Abstract
The importance of cholinergic neurons projecting from the medial septum (MS) of the basal forebrain to the hippocampus in memory function has been controversial. The aim of this study was to determine whether loss of cholinergic neurons in the MS disrupts object and/or object location recognition in male Sprague-Dawley rats. Animals received intraseptal injections of either vehicle, or the selective cholinergic immunotoxin 192 IgG-saporin (SAP). 14 days later, rats were tested for novel object recognition (NOR). Twenty-four hours later, these same rats were tested for object location recognition (OLR) (recognition of a familiar object moved to a novel location). Intraseptal injections of SAP produced an 86% decrease in choline acetyltransferase (ChAT) activity in the hippocampus, and a 31% decrease in ChAT activity in the frontal cortex. SAP lesion had no significant effect on NOR, but produced a significant impairment in OLR in these same rats. The results support a role for septo-hippocampal cholinergic projections in memory for the location of objects, but not for novel object recognition.
Keywords: hippocampus, cholinergic denervation, object recognition, place recognition, memory
Introduction
The hippocampus plays an important role in a number of memory functions and cholinergic neurons of the medial septum and vertical limb of the diagonal band of Broca (MS) that project to the hippocampus have long been accorded an important role in supporting learning and memory [6,7,16]. However, the role of cholinergic inputs to the hippocampus with regard to memory function has been brought into question following the development of 192 IgG-saporin (SAP), an immunotoxin that selectively destroys cholinergic neurons of the basal forebrain [31]. For example, some studies report that selective cholinergic lesion of the MS produced deficits in rats tested on radial arm maze [23,37], T-maze [20,25] and plus maze [13], while other studies utilizing the Morris water maze [2,8,14] or radial arm water maze [19], did not show deficits.
Such inconsistencies may result from the nature of the particular lesion and/or differences in relative cognitive demands across testing paradigms. The novel object recognition task (NOR) is a simple one-trial learning task with minimal motivational components that exploits the natural tendency of rats to spend more time exploring novel objects than familiar ones. The task does not involve fear or reward and is not associated with high levels of stress or physical activity.
The role of cholinergic neurotransmission in NOR has been studied previously [1, 5, 30, 38]. However, to the extent that the function of cholinergic neurons of the basal forebrain in cognitive processes has not been fully resolved and the particular involvement of cholinergic neurons of the MS in recognition memory has not been studied directly, the goal of this investigation was to determine whether selective cholinergic lesion of the MS would affect NOR and/or OLR.
Materials and methods
All chemicals were purchased from Sigma. Inc, St. Louis, unless stated otherwise.
Animals
Sixty-eight male Sprague-Dawley (SD) rats weighing between 250 and 275g were purchased from Hilltop Lab Animals (Scottsdale, PA) and housed in a temperature and humidity controlled facility with a 12:12 H light/dark cycle with food and water available ad libitum. All experiments were conducted in accordance with N.I.H. guidelines for the care and use of laboratory animals and were approved by the Duquesne University IACUC.
Surgery
Following a one-week acclimation period to the environment of the animal facility, surgery was performed to inject SAP into the medial septum (MS). Rats were anesthetized using sodium pentobarbital (50 mg/Kg: IP), and placed into a stereotaxic frame (Stoelting, Wood dale, IL). An incision was made exposing the dorsal aspect of the skull and a small stainless steel cannula was lowered into the MS (coordinates from Bregma: AP + 0.2mm, L 0.0, DV -5.4mm from dura mater). Either SAP (0.22 μg/μl, Advanced Targeting Systems, Inc), or artificial CSF (CMA/Microdialysis), 1 μl was infused into the MS over 5 minutes at a rate of 0.2 μl/min. Following infusion, the cannula was left in place for 5 minutes to allow diffusion of the infusate into the tissue. After the withdrawal of the cannula, the incision was sutured closed and Ibuprofen (10 mg/Kg) administered IP for post-surgical analgesia. Based on previous studies demonstrating memory impairment following cholinergic lesion of the MS using the procedures above [17, 18, 20, 25], rats were allowed to recover for two weeks prior to post-surgical training and retention testing. To determine whether intraseptal infusion alone could impair performance in the NOR test, additional rats that did not undergo surgery (n=10) were also tested.
Behavioral testing
The object recognition task was conducted in an open-field arena (45 cm×60 cm×60cm) constructed of gray polyvinylchloride (PVC) plastic. Three floors were prepared, a bare floor that was used during acclimation, and two identical floors that contained brackets to fix objects into place in opposite corners of the floors 13 cm from the walls on each side. A dim diffusive light from two lamps was utilized to minimize shadows within the arena. A video camera (JVC), positioned over the arena recorded behavior during sample and test phases for later analysis by blind observers. The stimulus objects were made of glass, porcelain, or plastic with different shapes and textures, such as light bulbs, mugs, coffee cups, and saline bottles. The animals could not displace the objects nor hide in or under them. Objects were washed between trials to remove olfactory cues. Fourteen days following surgery, the rats were habituated to the arena by allowing them to explore the arena for a single 10 min period for four consecutive days. The rats then received one sample-exposure session per day for two consecutive days. During these sessions, the rat was placed into the arena with two identical sample objects in the same position on the floor for 5 min before being returned to its home cage. The same objects were used for both days (Figure 1).
Figure 1.
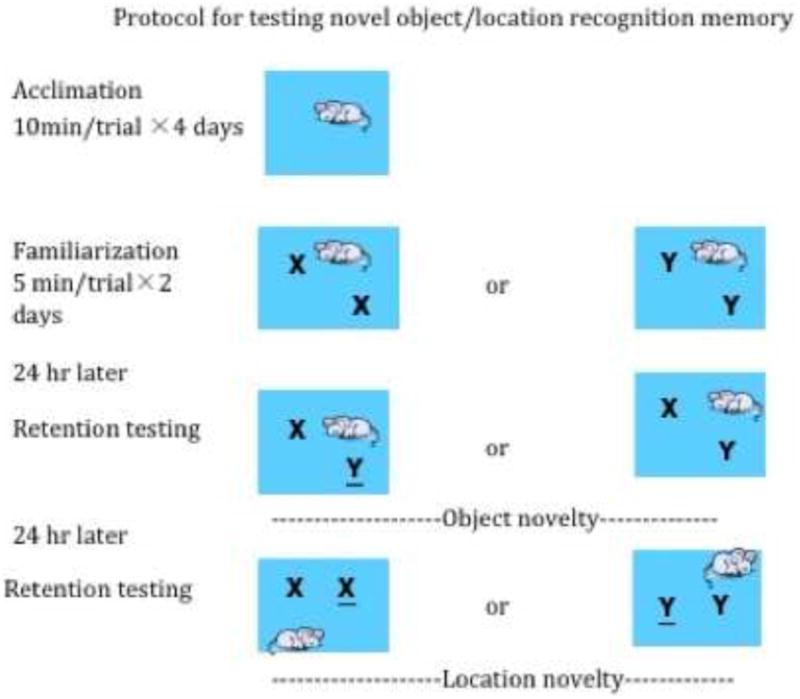
Protocol for testing novel object and novel location recognition memory. X and Y represent different objects; or objects in a novel location. In order to limit potential object bias, the same familiar and novel objects were used for half of each treatment group and switched for the other half.
Object recognition: After a delay of 24 hours, the rats were returned to the arena for 3 minutes, during which time they could examine the original sample object from the previous day and a novel object. The 24-hour delay in retention testing was selected based on previous studies demonstrating object and location recognition following this duration of delay (38). In order to limit potential object bias, the same familiar and novel objects were used for half of each treatment group and switched for the other half. For example, for half of the rats in a treatment group, a bottle was used as the familiar object, and a cup as the novel object, and for the other half of the same group, the cup would be the familiar object and the bottle the novel object.
Object location recognition: one day following the retention test for object recognition, rats were returned to the arena for 3 minutes, which now contained the two identical familiar objects: one at the previous location; and one at a novel location 30 cm equidistant from two opposing walls and 18 cm from the closest adjacent wall.
The exploration time for each object was recorded and the exploration ratio calculated by dividing the time spent exploring the novel object by the total time spent exploring both the novel and familiar objects. Results were reported as mean ± SEM. Object exploration criteria were as follows: The head of the rat must be within 4 cm and oriented within 45 degrees of the object with actions such as staring, touching or sniffing the object to be considered engaged in object exploration. Rats that spent a total of less than 10 sec exploring objects during the testing phase were excluded from data analysis, as were rats in the SAP group with normal hippocampal ChAT activity (within two standard deviations of the mean value for ChAT activity for control animals).
Choline Acetyltransferase (ChAT) assay
After completing the behavioral tests, rats were anesthetized with pentobarbital (100 mg/kg; IP), and euthanized by decapitation. Tissues from the hippocampus and frontal cortex were dissected from the brain and frozen. The extent and specificity of cholinergic lesions were verified by measuring ChAT activity in the hippocampus and frontal cortex as described previously [20,25]. Briefly, tissues were thawed and dissociated by sonication in a medium containing EDTA (10 mM) and Triton X-100 (0.5%) and diluted to a concentration of 10 mg tissue/ml. An aliquot of each sample was used for the determination of total protein [9]. Three 5 μl aliquots of each sample were incubated for 30 min at 37 °C in a medium containing [3H] acetyl-CoA (50,000–60,000 d.p.m./tube, final concentration 0.25 mM acetyl-CoA), choline chloride (10.0 mM), physostigmine sulfate (0.2 mM), NaCl (300 mM), sodium phosphate buffer (pH 7.4, 50 mM), and EDTA (10 mM). The reaction was terminated with 4 ml sodium phosphate buffer (10 mM) followed by the addition of 1.6 ml of acetonitrile containing 5 mg/ml tetrephenylboron. The amount of [3H] acetylcholine produced was determined by adding 8 ml of EconoFluor scintillation cocktail (Packard Instruments, Meriden, CT) and counting total cpm in the organic phase using an LKB beta-counter. Background was determined using identical tubes to which no sample was added. For each sample, the three reaction tubes containing sample were averaged and the difference between total cpm and background cpm was used to estimate the total amount of ACh produced per sample. ChAT activity was then calculated for each sample as pmol ACh manufactured/h/μg protein.
Data analysis
Comparisons between control and SAP treatment groups were made using Student's T-test (GraphPad Prism 5.01). P values less than 0.05 were considered significant.
Results
The effect of surgery on recognition memory for objects and locations
There were no significant differences in the mean exploration ratios between the non-surgery and aCSF groups for either NOR or OLR tests (0.66 vs 0.66 object; 0.64 vs 0.67 place). Based on the above findings, subsequent data analysis combined the data from animals infused with aCSF and those that did not undergo infusion into a single control group.
ChAT Activity
Of the 25 rats that received intraseptal injections of SAP, the hippocampi of 17 had substantially reduced levels of ChAT activity relative to controls (a reduction of at least 2 standard deviations), consistent with a substantial loss of cholinergic inputs (Table 1). The remaining nine rats appeared to have either no lesion or only a partial lesion of the septo-hippocampal tract and were excluded from further analysis. Of those rats that were included, SAP treatment produced average decreases of 85.8% and 30.8 % in ChAT activity in the hippocampus and frontal cortex relative to controls.
Table.
The effect of SAP on ChAT activity in the hippocampus and frontal cortex. Results are reported as Mean ± SEM.
| pMol ACh hr/ug protein | Control (n=26) | SAP (n=17) | ||
|---|---|---|---|---|
| Means ± sem. | Range | Means ± sem | Range | |
| Hippocampus | 38.93 ± 1.21 | 29.61 - 55.42 | 5.52 ± 0.74 * | 2.76 - 14.13 |
| Frontal cortex | 33.76 ± 0.96 | 24.81 - 41.26 | 23.35 ± 1.4 * | 12.99 - 36.15 |
Indicates a significant decrease compared to control animals.
The effect of SAP lesion on exploration time, object recognition memory and object location recognition memory
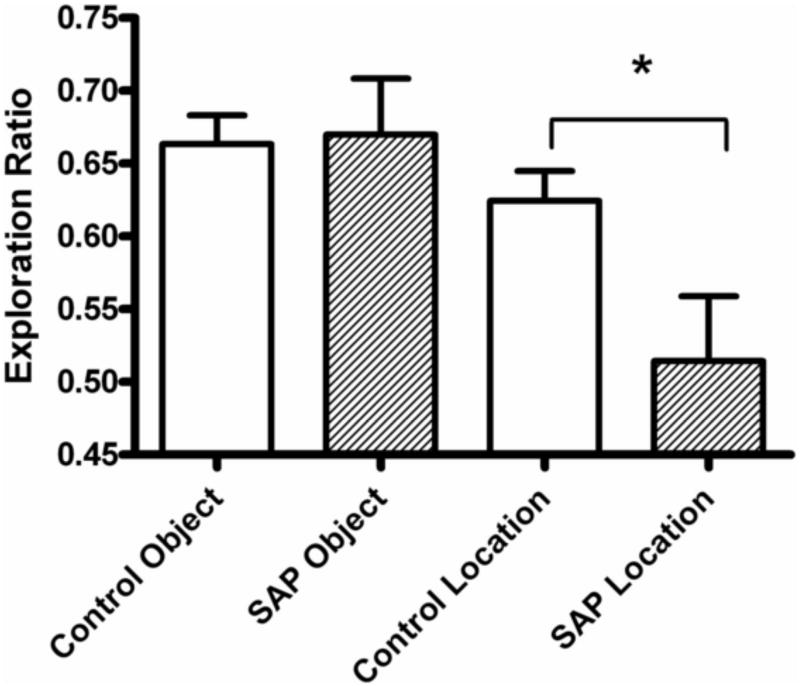
There was no significant difference (P =0.127) in total exploration times during object recognition testing between SAP treated (54.5±3.6 sec) vs. control rats (60.8±3.9 sec) indicating that cholinergic lesions did not affect the predisposition to explore objects. Moreover, there was no significant difference in the exploration ratios between control and SAP-treated rats (0.68±0.04 vs. 0.67±0.03, p = 0.866) indicating that lesion of cholinergic neurons of the MS had no effect on NOR (Fig 2). In contrast, compared to control rats, SAP-treated rats had a significantly lower exploration ratio (0.51±0.04 vs. 0.62±0.02, p = 0.013) when a familiar object was moved to a novel location. This result suggests that SAP-treated rats were significantly impaired in OLR memory. A linear regression analysis was performed to determine whether there was a correlation between hippocampal ChAT activity and exploration ratio. The result was not significant (R2 = 0.04; p = 0.126).
Figure 2.
The effect of SAP lesion of cholinergic neurons of the MS on novel object recognition and an altered spatial orientation of a familiar object as measured by exploration ratio (time spent exploring novel object or location divided by total exploration time). Lesioned rats retained memory of the familiar object but did not recognize a change in the location of a familiar object. Results are reported as Mean ± SEM. Lesioned rats retained memory of the familiar object(0.68±0.04 vs. 0.67±0.03, n=26) but did not recognize a change in the location of a familiar object (0.51±0.04 vs. 62±0.02, n=17). * Indicates a significant decrease in exploration ratio (p < 0.05).
Discussion
Our findings are consistent with a number of reports that selective cholinergic lesion of the MS by SAP produces impairment of spatial learning and memory tasks such as the water maze [14,23,34,37,41], T-maze [20,25] plus-maze [2,13], and the Cone-filed task [35]. The current study shows not only that septal cholinergic lesions impair OLR, but that NOR is spared, suggesting that cholinergic inputs to the hippocampus are selectively involved in spatial processing.
Cholinergic Innervation of the Frontal Cortex, NOR and OLR
Barker & Warburton [4], found that bilateral nonselective lesion of the medial prefrontal cortex using NMDA impaired OLR. In the current study, along with a substantial loss of ChAT activity in the hippocampus, there was a smaller but significant decrease in ChAT activity in the frontal cortex of SAP treated rats. An increase in ACh release in the frontal cortex of rats has been associated with being placed in novel environments and engaging exploratory behaviors [21]. Moreover, lesions of cholinergic neurons innervating the frontal cortex have been shown to impair object discrimination [5]. These effects may be related to the role that cholinergic neurons projecting to the frontal cortex play in attention and arousal [11,28]. That SAP treated animals in this study did not show a decrease in either total exploration time or impairment of object recognition suggests that the loss of cholinergic inputs reflected by the decreased ChAT activity in the frontal cortex was not substantial enough to affect attention or arousal, at least from the standpoint of motivation to explore. However, to the extent that object recognition and spatial memory are distinct functions, whether the decrease in cholinergic neurotransmission in the frontal cortex resulted in impairment in OLR following a delay of 24 hours cannot absolutely be ruled out. It is also possible that shorter delays in recognition testing may have resulted in less or no impairment.
Cholinergic Innervation of Temporal Lobe Structures, NOR and OLR
The role of cholinergic neurons of the basal forebrain that project to the hippocampus has been controversial, with some studies finding no effect on memory following selective cholinergic lesion [2,8,14,31] and other studies demonstrating impairment [13,17,20, 23,25, 37]. In a test of NOR while collecting microdialysis samples from the hippocampus, Ihalainen et al. [22], found increased ACh release during NOR testing.
Winters and Bussey proposed that NOR is mediated at least in part by cholinergic projections from the NBM to the PRh [38]. However, this theory was based on direct injections of SAP into the PRh with a resulting deficit in NOR and decreased ChAT immunoreactivity in the NBM. The results of a study by Paban et al. [30] were similar to results of the current investigation. However, in the Paban study both the MS and the NBM were leasoned. Contrary to the conclusions of Winters & Bussey [39], the Paban study suggested that cholinergic projections from the NBM to the PRh may not play an important role in NOR, but that cholinergic neurons of the basal forebrain were involved with OLR.
There is substantial evidence that the processing of object novelty occurs in the perirhinal cortex, while associate novelty is primarily a hippocampal function [3,26,27,33,39]. For example, a study by Winters et al. [40] of the functional dissociation between the effects of peri-postrhinal cortex and hippocampal lesions found that rats with non-selective hippocampal lesions were impaired in a spatial memory task, whereas rats with peri-postrhinal lesions were impaired in object recognition. The concept is further supported by the differential effect of selective perirhinal cortex lesion on spatial and object recognition memory reported by Barker et al. [3], where excitotoxic lesion of the PRh produced impaired object recognition but spared spatial recognition memory. Other studies, however, have reported that hippocampal lesions can impair recognition memory [10, 12]. Moreover, hippocampal lesion size, representational demands of the task and the specific task protocol could to play a role in such disparate results [5,10,15, 24, 32]. One advantage of the current study may be increased sensitivity resulting from combining the two tasks into the same experimental paradigm, thereby decreasing potential biases introduced by differing light sources, objects and exploration environment.
Summary
Our findings are the first to demonstrate that selective lesion of cholinergic neurons projecting from the medial septum to the hippocampus results in an impairment of memory for OLR. The results are consistent with the involvement of cholinergic afferents in spatial processing associated with recollecting spatial relationships but not processing associated with object recognition.
Highlights.
> Effect of medial septal cholinergic lesion on object and location recognition. > Selective cholinergic lesion of medial septum did not impair object recognition. > Selective cholinergic lesion of medial septum impaired object location recognition. > Septal cholinergic neurons are important for location but not object recognition.
Acknowledgments
The authors wish to acknowledge the excellent technical skills of Mrs. Christine Close and Mr. Douglas Nelson. This study was supported by NIH grants: AG16261 (DAJ) and AG021471 (RBG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe H, Ishida Y, Iwasaki T. Perirhinal N-methyl-D-aspartate and muscarinic systems participate in object recognition in rats. Neurosci Lett. 2004;356:191–194. doi: 10.1016/j.neulet.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 2.Bannon AW, Curzon P, Gunther KL, Decker MW. Effects of intraseptal injection of 192-IgG-saporine in long-Evens rats. Brain Res. 1996;718:25–36. doi: 10.1016/0006-8993(95)01568-x. [DOI] [PubMed] [Google Scholar]
- 3.Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: disconnection analysis of the role of the medial Prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartolini L, Casamenti G, Pepeu C. Aniracetam restores object recognition impaired by age, scopolamine and nucleus basalis lesions. Pharmacol Biochem Be. 1996;53:277–749. doi: 10.1016/0091-3057(95)02021-7. [DOI] [PubMed] [Google Scholar]
- 6.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 7.Bartus RT, Flicker C, Dean RL, Pontecorvo M, Figueiredo JC, Fisher SK. Selective memory loss following nucleus basalis lesions: long term behavioral recovery despite persistent cholinergic deficiencies. Pharmacol Biochem Be. 1985;23:125–135. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- 8.Baxter MG, David JB, Sobel TJ, Williams MJ, Gorman LK, Gallagher M. Intact spatial learning following lesions of basal forebrain cholinergic neurons. Neuroreport. 1996;7:1417–1420. doi: 10.1097/00001756-199605310-00019. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principal of protein dye binding. Anal Biochem. 1976;72:248–253. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory and the hippocampus. P Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzsaki G, Gage FH. In: The cholinergic nucleus basalis: a key structure in neocaortical arousal. Frotscher M, Misgeld U, editors. Central Cholinergic Synaptic Transmission; Birkhauser, Boston: 1989. pp. 159–171. [DOI] [PubMed] [Google Scholar]
- 13.Chang Q, Gold PE. Impaired and spared cholinergic functions in the hippocampus after lesions of the medial septum/vertical limb of the diagonal band with 192 IgG-Saporin. Hippocampus. 2004;14:170–179. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- 12.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2001;20:8853–8850. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dornan WA, McCambell AR, Tinker GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL. Comparison of site-specific injection into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192IgG-Saporin. Behav Brain Res. 1997;82:93–101. doi: 10.1016/s0166-4328(97)81112-6. [DOI] [PubMed] [Google Scholar]
- 15.Etchamendy N, Desmedt A, Cortes-Torrea C, Marighetto A, Jaffard R. Hippocampal lesions and discrimination performance of mice in the radial maze: sparing or impairment depending on the representational demands of the tasks. Hippocampus. 2003;13:197–211. doi: 10.1002/hipo.10055. [DOI] [PubMed] [Google Scholar]
- 16.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 17.Fitz NF, Gibbs RB, Johnson DA. Selective cholinergic lesion of the septal-hippocampal tract in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain Res Bull. 2008;77:356–360. doi: 10.1016/j.brainresbull.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitz NF, Gibbs RB, Johnson DA. Aversive attenuates impairment of acquisition in a delayed match to position T-maze task caused by a selective lesion of septal-hippocampal cholinergic projections. Brain Res Bull. 2006;69:600–665. doi: 10.1016/j.brainresbull.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher BR, Baxter MG, Guzowski JF, Shapiro ML, Rapp PR. Selective cholinergic depletion of the hippocampus spares both behaviorally induced Arc transcription and spatial learning and memory. Hippocampus. 2007;17:227–234. doi: 10.1002/hipo.20261. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem. 2007;88:19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovanini MG, Bartolini L, Kopf SR, Pepeu G. Acetylcholine release from the frontal cortex during exploratory activity. Brain Res. 1998;784:218–227. doi: 10.1016/s0006-8993(97)01161-x. [DOI] [PubMed] [Google Scholar]
- 22.Ihalainen J, Sarajarvi T, Kemppainen S, Keski-Rahkonen P, Lehtonen M, Tanila H. A novel delayed non-match to sample object recognition task that allows simultaneous in vivo microdialysis. J Neurosci Meth. 2010;189:210–215. doi: 10.1016/j.jneumeth.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Janis LS, Glasier MM, Fulop Z, Stein DG. Intraseptal injection of 192IgG-Saporin produce deficits for strategy selection in spatial-memory tasks. Behav Brain Res. 1998;90:23–34. doi: 10.1016/s0166-4328(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 24.Jo YS, Lee I. Disconnection of the hippocampus-perirhinal cortical circuits severely disrupts object-place paired associative memory. J Neurosci. 2010;30:9850–9858. doi: 10.1523/JNEUROSCI.1580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DA, Zambon NJ, Gibbs RB. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Res. 2002;943:132–141. doi: 10.1016/s0006-8993(02)02623-9. [DOI] [PubMed] [Google Scholar]
- 26.Kumaran D, Maguire EA. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007;17:735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- 27.Kumaran D, Maguire EA. Match-mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8524–8517. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGaughy J, Dalley JW, Morison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mumby DG. Object recognition. In: Kolb B, Whishaw IQ, editors. Behavior of the laboratory Rat: A handbook With Tests. Oxford University Press; New York: 2005. pp. 383–392. [Google Scholar]
- 30.Paban V, Chambon C, Jaffard M, Alescio-Lautier B. Behavioral effects of basal forebrain cholinergic lesions in young adult and aging rats. Behav Neurosci. 2005;19:933–45. doi: 10.1037/0735-7044.119.4.933. [DOI] [PubMed] [Google Scholar]
- 31.Parent MB, Baxter MG. Septohippocampal acetylcholine involved in but not necessary for learning and memory? Learn Memory. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzo DP, Thal LJ, Winker J. Mnemonic deficits in animals depend upon the degree of cholinergic deficit and task complexity. Exp Neurol. 2002;177:292–305. doi: 10.1006/exnr.2002.7993. [DOI] [PubMed] [Google Scholar]
- 33.Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. No effect of hippocampal lesions on perirhinal cortex-dependent feature-ambiguous visual discriminations. Hippocampus. 2006;16:421–430. doi: 10.1002/hipo.20170. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Barnes CA, Wenk GL, McNaughton BL. Differential effects of selective immunotoxic lesions of medial septal cholinergic cells on spatial working and reference memory. Behav Neurosci. 1996;110:1181–1186. doi: 10.1037//0735-7044.110.5.1181. [DOI] [PubMed] [Google Scholar]
- 35.Staay FJ, Bouger P, Lehmann O, Lazarus C, Cosquer B, Koening J, Stump V, Cassel JC. Long-term effects of immunotoxic cholinergic lesions in the septum on acquisition of the Cone-field task and noncognitive measures in rats. Hippocampus. 2006;16:1060–1079. doi: 10.1002/hipo.20229. [DOI] [PubMed] [Google Scholar]
- 37.Walshet TJ, Herzog CD, Gandhi C, Stackman RW, Wiley RG. Injection of 192 IgG-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res. 1996;726:69–79. [PubMed] [Google Scholar]
- 38.Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winters BD, Bussey TJ. Removal of cholinergic input to the perirhinal cortex disrupts object recognition but not spatial working memory in the rat. Eur J Neurosci. 2005;21:2263–2270. doi: 10.1111/j.1460-9568.2005.04055.x. [DOI] [PubMed] [Google Scholar]
- 40.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of objects recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrenn CC, Lappi DA, Wiley RG. Threshold relationship between lesion extent of the cholinergic basal forebrain in the rat and working memory impairment in the radial maze. Brain Res. 1999;847:284–298. doi: 10.1016/s0006-8993(99)02099-5. [DOI] [PubMed] [Google Scholar]