Abstract
Antibodies directed at non-gal xenoantigens are responsible for acute humoral xenograft rejection when gal knockout (GalTKO) pig organs are transplanted into non-human primates. We generated IgM and IgG gene libraries using peripheral blood lymphocytes of rhesus monkeys initiating active xenoantibody responses after immunization with GalTKO pig endothelial cells and used these libraries to identify IgVH genes that encode antibody responses to non-gal pig xenoantigens. Immunoglobulin genes derived from the IGHV3-21 germline progenitor encode xenoantibodies directed at non-gal xenoantigens. Transduction of GalTKO cells with lentiviral vectors expressing the porcine α1,3 galactosyltransferase gene responsible for gal carbohydrate expression results in a higher level of binding of “anti-non-gal” xenoantibodies to transduced GalTKO cells expressing the gal carbohydrate, suggesting that anti-non-gal xenoantibodies crossreact with carbohydrate xenoantigens. The galactosyltransferase 2 gene encoding isoglobotriaosylceramide synthase (iGb3 synthase) is not expressed in GalTKO pig cells. Our results demonstrate that anti-non-gal xenoantibodies in primates are encoded by IgVH genes that are restricted to IGHV3-21 and bind to an epitope that is structurally related to but distinct from the Gal carbohydrate.
Keywords: Primate; Xenotransplantation; Pig; α1,3 galactosyltransferase gene knockout; Xenoantibodies; VH3 Immunoglobulin Genes
Introduction
Pigs are considered to have potential as a supply of donor organs for transplantation into humans, but an aggressive humoral immune response prevents graft acceptance. Wild type pig organs are rejected by natural xenoantibodies that bind to the gal α1,3 gal carbohydrate xenoantigen1–7 and initiate complement-mediated cytotoxicity. Transgenic pigs expressing complement regulatory proteins such as human decay accelerating factor (hDAF) are not hyperacutely rejected but undergo acute humoral xenograft rejection8–10. Xenoreactive antibodies that initiate the rejection of xenografts from wild type pig donors and hDAF transgenic pig organs are encoded by a restricted number of germline progenitors11–15. The IgVH genes encoding these xenoantibodies in primates are closely related alleles of IGHV3-11, the germline progenitor encoding induced xenoantibodies in humans following placement on a bioartificial liver13–15.
The most recent advance in this field was the development of GalTKO pigs in which the porcine α1,3 galactosyltransferase gene was functionally inactivated16–18. GalTKO pig kidneys and hearts undergo acute humoral xenograft rejection (AHXR) and thrombosis at 3–6 months post-transplantation in primates despite extensive immunosuppression19–20. The nature of the anti-non-gal xenoantibodies responsible for delayed rejection and the target xenoantigens that initiate this response have not yet been characterized. It has been hypothesized that carbohydrate xenoantigens may be important targets of induced antibodies to GalTKO organs, but the issue is highly controversial21–25. Inactivation of the porcine α1,3 galactosyltransferase gene is not sufficient to prevent low-level expression of the gal carbohydrate in at least some lines of GalTKO pig cells26. The controversy arises over the fact that the IB4 lectin does not detect this residual gal carbohydrate whereas select anti-gal monoclonal antibodies do bind to cells from gal knockout donors22,23. It has been proposed that iGb3 synthase, a member of the ABO blood group glycosyltransferase family22 which is expressed in GalTKO mice, may be expressed in GalTKO pigs, resulting in low-level Gal carbohydrate expression on these cells. In this manuscript, we show that although anti-non-gal xenoantibodies can bind to carbohydrate xenoantigens, iGb3 synthase is not expressed in GalTKO pig cells. The restricted group of anti-non-gal xenoantibodies that are induced in response to GalTKO xenoantigens in vivo is closely related in structure, but distinct from the anti-gal xenoantibodies that reject wild type pig organ xenografts.
Materials and Methods
Animals
Three juvenile captive-bred rhesus monkeys (Macaca mulatta) 2–3 years of age (3.3–3.6 Kg in weight) were obtained from the California National Primate Research Center primate colony. The animals were housed and all surgical and sampling procedures were conducted at the California National Primate Research Center (CNPRC). These studies were reviewed and approved by the Animal Care and Use Committee of the CNPRC at the University of California, Davis.
Flow cytometry to identify binding to GalTKO and wild type pig cells
The GalTKO fetal pig fibroblasts and GalTKO endothelial cells (PEGK042) were kindly provided by Dr. David Sachs at Massachusetts General Hospital. Wild type minipig kidney cells (MPK cells) were obtained from the ATCC (Manassas, VA). Binding of anti-non-gal xenoantibodies present in the serum of rhesus monkeys at day 0, 8 and 21 post-immunization was analyzed by flow cytometry that was performed on a FACSCalibur cytometer. Each serum sample was diluted 1:10 for labeling. The secondary anti IgM-FITC antibody was obtained from Jackson Immunoresearch (West Grove, PA, #109096129) and the anti-IgG antibody used for these studies was purchased from BDPharmingen (San Diego, CA, #555787). Aquisition was done using CellQuest software and the analysis was done using FloJo (BectonDickinson, San Jose, CA). Labeled cells were identified by setting the gates to exclude non-specific binding that was determined using appropriate isotype-matched antibody controls (BDPharmingen). Background labeling was subtracted in the determination of the percentage of labeled cells.
ELISA for detection of carbohydrate binding
Binding of xenoantibodies to purified carbohydrates was examined by ELISA. Briefly, 96-well plates were coated with Gal α 1-3Gal β 1-4Glc-BSA (V-Labs Inc, Covington, LA, #NGP0330), Gal α 1-3Gal β 1-4GlcNAc-BSA (V-Labs Inc #NGP0334), or N-Acetyllactosamine-BSA (V-Labs Inc #NGP0201), at 5μg/ml in PBS and incubated overnight at 4°C. After washing with PBS, 0.1% Tween, the plates were then blocked with PBS, 1% BSA, 0.5% Tween for 1–3 hours. Serum samples were diluted 1:20 in assay buffer (PBS, 0.5% Tween), added in duplicate, and incubated at room temperature for 1 hour. The plates were then washed. To detect the bound antibodies, peroxidase-conjugated anti- human IgM and IgG (Sigma, St. Louis, MO) were added at 1:1600 and 1:400, respectively, and incubated for 1 hour at room temperature. Following additional washes, the reaction was developed with SureBlue™ TMB Peroxidase Substrate (KPL, Gaithersburg, MA) and stopped with 1N HCl. The OD was read at 450nm.
Blocking with soluble carbohydrates to inhibit binding of xenoantibodies to GalT−/− pig cells
The ability of soluble carbohydrates to block the binding of anti-non-gal antibodies found in serum to ELISA plates coated with PEGK042 GalT−/− pig endothelial cells was assessed by inhibition ELISA27,32,34. Soluble carbohydrates Gal α 1–3Gal β 1-4Glc-BSA (V-Labs Inc #NGP0330) or Gal α 1-3Gal β 1-4GlcNAc-BSA (V-Labs Inc #NGP0334), both at a concentration of 4.45mM, were incubated overnight with induced xenoantibodies obtained from the serum of monkeys immunized with GalTKO pig endothelial cells. Serum incubated with PBS overnight was used as a control. The serum was used at a 1:50 dilution and overnight incubation was done at 4°C. The primary antibodies were then incubated with GalTKO endothelial cells on blocked, precoated plates for 1 hour at room temperature before washing 3 times with PBS, 0.05% Tween. Antibody binding was detected using peroxidase conjugated anti-human IgG (Sigma, A2290) at 1:400 and incubated for 1 hour. Following another wash as above, the reaction was developed with SureBlue™ TMB Peroxidase Substrate (KPL, Gaithersburg, MA) and stopped with 1N HCl. The OD was read at 450nm.
Preparation of cDNA libraries
Peripheral blood leukocytes (PBLs) were Ficoll-separated from the whole blood of rhesus monkeys at days 0, 8 and 21 post-immunization with 60 million pig GalTKO endothelial cells iv. The induction of a strong anti-non-gal xenoantibody response was confirmed by flow cytometry and cDNA libraries were prepared from peripheral blood B cells that were isolated at each timepoint. RNA was prepared using the RNeasy Kit, (Qiagen, Valencia, CA) followed by synthesis of cDNA using Sensicript reverse transcriptase (Qiagen). The cDNA libraries of genes encoding xenoantibodies were constructed and PCR amplifications were performed as previously described14–15. Briefly, the PCR was performed in a Perkin-Elmer 9600 GeneAmp PCR System Thermocycler for 1 cycle of 94°C 5 min. followed by 28 cycles of 94°C for 30 seconds, 53°C for 30 seconds, 72°C for 60 seconds, and one cycle of 72° C for 7 minutes. The nested PCR amplification was done using Cγ, Cμ and upstream primers under conditions that were previously established by our laboratory for isolation and cloning of Ig genes in rhesus monkeys14–15. The PCR products were verified by size on a 2% agarose gel, cloned into the TA 2.1 vector (Invitrogen, Carlsbad, CA) and transformed into competent cells. We grew and sequenced a minimum of 40 cDNA clones from each individual cDNA library and compared day 0 with post-immunization libraries for the frequency of expression of IgVH genes encoded by specific germline progenitors.
DNA sequence analysis
The DNA was prepared using the QIAPrep Spin MiniPrep Kit (Qiagen) and was sequenced using the ALFexpress™ automated DNA sequencer and AutoRead™ Sequencing Kit (Pharmacia Biotech, Piscataway, NJ).
Expression of the cDNA clone encoding porcine α1,3 galactosyltransferase in GalTKO pig endothelial cells
In order to determine whether induced anti-non-gal xenoantibodies can bind to carbohydrate xenoantigens, we transduced GalTKO pig cells with lentiviral vectors expressing porcine α1,3 galactosyltransferase (pCSO-rre-cppt-MCU3-α1,3gal-WPRE) or a control lentivector (αGT in reverse orientation)30. The GalTKO pig cells were plated at 1×105 cells per well and were transduced in 0.5 ml of Medium 199 (Invitrogen, Carlsbad, CA). Expression of the gal carbohydrate on transduced cells was verified by flow cytometry 72 hours post-infection. Experiments were repeated twice and transductions were performed in triplicate.
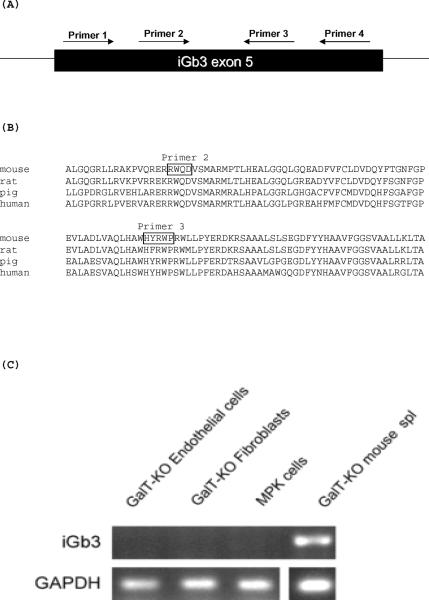
PCR for analysis of iGb3 expression
Four different primers from iGb3 exon 5, used in three primer pair sets, were used to detect iGb3 expression. The forward iGb3 primers used were 5' CTGGAGAAGTACCTGGAACACTTC 3' and 5' GGCGCTGGCAGGAC 3'. The reverse primers were 5' CGGCCAGCGGTAGTG 3' and 5' CAGTGCGCCGTCAG 3'. The primers identify a region of the cDNA and/or genomic DNA for iGb3 that is identical in various species (Genbank accession numbers NM_001009819, AF248543, NM_001080438 and references 28,29 for the pig genomic sequence). The PCR conditions used were 94°C for 5 min., 35 cycles of 94°C for 30 sec., 48°C, 51°C or 53°C for 30 sec., 72°C for 30 sec., followed by one cycle of 72°C for 7 min. The primers used to detect GAPDH as a control were 5' AGTATGACAACAGCCTCAAGATCA 3' and 5' ACAGTCTTCTGGGTGGCAGTGATG 3' for the pig, and 5' TGCACCACCAACTGCTTAGCC 3' and 5' GGCATGGACTGTGGTCATGAG 3' for the mouse. The PCR conditions used for GAPDH were 94°C from 5 min., 35 cycles of 94°C for 30 sec., 55°C for 30 sec., 72°C for 30 sec., followed by one cycle of 72°C for 7 min. All PCR products were visualized on a 2% agarose gel.
Statistics
Data is presented as the mean +/− standard deviation.
Results
Anti-non-gal xenoantibodies pre-exist at low to moderate levels prior to exposure to pig cells
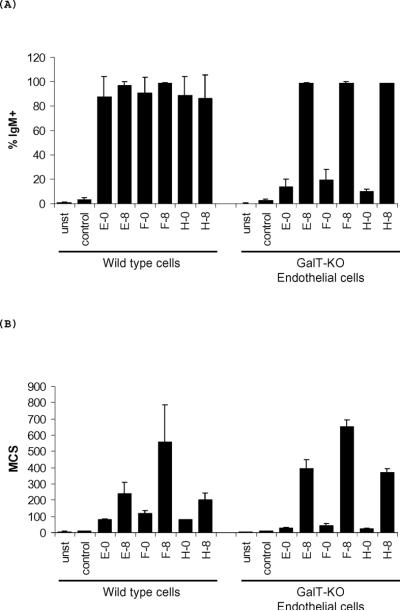
The levels of pre-formed xenoantibodies directed at non-gal xenoantigens expressed on GalTKO pig endothelial cells in 2–3 year old rhesus monkeys ranged from 6–14% (Figure 1). The mean channel shift (MCS) ranged between 14 and 23. The levels of pre-existing xenoantibodies that bind to GalTKO pig cells were considerably lower when compared with pre-existing natural antibody binding to wild type pig cells (Figure 1). Pre-existing IgM xenoantibodies bound to 8% (Mean channel shift of 35) of GalTKO pig cells and to 78–99% (MCS of 69–80) of wild type pig cells (Figures 1 and 2). Natural antibodies that may prevent acceptance of GalTKO xenografts are present but at very low levels in rhesus monkeys.
Figure 1. Levels of antibody binding to wild type and GalTKO endothelial cells.
(A) Percent binding of IgM xenoantibodies pre and post-immunization was determined by flow cytometry at Day 0 and Day 8 (n=3). (B) Binding of IgM xenoantibodies shown as mean channel shift at Day 0 and Day 8 (n=3).
Figure 2. Levels of IgM and IgG xenoantibody binding to wild type and GalTKO pig cells at days 0, 8, and 21 as demonstrated by flow cytometry.
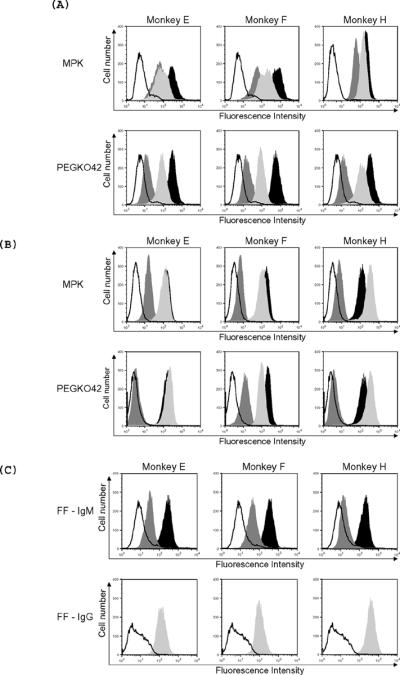
The tracing that is not shaded indicates background binding of secondary antibody alone. Dark gray, black, and light gray filled areas indicate binding at days 0, 8, and 21, respectively. IgM xenoantibody binding to MPK cells (wild type minipig kidney cells) and to PEGKO42 (GalTKO endothelial cells) (A); IgG xenoantibody binding to MPK and to GalTKO endothelial cells (B) and binding of IgM and IgG xenoantibodies to GalTKO fetal fibroblasts (FF, section C) are shown.
Germline IgVH progenitors encoding anti-non-gal xenoantibodies in monkeys immunized with GalTKO pig cells are restricted
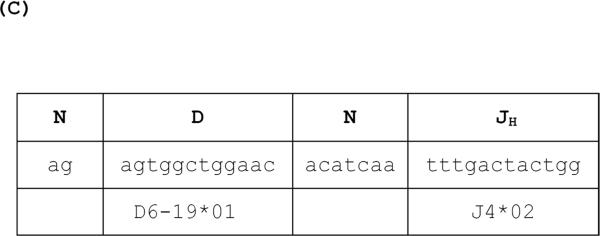
The cDNA libraries were produced from peripheral blood lymphocytes of three monkeys that demonstrated high levels of IgM and IgG xenoantibodies at days 8 and 21, as shown by flow cytometry (Figure 2). The generation of IgM and IgG cDNA libraries from each timepoint (n=3) allowed us to determine the relative percentage of IgVH gene usage prior to and following exposure to pig cells. IgVH gene usage in untreated control monkeys was comparable to established frequencies reported in the literature31. The data obtained from monkey samples post-xenoantigen exposure showed that immunoglobulin genes encoded by the IGHV3-21 germline progenitor were increased in frequency from 1–3% at day 0 to 72–84% of VH3 gene usage at day 8. The frequency of Ig gene usage was based on sequencing a minimum of 40 genes in each individual cDNA library. The nucleotide and amino acid sequence of the genes encoding anti-non-gal xenoantibodies is shown in Figures 3 and 4. The IGHV3-11*01 gene that encodes anti-gal xenoantibodies to wild type pig xenografts was the second closest germline by a difference of only 0.5%. The V3-21 germline gene is also 91% similar to the IGHV3-11*03 allele that encodes anti-non-gal xenoantibodies to hDAF transgenic pig hearts in monkeys treated with GAS914, a reagent that removes anti-gal xenoantibodies before transplantation9,29. The IGHV3-11*01,V3-11*03 and V3-21 germline genes are evolutionarily and structurally-related and they all have the ability to bind to carbohydrate xenoantigens13,15,29,30 (Figure 5). The amino acid substitutions identified in the sequence of the genes encoding IgM anti-non-gal xenoantibodies could be detected prior to xenoantigen exposure, indicating that these natural antibodies pre-exist at low levels and are expanded during the course of an immune response to GalTKO pig cells. The xenoantibody response was not a polyclonal activation of genes encoded by several germline progenitors, but demonstrated a restricted usage of VH genes encoded by IGHV3-21. This germline gene encodes xenoantibodies with a 1–3 canonical structure similar to the structure of the Ig genes encoding xenoantibodies to the gal carbohydrate.
Figure 3. Comparison of nucleotide sequence of the gene encoding IgG and IgM xenoantibodies to GalTKO xenoantigens.
(A) IgM sequence comparison. (B) IgG sequence comparison. (−) Identical residues to initial line which is the human germline gene. CDR1 and CDR2 are complementarity determining regions. FR1, FR2, and FR3 are framework regions. (C) the CDR3 region of anti-non-gal IgG xenoantibodies.
Figure 4. Amino acid sequence of the genes encoding IgM and IgG anti-non-gal xenoantibodies in rhesus monkeys.
(−) Identical residues when compared with the closest human germline gene. CDR1 and CDR2 are complementarity determining regions. FR1, FR2, and FR3 are framework regions.
Figure 5.
Comparison of the amino acid sequences of IgVH germline progenitors that encode anti-gal and anti-non-gal xenoantibodies in primates.
The CDR3 usage in IgVH genes encoding anti-non-gal xenoantibodies is unique and restricted
In order to further understand the unique features of these closely related xenoantibodies, we carefully examined the sequence and structure of the CDR3, a region that often contributes to binding specificity. The VH genes encoding IgM xenoantibodies utilized J4*02 at day 8 but did not demonstrate preferential use of a single D gene. The IgG xenoantibodies directed at non-gal xenoantigens demonstrated a selective expansion of antibodies that utilize D6-19*01 and J4*02 (Figure 3C). The CDR3 sequence of xenoantibodies induced in response to wild type pig xenografts, hDAF transgenic xenografts and GalTKO xenoantigens differ from each other significantly13–15, 32. The contribution of the CDR3 region to the specificity and affinity of non-gal xenoantibodies for antigens expressed on GalTKO cells remains to be determined.
Induced anti-non-gal xenoantibodies bind to a carbohydrate xenoantigen
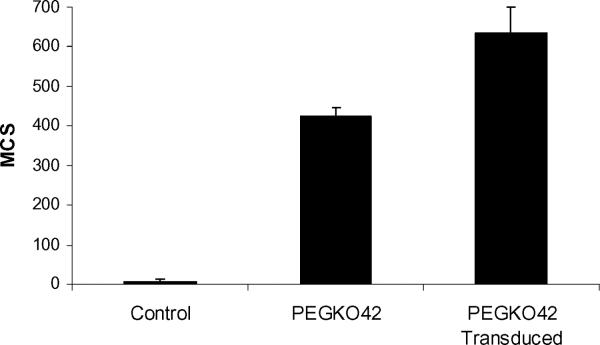
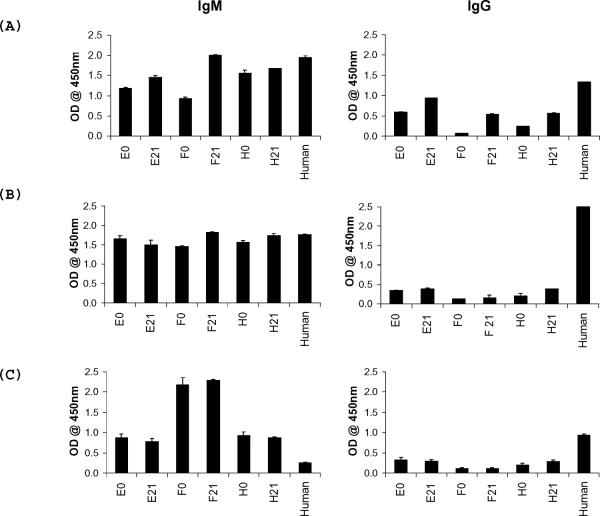
PEGK042 GalTKO pig endothelial cells were transduced with the gene encoding porcine α1,3 galactosyltransferase that was cloned into a lentiviral vector to determine whether anti-non-gal xenoantibodies have the ability to bind to carbohydrate xenoantigens. We reasoned that if the induced xenoantibodies were structurally unrelated to the gal carbohydrate xenoantigen, we would expect to see no change in binding of these antibodies when comparing transduced and non-transduced GalTKO cells. Our results showed a substantial increase in binding to GalTKO cells to which the gal carbohydrate had been re-introduced (Figure 6). Xenoantibodies induced after immunization also showed an increase in binding to purified gal carbohydrate Gal α 1-3Gal β 1-4GlcNAc, but not to Gal α 1-3Gal β 1-4Glc or to N-Acetyllactosamine when examined directly by ELISA (Figure 7). Soluble gal carbohydrate was not effective, however, in inhibiting the functional interaction of induced xenoantibodies with GalTKO endothelial cells. These data demonstrate that anti-non-gal xenoantibodies induced in vivo have the ability to bind to a carbohydrate xenoantigen that is structurally similar to, but distinct from, the gal carbohydrate.
Figure 6. Anti-non-gal xenoantibodies bind to a carbohydrate xenoantigen that is structurally similar to the gal carbohydrate.
GalTKO cells that are genetically modified to express the gal carbohydrate after transduction with viral vectors expressing the gene encoding porcine α 1,3 galactosyltransferase show higher levels of binding of anti-non-gal xenoantibodies when compared with non-tranduced GalTKO cells. Positive binding was evaluated by mean channel shift. PEGKO42 cells are GalTKO pig endothelial cells. Data shown is representative of three independent transductions +/− standard deviation.
Figure 7. Binding of non-gal xenoantibodies to carbohydrates.
Binding activity to carbohydrate antigens was examined in three monkeys (E, F and H) by ELISA. IgM and IgG xenoantibodies induced after immunization with GalTKO endothelial cells show an increase in binding to Gal α 1–3Gal β 1–4GlcNAc (A) but not Gal α 1–3Gal β 1–4Glc (B) or N-Acetyllactosamine (C). Binding of xenoantibodies in normal human serum was included as a positive control. Data are presented +/− standard deviation.
Galactosyltransferase 2 (iGb3) is not expressed in GalTKO pig endothelial cells or fetal fibroblasts
We next sought to directly determine whether iGb3 synthase, the enzyme responsible for the expression of residual Gal carbohydrate on GalTKO mouse cells, might be expressed in GalTKO pig cells. We designed a series of primers based on conserved regions in the galactosyltransferase 2 gene (A3galt2 or iGb3 synthase) in humans, rats, and mice (Figure 8). The primer sequences that we selected identified a gene segment within exon 5 of the genomic iGb3 sequence in the pig28,29 that is conserved across species (Figure 8). Our data confirms that expression of the gene encoding this transferase is detected in both GalTKO mice and wild type mice by PCR. We cloned and sequenced the gene segments that were amplified using each set of our primer pairs in mice and confirmed that the sequenced gene was identical to the sequence for mouse iGb3 synthase (accession number NM_001009819), indicating that our primers were designed correctly. We did not detect expression of this gene in GalTKO pig cells, GalTKO fetal pig fibroblasts or in minipig kidney cells (MPK) from wild type pigs (Figure 8). These data suggest that iGb3 synthase is not expressed in GalTKO pig cells and the relevant xenoantigen is similar in structure to but distinct from the gal carbohydrate.
Figure 8. Expression of galactosyltransferase 2 (iGb3 synthase) is not detected in GalTKO pig cells but is detected in GalT-KO mouse cells.
A series of primers were generated that anneal within exon 5 of galactosyltransferase 2 (A). The primers identify a region of the cDNA and/or genomic DNA for iGb3 that is identical in various species (Genbank accession numbers NM_001009819, AF248543, NM_001080438 and reference xx for the pig genomic sequence. (B) iGb3 synthase expression was confirmed by PCR in GalT-KO mouse cells as reported in earlier studies22 but was not detected in GalTKO pig endothelial cells or GalTKO fetal fibroblasts.
Discussion
The major obstacle in xenotransplantation is the antibody-mediated destruction of the xenograft. Genetically modified porcine organ donors that lack the gal α1,3 gal carbohydrate xenoantigen do not undergo hyperacute rejection in non-human primates, but acute humoral rejection occurs after three to six months despite intense immunosuppressive treatments19–20. In this study, we have identified the previously uncharacterized antibodies and the germline progenitors that are induced in response to GalTKO pig cells in rhesus monkeys. The xenoantibody response is restricted and is encoded by V3-21 germline progenitors in the VH3 family. Induced anti-non-gal xenoantibodies bind to a carbohydrate xenoantigen that is structurally similar to but distinct from the Gal carbohydrate and is not the produced by iGb3 synthase.
The novel finding that the germline progenitors encoding xenoantibodies to GalTKO, hDAF transgenic and wild type pig xenoantigens differ in sequence by less than 5% suggests that the structure of the target xenoantigens is similar despite genetic modification. The germline progenitors IGHV3-11*01, IGHV3-11*03 and V3-21 encoding induced antibody responses to wild type, hDAF-expressing and GalTKO pig cells are distinguishable by specific amino acid substitutions at positions 31, 33, 50 and 58. Our laboratory and others have recently identified these sites as relevant for the specificity of xenoantigen/carbohydrate interaction using computer-simulated models23,34. The ability of anti-non-gal xenoantibodies to show enhanced binding to GalTKO cells induced to express high levels of the gal carbohydrate further suggests that carbohydrate xenoantigens play a key role in the xenoantibody response to GalTKO cells. Anti-carbohydrate antibodies are evolutionarily conserved in humans and non-human primates as a consequence of their function in innate immune responses35–37. Our studies support the concept that the canonical structure of anti-carbohydrate antibodies has been conserved and determines the selection of VH3 genes in the induced antibody repertoire after xenotransplantation13–15,35,37–41. Further selection for antibodies with an optimal fit for relevant carbohydrate xenoantigens results in restricted usage of specific germline progenitors in combination with a unique CDR3. Pig cells whose surface antigenicity has been altered as a consequence of remodeling after genetic modification may induce selection of structurally-related but distinct xenoantibodies.
The V3-21 germline progenitor is an optimal fit for xenoantigens expressed on GalTKO cells but is not expressed at high levels in the natural antibody repertoire of untreated monkeys and/or humans. The most-closely related genes that we were able to identify in Genebank encode antibodies to glycolipids35 and human antibodies to the Gal/GalNAc lectin expressed on the intestinal parasite Entamoeba histolytica33. Anti-non-gal xenoantibodies are similar to the antibodies that encode anti-glycolipid responses in humans, but differ in D–J usage35. The unique contribution of the CDR3 to the specificity of the xenoantibody responses has previously been reported from studies done in our laboratory in which we defined IgVH genes encoding xenoantibody responses in monkeys after transplantation of wild type and genetically modified hDAF transgenic pig organs14–15,32. The CDR3 region of xenoantibodies appears to play a role in the fine specificity of xenoantibody/xenoantigen interaction. Our data supports the concept that “signature” antibody repertoires of clonally expanded Ig genes can be used to identify antibodies with unique specificities35. Importantly, based on information available in the literature on the pattern of Ig gene usage for specific types of responses, it appears that elimination of xenoantibodies to promote xenograft survival may be feasible without significant interference with the majority of innate immune responses that protect humans from commonly occurring infectious agents35.
Our structural and functional data is entirely consistent with the hypothesis that anti-non-gal xenoantibodies are specific for a xenoantigen that is expressed on a glycolipid such as iGb322, and we were therefore surprised to find that iGb3 synthase could not be detected in the GalTKO pig cells examined in our study. We designed several sets of primers using conserved segments of the iGb3 gene that are identical in other species and present in the genomic pig sequence, but could not detect iGb3 sythase expression in the GalTKO pig cells that we used in this study. A similar conclusion, however, was recently reported by two other laboratories using different experimental designs43–44. Breimer's laboratory showed that gal α1,3 gal glycolipids are not expressed on GalTKO pig tissues and they detected no new compensatory glycolipid compounds that bind to xenoantibodies after genetic manipulation43. Since we do detect iGb3 synthase in GalT knockout mice, it appears that this is yet another example documenting specific differences that limit the utility of small animal models in xenotransplantation. These findings emphasize the need to validate information provided from rodents in higher species, which represent more relevant preclinical models.
Although there is still no information on the target xenoantigen(s) that initiate rejection of GalTKO cells, the relevant antigens appear to be xenoreactive carbohydrates21,24,44. The similarity in the structure of the xenoantibodies that initiate rejection of porcine xenografts provides new opportunities for the design of novel approaches to limit xenoantibody-mediated rejection. Structure-based drug design or the elimination of B cells producing xenoantibodies may be useful as adjunct therapeutics to prolong xenograft survival45. Specific elimination of anti-gal B cells using alpha-gal linked toxins in mice has been performed without toxicity to the mice and without affecting production of antibodies with other specificities46. Similar techniques could be developed and applied to suppress the population of B cells producing anti-non-gal antibodies in primates. Pre-existing anti-non-gal xenoantibodies to GalTKO donors are present in significantly lower levels when compared with antibodies directed at the gal carbohydrate47,48. This will be important in efforts to keep these antibodies at low levels to induce a state of accommodation49 and/or in attempts to achieve tolerance by the induction of chimerism prior to transplantation50. The comorbid conditions, drug toxicities, and adverse effects that are associated with the use of extensive immunosuppression could then be minimized, improving patient and graft survival.
Acknowledgements
GalTKO pig endothelial cells and fetal pig fibroblasts were kindly provided by Dr. David Sachs, Massachusetts General Hospital. This work was supported by NIH grant RO1AI52079 (MKJ).
Abbreviations
- GalTKO
α1,3 galactosyltransferase gene knockout
- CDR
complementarity determining region
- gal α (1,3) gal
galactose α (1,3) galactose
- VH
variable region of the immunoglobulin heavy chain gene
References
- 1.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IFC. Anti-pig IgM antibo human serum react predominantly with Galα(1–3)Gal epitopes. Proc. Natl. Acad. Sci. USA. 1993;90:11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991;52:214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Parker W, Bruno D, Holzknecht ZE, Platt JL. Characterization and affinity isolation of xenoreactive human natural antibodies. J. Immunol. 1994;153:3791–3803. [PubMed] [Google Scholar]
- 4.Galili U, LaTemple DC, Walgenbach AW, Stone KR. Porcine and bovine cartilage transplants in cynomolgus monkey: II. Changes in anti-gal response during chronic rejection. Transplantation. 1997;63:646–651. doi: 10.1097/00007890-199703150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Galili U, Minanov OP, Michler RE, Stone KR. High-affinity anti-Gal immunoglobulin G in chronic rejection of xenografts. Xenotransplantation. 1997;4:127–131. [Google Scholar]
- 6.Stone KR, Ayala G, Goldstein J, Hurst R, Walgenbach A, Galili U. Porcine cartilage transplants in the cynomolgus monkey. III. Transplantation of α-galactosidase-treated porcine cartilage. Transplantation. 1998;65:1577–1583. doi: 10.1097/00007890-199806270-00007. [DOI] [PubMed] [Google Scholar]
- 7.Galili U, Shohet SB, Kobrin E, Stults CLM, Macher BA. Man, apes, and old world monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 8.Chen G, Sun H, Yang H, Kubelik D, Garcia B, Luo Y, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81(2):273–83. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 9.Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation. 2004;11(6):531–5. doi: 10.1111/j.1399-3089.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez P, Montoya MJ, Rios A, Garcia Palenciano C, Majado M, Chavez R, et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase) Transplant Proc. 2005 Nov;37(9):4103–6. doi: 10.1016/j.transproceed.2005.09.186. [DOI] [PubMed] [Google Scholar]
- 11.Nozawa S, Xing P-X, Wu GD, Gochi E, Kearns-Jonker M, Swensson J, et al. Characteristics of immunoglobulin gene usage of the xenoantibody binding to Gal-α(1,3)Gal target antigens in the Gal knockout mouse. Transplantation. 2001;72:147–155. doi: 10.1097/00007890-200107150-00028. [DOI] [PubMed] [Google Scholar]
- 12.Kearns-Jonker M, Fraiman M, Chu W, Gochi E, Michel J, Wu G–D, Cramer DV. Xenoantibodies to pig endothelium are expressed in germline configuration and share a conserved immunoglobulin VH gene structure with antibodies to common infectious agents. Transplantation. 1998;65:1515–1519. doi: 10.1097/00007890-199806150-00023. [DOI] [PubMed] [Google Scholar]
- 13.Kearns-Jonker M, Swensson J, Ghiuzeli C, Chu W, Osame Y, Baquerizo A, Demetriou A, Cramer DV. The human antibody response to porcine xenoantigens is encoded by IGHV3-11 and IGHV3-74 IgVH germline progenitors. J. Immunol. 1999;163:4399–4412. [PubMed] [Google Scholar]
- 14.Kleihauer A, Gregory CR, Borie D, Kyles AE, Shulkin IS, Patanwala I, Zahorsky-Reeves J, Starnes VA, Mullen Y, Todorov I, Kearns-Jonker M. Identification of the VH genes encoding xenoantibodies in non-immunosuppressed rhesus monkeys. Immunology. 2005;116:89–102. doi: 10.1111/j.1365-2567.2005.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahorsky-Reeves J, Gregory C, Cramer DV, Kyles AE, Borie DC, Christe KL, Starnes VA, Kearns-Jonker M. Similarities in the immunoglobulin response and VH gene usage in rhesus monkeys and humans exposed to porcine hepatocytes. BMC Immunol. 2006;7(1):3. doi: 10.1186/1471-2172-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–422. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 18.Nottle MB, Beebe LFS, Harrison SJ, McIlfatrick SM, Ashman RJ, O'Connell PJ, et al. Production of homozygous α1,3 galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation. 2007;14:339–344. doi: 10.1111/j.1399-3089.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O'Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005 Jan;11(1):32–4. doi: 10.1038/nm1172. Epub 2004 Dec 26. [DOI] [PubMed] [Google Scholar]
- 20.Kuwaki K, Tseng YL, Dor FL, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005 Jan;11(1):29–31. doi: 10.1038/nm1171. Epub 2004 Dec 26. [DOI] [PubMed] [Google Scholar]
- 21.Sandrin M. Gal knockout pigs: any more carbohydrates? Transplantation. 2007;84(1):8–9. doi: 10.1097/01.tp.0000269728.11879.f6. [DOI] [PubMed] [Google Scholar]
- 22.Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. The molecular basis for gal alpha(1,3)gal expression in animals with a deletion of the alpha 1,3 galactosyltransferase gene. J Immunol. 2006 Feb 15;176(4):2448–54. doi: 10.4049/jimmunol.176.4.2448. [DOI] [PubMed] [Google Scholar]
- 23.Milland J, Yuriev E, Xing P, McKenzie IFC, Ramsland P, Sandrin M. Carbohydrate residues downstream of the terminal Gal α1,3 Gal epitope modulate the specificity of xenoreactive antibodies. Immunology and Cell Biology. 2007:1–10. doi: 10.1038/sj.icb.7100111. [DOI] [PubMed] [Google Scholar]
- 24.Ezzelarab M, Ayares D, Cooper DKC. Carbohydrates in xenotransplantation. Immunology and Cell Biology. 2005;83:396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhong R. Gal knockout and beyond. American Journal of Transplantation. 2007;7:5–11. doi: 10.1111/j.1600-6143.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Naziruddin B, Cui C, Martin MJ, Xu H, Wan H, et al. Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75(4):430–6. doi: 10.1097/01.TP.0000053615.98201.77. [DOI] [PubMed] [Google Scholar]
- 27.Magnusson S, Romano E, Hallberg E, Wadenvik H, Breimer M. Soluble saccharides block the inhibition of agonist-induced human platelet aggregation observed after in vitro incubation of human platelet-rich plasma with porcine aortic endothelial cells. Transpl. Int. 1998;11:345–352. doi: 10.1007/s001470050156. [DOI] [PubMed] [Google Scholar]
- 28.Sandrin M. International Patent Application Number WO 02/081688 A1. The Austin Research Institute; 2002. DNA Molecules Encoding IGB3 Synthase, and uses thereof for the disruption of glycosyltransferase genes in xenotransplantation tissues and organs. [Google Scholar]
- 29.Koike C. US Patent Application Publication Number US/2005/0155095 A1. University of Pittsburgh; 2005. Porcine isogloboside 3 synthase protein, cDNA, genomic organization and regulatory region. [Google Scholar]
- 30.Fischer-Lougheed J, Tarantal A, Shulkin I, Mitsuhashi Noboru, Kohn DB, Lee C, Kearns-Jonker M. Gene therapy to inhibit xenoantibody production using lentiviral vectors in non-human primates. Gene Therapy. 2007;14(1):49–57. doi: 10.1038/sj.gt.3302818. [DOI] [PubMed] [Google Scholar]
- 31.Bible J, Howard W, Robbins H, Dunn-Walters D. IGHV1, IGHV5 and IGHV7 subgroup genes in the Rhesus macaque. Immunogenetics. 2003;54:867–873. doi: 10.1007/s00251-003-0536-2. [DOI] [PubMed] [Google Scholar]
- 32.Zahorsky-Reeves JL, Kearns-Jonker MK, Lam TL, Jackson JR, Morris RE, Starnes VA, Cramer DV. The xenoantibody response and immunoglobulin gene expression profile of cynomolgus monkeys transplanted with hDAF-transgenic porcine hearts. Xenotransplantation. 2007;14(2):135–144. doi: 10.1111/j.1399-3089.2007.00381.x. [DOI] [PubMed] [Google Scholar]
- 33.Tachibana H, Watanabe K, Cheng X, Tsukamoto H, Kaneda Y, Takeuchi T, Ihara S, Petri W., Jr VH3 gene usage in neutralizing human antibodies specific for the Entamoeba histolytica Gal/GalNAc lectin heavy subunit. Infect. Immun. 2003;71(8):4313–9. doi: 10.1128/IAI.71.8.4313-4319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kearns-Jonker M, Barteneva N, Mencel R, Hussain N, Shulkin I, Xu A, Yew M, Cramer DV. Use of molecular modeling and site-directed mutagenesis to define the structural basis for the immune response to carbohydrate xenoantigens. BMC Immunology. 2007;8(1):3. doi: 10.1186/1471-2172-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnaout R. Specificity and overlap in gene segment-defined antibody repertoires. BMC Genomics. 2005;6:148. doi: 10.1186/1471-2164-6-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen H, Sato N, MacKenzie C, Brade L, Kosma P, Brade H, Evans S. Germline antibody recognition of distinct carbohydrate epitopes. Nat Struct Biol. 2003 Dec;10(12):1019–25. doi: 10.1038/nsb1014. [DOI] [PubMed] [Google Scholar]
- 37.Ohlin M, Zouali M. The human antibody repertoire to infectious agents: implications for disease pathogenesis. Molecular Immunology. 2003;40:1–11. doi: 10.1016/s0161-5890(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 38.Tomlinson IM, Walter G, Marks JD, Llewelyn MB, Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J.Mol.Biol. 1992;227:776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- 39.Oliva B, Bates P, Querol E, Aviles F, Sternberg M. Automated classification of antibody complementarity determining region 3 of the heavy chain (H3) loops into canonical forms and its application to protein structure prediction. J Mol Biol. 1998 Jun 26;279(5):1193–210. doi: 10.1006/jmbi.1998.1847. [DOI] [PubMed] [Google Scholar]
- 40.Morea V, Tramontano A, Rustici M, Chothia C, Lesk A. Conformations of the third hypervariable region in the VH domain of immunoglobulins. J Mol Biol. 1998 Jan 16;275(2):269–94. doi: 10.1006/jmbi.1997.1442. [DOI] [PubMed] [Google Scholar]
- 41.Oliva B, Bates P, Querol E, Aviles F, Sternberg M. Automated classification of antibody complementarity determining region 3 of the heavy chain (H3) loops into canonical forms and its application to protein structure prediction. J Mol Biol. 1998;279(5):1193–210. doi: 10.1006/jmbi.1998.1847. [DOI] [PubMed] [Google Scholar]
- 42.Morea V, Tramontano A, Rustici M, Chothia C, Lesk A. Conformations of the third hypervariable region in the VH domain of immunoglobulins. J Mol Biol. 1998;275(2):269–94. doi: 10.1006/jmbi.1997.1442. [DOI] [PubMed] [Google Scholar]
- 43.Diswall M, Angstrom J, Schuurman H, Dor F, Rydberg L, Breimer M. Studies on glycolipid antigens in small intestine and pancreas from α1,3 galactosyltransferase knockout miniature swine. Xenotransplantation. 2007;84(10):1348–1356. doi: 10.1097/01.tp.0000287599.46165.15. [DOI] [PubMed] [Google Scholar]
- 44.Soethre M, Baumann B, Fung M, Seebach J, Mollnes E. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal deficient endothelial cells. Transplantation. 2007;84(2):244–250. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 45.Fischer-Lougheed J, Gregory C, White Z, Shulkin I, Gunthart M, Kearns-Jonker M. Identification of an anti-idiotypic antibody that defines B cell subset(s) producing xenoantibodies in primates. Immunology. 2007 Oct 4; doi: 10.1111/j.1365-2567.2007.02704.x. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanemura M, Ogawa H, Yin DP, Chen ZC, DiSesa VJ, Galili U. Elimination of anti-Gal B cells by alpha-Gal ricin1. Transplantation. 2002;73:1859–1868. doi: 10.1097/00007890-200206270-00002. [DOI] [PubMed] [Google Scholar]
- 47.Ezzelarab M, Hara H, Busch J, Rood PP, Zhu X, Ibrahim Z, et al. Antibodies directed to pig non-Gal antigens in naïve and sensitized baboons. Xenotransplantation. 2006;13(5):400–407. doi: 10.1111/j.1399-3089.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 48.Rood PP, Hara H, Busch JL, Ezzelarab M, Zhu X, Ball S, et al. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transpl. Int. 2006;19(2):158–165. doi: 10.1111/j.1432-2277.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 49.Tang AH, Platt JL. Accommodation of grafts: implications for health and disease. Human Immunology. 2007;68(8):645–651. doi: 10.1016/j.humimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sykes M. Mechanisms of tolerance induced via mixed chimerism. Front Biosci. 2007;12:2922–34. doi: 10.2741/2282. [DOI] [PubMed] [Google Scholar]