Tissue factor pathway inhibitor (TFPI) is a multivalent Kunitz-type protease inhibitor that regulates tissue factor (TF)-induced coagulation by inhibiting factor Xa (FXa) and the factor VIIa (FVIIa)/TF complex [1]. Full-length TFPIα (FL-TFPIα) is a 276-residue glycoprotein with an acidic amino-terminus followed by three tandem Kunitz-type domains and a basic carboxy-terminus. In man, approximately 80% of circulating TFPI is tightly bound to plasma lipoproteins (LDL>HDL>VLDL) and lacks the third Kunitz domain (K3) and carboxy-terminus of TFPIα [2]. The remaining TFPI consists of forms that contain the third Kunitz domain, but lack the carboxy-terminus of TFPIα (TFPIα-desCTP), and FL-TFPIα, which is responsible for most of the anticoagulant activity of TFPI in plasma [3,4].
Factor V (FV) is a 330 kDa single chain glycoprotein consisting of two tandem A domains, followed by a B domain, a third A domain, and two C domains. During blood coagulation, FV is proteolytically activated to FVa resulting in the release of the B domain. Duckers et al. showed that TFPIα levels are ∼70% lower in the plasma of patients with severe FV deficiency relative to normal plasma and that the level of TFPIα in normal plasma is reduced by ∼60 - 90% following immuno-precipitation of FV [5]. Using surface plasmon resonance, they demonstrated direct binding of FV to immobilized recombinant FL-TFPIα with half-maximal binding at 13.5 nM FV. TFPIα may also interact with FVa as previous functional studies have shown that FVa enhanced TFPIα inhibition of FXa in the presence of phospholipids and calcium ions [3].
Ligand blot experiments (Figure 1A) showed that both FV and FVa bind FL-TFPIα, whether the FL-TFPIα was expressed in E. coli or mammalian cells. Neither FV nor FVa, however, recognized TFPIα1-252 [3], which lacks the basic carboxy-terminus of TFPIα. Control experiments showed that FXa binds to all the forms of TFPI and that our anti-CTP antibody is selective for FL-TFPIα. The binding of FV(a) to FL-TFPIα was not affected by EDTA (not shown). Thus, FL-TFPIα is bound by both FV and its activated form and this binding requires the carboxyterminus of FL-TFPIα, but not divalent cations or posttranslational modification of FL-TFPIα.
Fig. 1. Interaction of TFPI with Factor V and Factor Va.
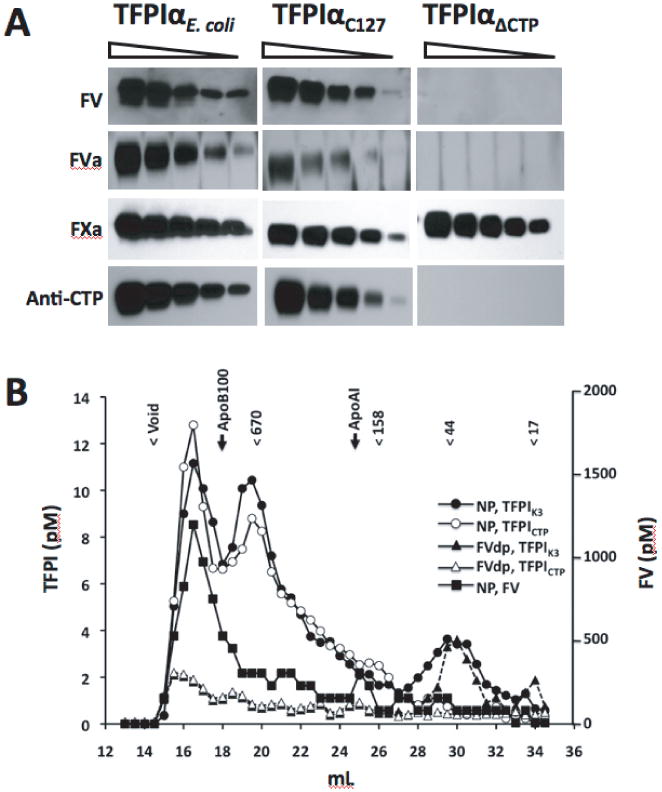
(A) Ligand and western blotting of TFPI. The production and purification of recombinant FL-TFPIα from Escherichia coli (TFPIαE. coli), and recombinant FL-TFPIα and TFPIα1-252 (TFPIαΔCTP) in mammalian C127 cells have been described [3,8,9]. Two-fold serial dilutions of TFPI proteins, starting with 2.0 μg (left lanes) and ending with 0.125 μg (right lanes) were electrophoresed in 10-20% SDS-polyacrylamide gradient gels (Invitrogen, Grand Island, NY, USA) and transferred onto polyvinyldiene fluoride (PVDF) membranes (Immobilon-P; Millipore, Billerica, MA, USA). Identical membranes were blocked with 5% non-fat milk in HST (20 mM Hepes, 100 mM NaCl, and 0.1% Tween 20, pH 7.4) and initially probed with either FV or FVa. In one case, the membrane was incubated with FV (40 ng/mL; Haematologic Technologies Inc; HTI, Essex Junction, VT, USA) conjugated to HRP (Lightning-Link, Innova Biosciences Ltd, Babraham, Cambridge, UK) for 1 hour at room temperature. The membrane was washed with HST and the HRP-conjugated FV was detected with the Lumi-LightPlus Western blotting substrate Kit (Roche Diagnostics, Indianapolis, IN, USA). In the second case, the membrane was incubated with FVa (HTI) at 2 μg/mL in HSAT (HST containing 3% bovine serum albumin) for 2 hours at room temperature. The membrane was washed with HST and incubated for 2 hours with polyclonal sheep anti-human FV antibody (Affinity Biologicals, Ancaster, ON, Canada). HRP-conjugated donkey anti-sheep antibody (Jackson Immunolaboratories Research, West Grove, PA, USA) and Lumi-LightPlus Western blotting substrate Kit (Roche) were then used to detect the primary antibody. The membrane used for probing with FVa was subsequently stripped with 100 mM glycine, pH 2.2, at room temperature for 30 minutes, and then incubated with FXa (Enzyme Research Laboratories; ERL, South Bend, IN, USA) (0.1 μg/ml in HSAT) for 1 hour. The membrane was washed 3 × 10 minutes with HST and incubated with a polyclonal goat anti-FX antibody (HTI) (1 μg/ml) in HST containing 1% fat-free milk for 1 hour, after which HRP-rabbit anti-goat antibody (Sigma-Aldrich, St. Louis, MO, USA) and the Lumi-LightPlus Western blotting substrate Kit were then used to detect the primary antibody. The same membrane was again stripped and incubated with rabbit polyclonal anti-TFPICTP antibody (0.05 μg/ml) for 1 hour in HST containing 1% fat-free milk. HRP-goat anti-rabbit IgG (Sigma-Aldrich) and Lumi-LightPlus Western blotting substrate Kit were then used to detect the primary antibody. (B) Size exclusion chromatography of TFPI in plasma. Citrated pooled normal plasma (NP) was from George King Biomedical, Overland Park, KS, USA. FV-depleted plasma (FVdp) was prepared from NP essentially as described [5] except that the antibody used was polyclonal sheep anti-human FV antibody (Affinity Biologicals). To run the chromatography, two 10 mm × 300 mm Superdex 200 GL columns (GE Healthcare, Piscataway, NJ, USA) were connected in series and equilibrated with Hepes saline buffer (HS; 20 mM Hepes, 100 mM NaCl, pH 7.4). NP or FVdp (0.5 mL) was applied, and the chromatography performed at a flow rate of 0.5 mL/minute at room temperature. Fractions (0.5 mL) were collected and assayed using the Quantikine Human TFPI Immunoassay kit (R&D Systems, Inc., Minneapolis, MN, USA), which uses an anti-K3 capture antibody and a horse radish peroxidase (HRP)-labeled polyclonal anti-TFPI for the detection of TFPI forms containing an intact K3 domain. An in-house ELISA was developed to selectively measure FL-TFPIα, by using a polyclonal antibody that was developed in rabbit against the TFPIα C-terminal peptide KIAYEEIFVKNM (TFPIα265-276) [9], affinity purified as capture antibody and HRP-labeled rabbit polyclonal anti-TFPI1-160 antibody for detection. Briefly, at room temperature, 50 μL of capture antibody (40 μg/mL in 50 mM sodium carbonate, pH 9.5) was coated onto wells of high binding 96-well plates (Fisher Scientific) overnight. All subsequent incubation steps were carried out at room temperature. Wells were blocked with HSAT for 1 hour. After washing the wells five times with HST, samples diluted in HSAT (100 μL) were applied to the wells and incubated for 4 hours. Thereafter, wells were washed 5 times with HST and 100 μL of detection antibody in HSAT (2 μg/mL) was applied and incubated for 1 hour. The wells were washed 5 times with HST, and 100 μL 3,3′,5,5′-tetramethylbenzidine liquid substrate system (Sigma-Aldrich) was added. After 5 minutes, 50 μL of 0.5M H2SO4 was added to stop the reaction, and the absorbance at 450 nm was measured with a Vmax microtitre plate reader (Molecular Devices, Sunnyvale, CA, USA). Purified recombinant FL-TFPIα expressed in E. coli (TFPIαE. coli)[9] was used as standard and produced a linear response between concentrations of 0 and 100 pM. Positions of the column void volume and the elution peaks of molecular weight markers in kDa (Bio-Rad Laboratories, Hercules, CA, USA), apolipoprotein B (LDL) and apolipoprotein AI (HDL) (Human Apo AI and Apo B Duplex ELISA Kit, Cell Biolabs Inc., San Diego, CA, USA) are indicated at the top of the profiles. FV was assayed using the Matched-Pair Antibody Sets for ELISA of human FV (ERL) according to the protocol of the manufacturer.
Upon size exclusion chromatography of pooled normal plasma (NP) in 0.1 M NaCl, 0.02 M Hepes, pH 7.4 (Figure 1B), three significant peaks of TFPIα are detected by an immunoassay using an anti-K3 domain antibody. The first two peaks, which elute at high apparent molecular weights (>1000 kDa and ∼ 700 kDa, respectively), are also recognized by the anti-CTP antibody, and are consistent with FL-TFPIα bound to other plasma moieties. The third peak elutes at a size consistent with unbound TFPI and is not recognized by the anti-CTP antibody, suggesting that it consists of TFPIα-desCTP. Various individual plasmas showed profiles similar to the elution profile for pooled NP presented in Figure 1B (data not shown), although the relative amplitude of the peaks did show some variability (less than two-fold), perhaps related to the known variability of plasma TFPI levels across individuals [2]. On gel filtration of NP under high salt conditions (1 M NaCl), both TFPIα-desCTP and FL-TFPIα elute at their anticipated sizes of ∼ 40 kDa (data not shown and [6]). The dissociation of FL-TFPIα from the high molecular weight complexes in high salt suggests an ionic interaction, consistent with a role for the charged carboxyterminus of FL-TFPIα. Notably, the substantially carboxyterminal truncated forms of TFPI that circulate tightly bound to plasma lipoproteins remain associated under high salt conditions [2,6,7] and the high molecular weight FL-TFPIα peaks do not co-migrate with LDL (apolipoprotein B) or HDL (apolipoprotein AI) (Figure 1B).
Immuno-depletion of FV from plasma reduced the FV levels by >95% and resulted in a significant reduction of the FL-TFPIα level (>80%), as reported by others (5). Gel filtration of the FV-depleted plasma demonstrated a striking reduction in the peaks of FL-TFPIα migrating at high apparent molecular weights (Figure 1B). Immuno-depleted FVII plasma, used as a control, showed a profile similar to normal plasma (data not shown). The plasma constituents with which FL-TFPIα associates in the high molecular weight peaks identified on gel filtration are not known, but FV appears to be involved based on the chromatography profile of FL-TFPIα following FV immunodepletion from plasma.
Acknowledgments
This work was supported by the National Institute of Health (grant HL77193; G.J.B.).
Footnotes
Disclosure of Conflict of Interests: The authors state that they have no conflict of interest.
References
- 1.Broze GJ, Jr, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71:335–43. [PubMed] [Google Scholar]
- 2.Broze GJ, Jr, Lange GW, Duffin KL, MacPhail L. Heterogeneity of plasma tissue factor pathway inhibitor. Blood Coag Fibrin. 1994;5:551–9. [PubMed] [Google Scholar]
- 3.Wesselschmidt R, Likert K, Huang Z, MacPhail L, Broze GJ., Jr Structural requirements for tissue factor pathway inhibitor interactions with factor Xa and heparin. Blood Coag Fibrin. 1993;4:661–9. [PubMed] [Google Scholar]
- 4.Wesselschmidt R, Likert K, Girard T, Wun T, Broze GJ., Jr Tissue factor pathway inhibitor: the carboxy terminus is required for optimal inhibition of factor Xa. Blood. 1992;79:2004–10. [PubMed] [Google Scholar]
- 5.Duckers C, Simioni P, Spiezia L, Radu C, Gavasso S, Rosing J, Castoldi E. Low plasma levels of tissue factor pathway inhibitor in patients with congenital factor V deficiency. Blood. 2008;112:3615–23. doi: 10.1182/blood-2008-06-162453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokawa T, Enjyoji K, Kumeda K, Kamikubo Y, Harada-Shiba M, Koh H, Tsushima M, Yamamoto A, Kato H. Measurement of the free form of TFPI antigen in hyperlipidemia. Relationship between free and endothelial cell-associated forms of TFPI. Arterioscler Thromb Vasc Biol. 1996;16:802–8. doi: 10.1161/01.atv.16.6.802. [DOI] [PubMed] [Google Scholar]
- 7.Lesnik P, Vonica A, Guerin M, Moreau M, Chapman MJ. Anticoagulant activity of tissue factor pathway inhibitor in human plasma is preferentially associated with dense subspecies of LDL and HDL and with Lp(a) Arterioscler Thromb Vasc Biol. 1993;13:1066–75. doi: 10.1161/01.atv.13.7.1066. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Collier JA, Palmier MO, Kretzmer KK, Bishop BF, Combs RG, Obukowicz MG, Frazier RB, Bild GS, Joy WD, Hill SR, Duffin ME, Gustafson ME, Junger KD, Grabner RW, Galluppi GR, Wun TC. Refold and characterization of tissue factor pathway inhibitor expressed in Escherichia coli. Thromb Haemost. 1994;71:339–46. [PubMed] [Google Scholar]
- 9.Girard TJ, Warren LA, Novotny WF, Bejcek BE, Miletich JP, Broze GJ., Jr Identification of the 1.4kb and 4.0 kb messages for the lipoprotein associated coagulation inhibitor and expression of the encoded protein. Thromb Res. 1989;55:37–50. doi: 10.1016/0049-3848(89)90454-4. [DOI] [PubMed] [Google Scholar]


