of MDA-MB-231 xenografts permitted an evaluation of micrometastasis to mouse organs from the primary tumor. KX-01 alone or in combination with paclitaxel resulted in complete absence of detectable micrometastasis to the lung and liver. It should be noted that the reduction in micrometastasis could be attributed, in part, to the reduced primary tumor burden in the treated animals. Because the tumor xenografts had to be terminated within 30-40 days due to the large size of the primary tumors, micrometastases to mouse organs were limited and sectioning/H&E staining of mouse organs to quantitate micrometastases was not possible. As an alternate approach to measure very early micrometastases, we quantitated levels of human chromosome 17 microsatellite regions by PCR using an established procedure (21, 37) that we have previously employed (38). Although precaution was taken to rinse mouse organs thoroughly with PBS to remove blood and blood clots prior to DNA isolation, a caveat to this procedure is the presence of any circulating tumor cells in the vasculature of the organs that could contribute to the DNA quantitation. Future studies will evaluate KX-01 in impacting multiple steps of the metastatic process using appropriate metastatic models and histopathologic methods to quantitate metastatic burden.
To determine whether KX-01 could synergize with cytotoxic drugs other than paclitaxel, doxorubicin (a topoisomerase II inhibitor widely used for the treatment of breast cancer) was used in combinational treatments. In ER/PR/HER2/neu negative cell lines, lower concentrations of KX-01 (10, 25 nM) synergized with doxorubicin for cell growth inhibition (Suppl.Table1B). However at higher KX-01 concentrations (50 nM), combination with doxorubicin resulted in antagonism with CI values above 1.0 (Suppl.Table1B). In MDA-MB-231 and MDA-MB-157 tumor xenograft experiments, combination of KX-01+doxorubicin resulted in significant tumor growth inhibition that was greater than either drug alone (Suppl.Fig.2B&C). However unlike combination with paclitaxel, the anti-metastatic effect of KX-01 in vivo was antagonized by combination with doxorubicin (Suppl.Fig.5B). Whereas KX-01 alone reduced lung metastasis, when combined with doxorubicin there was no significant difference in lung metastasis compared to vehicle. It is unknown why combination of KX-01 with doxorubicin did not result in complete synergy for cell growth inhibition and reduced metastasis across a broad concentration range of KX-01 as was demonstrated for combination with paclitaxel. The two cytotoxic agents have different MOA's that likely underlie the efficacy when combined with KX-01. As noted above, the synergy between KX-01 and paclitaxel may be due to targeting two different mechanisms for microtubule inhibition. Doxorubicin as a DNA damaging agent may not complement KX-01 as effectively. It will be important to identify concentrations of doxorubicin+KX-01 that will exhibit desired clinical outcomes. Additional studies are needed to identify the mechanisms underlying the mixed efficacy of doxorubicin combined with KX-01.
ER/PR/HER2 negative breast cancer represents 15% of breast cancers but this tumor subtype expresses elevated Src kinase compared to ER-positive breast cancer (39, 40). ER/PR/HER2 negative breast cancer presents more frequently in younger women and in African American women (41). In urban hospitals that treat a larger proportion of African American women, the incidence of ER/PR/HER2 negative breast cancer is higher than 15%. Patients who present with this tumor subtype represent an unmet medical need for clinical care of breast cancer. Although it is unlikely that inhibition of Src alone would significantly impact patient care, the present study demonstrated that targeting three MOA's (Src inhibition, tubulin disruption, and tubulin stabilization) can achieve greater efficacy in tumor growth inhibition than treatment with compounds that would target only a single MOA. Furthermore, paclitaxel dose is limited in the clinic due to neurotoxicity. The inclusion of KX-01 with paclitaxel therapy may permit lower doses of paclitaxel to be used while maintaining or improving efficacy.
Supplementary Material
Acknowledgments
Grant Support: R01DK068432, Breast Cancer Relief Foundation, New Orleans, LA, Kinex Pharmaceuticals LLC, Buffalo, NY, Clinical and Translational Research, Education and Commercialization Project, Tulane to B.G.R.
D. Hangauer is Chief Scientific Officer for Kinex Pharmaceuticals LLC that supplied KX-01 and dasatinib for this study. B. G. Rowan received a $25,000 research grant from Kinex Pharmaceuticals to support the research presented in this study.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed by other authors.
References
- 1.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 2.Rodler E, Korde L, Gralow J. Current treatment options in triple negative breast cancer. Breast Dis. 2010;32:99–122. doi: 10.3233/BD-2010-0304. [DOI] [PubMed] [Google Scholar]
- 3.Nabholtz JM, Gelmon K, Bontenbal M, Spielmann M, Catimel G, Conte P, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1858–67. doi: 10.1200/JCO.1996.14.6.1858. [DOI] [PubMed] [Google Scholar]
- 4.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 5.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto M, Takamura M, Ino Y, Miura A, Genda T, Hirohashi S. Involvement of c-Src in carcinoma cell motility and metastasis. Jpn J Cancer Res. 2001;92:941–6. doi: 10.1111/j.1349-7006.2001.tb01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–6. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Bengala C, Ibrahim N, Roche H, Sparano J, Strauss LC, et al. Dasatinib as a Single Agent in Triple-Negative Breast Cancer: Results of an Open-Label Phase 2 Study. Clin Cancer Res. 2011;17:6905–13. doi: 10.1158/1078-0432.CCR-11-0288. [DOI] [PubMed] [Google Scholar]
- 10.Gucalp A, Sparano JA, Caravelli J, Santamauro J, Patil S, Abbruzzi A, et al. Phase II Trial of Saracatinib (AZD0530), an Oral SRC-inhibitor for the Treatment of Patients with Hormone Receptor-negative Metastatic Breast Cancer. Clin Breast Cancer. 2011;11:306–11. doi: 10.1016/j.clbc.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor alpha positive breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1513-3. [DOI] [PubMed] [Google Scholar]
- 12.Fallah-Tafti A, Foroumadi A, Tiwari R, Shirazi AN, Hangauer DG, Bu Y, et al. Thiazolyl N-benzyl-substituted acetamide derivatives: synthesis, Src kinase inhibitory and anticancer activities. Eur J Med Chem. 2011;46:4853–8. doi: 10.1016/j.ejmech.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 13.ClinicalTrial.gov [homepage on the Internet]. Identifier: NCT00658970.
- 14.ClinicalTrial.gov [homepage on the Internet]. Identifier: NCT01074138
- 15.ClinicalTrial.gov [homepage on the Internet]. Identifier: NCT01397799
- 16.Hangauer D, Smolinski M, Bu Y, Kazim AL, Qu J. Photoaffinity labeling studies to better define the mechanism of action for Phase II oncology drug KX2-391. 239th ACS National Meeting; San Francisco, CA, United States. 2010. [Google Scholar]
- 17.Tryfonopoulos D, Walsh S, Collins DM, Flanagan L, Quinn C, Corkery B, et al. Src: a potential target for the treatment of triple-negative breast cancer. Ann Oncol. 2011;22:2234–40. doi: 10.1093/annonc/mdq757. [DOI] [PubMed] [Google Scholar]
- 18.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 19.Bijman MN, van Nieuw Amerongen GP, Laurens N, van Hinsbergh VW, Boven E. Microtubule-targeting agents inhibit angiogenesis at subtoxic concentrations, a process associated with inhibition of Rac1 and Cdc42 activity and changes in the endothelial cytoskeleton. Mol Cancer Ther. 2006;5:2348–57. doi: 10.1158/1535-7163.MCT-06-0242. [DOI] [PubMed] [Google Scholar]
- 20.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker M, Nitsche A, Neumann C, Aumann J, Junghahn I, Fichtner I. Sensitive PCR method for the detection and real-time quantification of human cells in xenotransplantation systems. Br J Cancer. 2002;87:1328–35. doi: 10.1038/sj.bjc.6600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 24.Yen WC, Lamph WW. The selective retinoid × receptor agonist bexarotene (LGD1069, Targretin) prevents and overcomes multidrug resistance in advanced breast carcinoma. Mol Cancer Ther. 2005;4:824–34. doi: 10.1158/1535-7163.MCT-05-0018. [DOI] [PubMed] [Google Scholar]
- 25.Naumova E, Ubezio P, Garofalo A, Borsotti P, Cassis L, Riccardi E, et al. The vascular targeting property of paclitaxel is enhanced by SU6668, a receptor tyrosine kinase inhibitor, causing apoptosis of endothelial cells and inhibition of angiogenesis. Clin Cancer Res. 2006;12:1839–49. doi: 10.1158/1078-0432.CCR-05-1615. [DOI] [PubMed] [Google Scholar]
- 26.Hangauer D, Smolinski M, Bu Y, Teegarden P, Qu J, Kazim L, Hegab T, Quinn J, Gao L, Gelman I. Keystone Symposia, New Directions in Small Molecule Drug Discovery. Whistler; British Columbia: 2010. Apr 22, Discovery of KX2-391: A Phase II Src Signaling Inhibitor with a Second MOA. [Google Scholar]
- 27.Rugo HS, Thomas ES, Lee RK, Fein LE, Peck R, Verrill M, et al. Combination therapy with the novel epothilone B analog, ixabepilone, plus capecitabine has efficacy in ER/PR/HER2-negative breast cancer resistant to anthracyclines and taxanes. SABCS. 2007;2007 [Google Scholar]
- 28.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 29.Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010;16:3526–32. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 30.Fornier MN, Morris PG, Abbruzzi A, D'Andrea G, Gilewski T, Bromberg J, et al. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H, Ganguly A, Cabral F. Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. J Biol Chem. 2010;285:32242–50. doi: 10.1074/jbc.M110.160820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balzer EM, Whipple RA, Thompson K, Boggs AE, Slovic J, Cho EH, et al. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29:6402–8. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frame MC, Fincham VJ, Carragher NO, Wyke JA. v-Src's hold over actin and cell adhesions. Nat Rev Mol Cell Biol. 2002;3:233–45. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- 34.Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–49. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 35.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias D, Di Pietroantonio D. Surgery for liver metastases from breast cancer. HPB (Oxford) 2006;8:97–9. doi: 10.1080/13651820500471871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 38.Marsden CG, Wright MJ, Carrier L, Moroz K, Pochampally R, Rowan BG. A novel in vivo model for the study of human breast cancer metastasis using primary breast tumor-initiating cells from patient biopsies. BMC Cancer. 2012;12:10. doi: 10.1186/1471-2407-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu I, Arnaout A, Loiseau S, Sun J, Seth A, McMahon C, et al. Src promotes estrogen-dependent estrogen receptor alpha proteolysis in human breast cancer. J Clin Invest. 2007;117:2205–15. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsberger B, Tan BA, Mitchell TJ, Brown SB, Mallon EA, Tovey SM, et al. Is expression or activation of Src kinase associated with cancer-specific survival in ER-, PR- and HER2-negative breast cancer patients? Am J Pathol. 2009;175:1389–97. doi: 10.2353/ajpath.2009.090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–66. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.