Abstract
Background
Using human MV models derived from three-dimensional (3D) echocardiography, finite element analysis (FEA) was used to predict mechanical leaflet and chordal stress. Subsequently, valve geometries were altered to examine the effects on stresses of: (1) varying coaptation area, (2) varying non-coapted leaflet tissue area, and (3) varying interleaflet coefficient of friction (μ).
Methods
Three human MV models were loaded with a transvalvular pressure of 80 mmHg using FEA. Initially leaflet coaptation was set to 10%, 50%, or 100% of actual coaptation length to test the influence of coaptation length on stress distribution. Next, leaflet surface areas were augmented by 1% overall and by 2% in the noncoapted “belly” region to test the influence of increased leaflet billowing without changing the gross geometry of the MV. Finally, the coefficient of friction between the coapted leaflets was set to μ = 0, 0.05, or 0.3, to assess the influence of friction on MV function.
Results
Leaflet coaptation length did not affect stress distribution in either the coapted or noncoapted leaflet regions: peak leaflet stress was 0.36±0.17 MPa at 100%, 0.35±0.14 MPa at 50%, and 0.35±0.15 MPa at 10% coaptation lengths (P=0.85). Similarly, coaptation length did not affect peak chordal tension (P=0.74). Increasing the noncoapted leaflet area decreased the peak valvular stresses by 5±2% (P=0.02). Varying the coefficient of friction between leaflets did not alter leaflet or chordal stress distribution (P=0.18).
Conclusions
Redundant MV leaflet tissue reduces mechanical stress on the noncoapted leaflets; the extent of coaptation or frictional inter-leaflet interaction does not independently influence leaflet stresses. Repair techniques which increase or preserve noncoapted leaflet area may decrease mechanical stresses and thereby enhance repair durability.
Keywords: Echocardiography, Mitral valve, Mitral valve repair, Modeling
Introduction
Degenerative pathologies are the most common causes of mitral regurgitation (MR).[1] Mitral repair has become the procedure of choice for symptomatic patients and asymptomatic patients with evidence of left ventricular dilatation. [2] Recent studies have documented a higher than expected recurrence of significant MR after valve repair. [3-9] Recurrence rates may be as high as 2-4% per year depending on the definition of recurrent MR. [4,5] Recurrence of MR after valve repair for degenerative disease may be a manifestation of elevated valve stress; therefore, surgical techniques that limit MV stress may be expected to improve MV repair durability. [6,9]
We sought to better understand how MV leaflet geometry and physical parameters affect leaflet and chordal stress distribution. Transesophageal real-time 3D echocardiography (rt-3DE) and finite element analysis (FEA) were utilized to examine the change in pressure-load-induced MV leaflet and chordal stress as the results of variation in: (1) leaflet coaptation area, (2) the amount of redundant noncoapted leaflet tissue, and (3) the magnitude of frictional interaction between the coapting portions of the anterior and posterior mitral leaflets.
Patients and Methods
Patients
Intraoperative real-time three-dimensional transesophageal echocardiography (rt-3DE) was performed in three human adult subjects undergoing cardiac surgery for pathology unrelated to the MV. All patients had preserved ejection fraction and competent nonstenotic MV’s. One patient underwent coronary artery bypass grafting (CABG) alone, one underwent transcatheter aortic valve implantation, and one underwent combine CABG and aortic valve replacement. The rt-3DE data sets were acquired with a Philips IE 33 (Philips, Andover, Massachusetts) equipped with an X7-2T transesophageal matrix array transducer probe. Electrocardiographically gated full volume (FV) images were acquired over 4 cardiac cycles. Care was taken to image the entire mitral apparatus throughout the cardiac cycle. The protocol was approved by the University of Pennsylvania School of Medicine institutional review board, and informed consent obtained from all patients.
Image Analysis
Each full-volume data set was then exported to an Echo-View 5.4 (Tomtec Imaging Systems, Munich, Germany) software workstation for image analysis. Manual segmentation and semi – automatic reconstruction of the MV annulus, anterior (AL) and posterior leaflets (PL), and the coaptation zone was performed as previously described. [10-13] Anterior and posterior papillary muscles tips were identified through visual inspection. All analysis was performed at mid-systole to minimize load variability among subjects.
Stress Analysis Using Finite Element Method
Three normal human MV’s models were imported into the explicit integration scheme of a commercial FEA program (ABAQUS/Explicit 6.3, HKS Inc. Pawtucket, RI) for stress analysis, as previously described by our laboratory.[14] That prior study provided a framework for the application of in vivo MV geometry and FEA to MV physiology, pathophysiology, and surgical repair, and is applied here. The AL was represented using a total of between 600 and 1000 nodes and between 1200 and 1500 triangular elements; the PL was represented with between 400 and 600 nodes and between 700 and 1000 elements (Figure 1). The triangulated leaflet surfaces were modeled as thin shell (type S3R) with uniform thicknesses of 1.4 mm for the anterior leaflet and 1.1 mm for the posterior leaflet. Leaflet tissue was assumed to be orthotropic and linearly elastic, with Young’s modulus determined from excised porcine tissue data (Table 1). [15] The coaptation area between anterior and posterior leaflet was defined as an interface pair with coefficient of friction μ. Thirty-two chordae originating from each papillary muscle head were inserted symmetrically into the anterior and posterior leaflets along the free edges of the leaflets (primary chordae), or more peripherally (secondary chordae). [16] Papillary muscle heads were models as single points hinged in space associated with rotational freedom only. Chordae tendinae were represented by strings connecting the papillary muscle tips to the insertion points on the leaflets, and modeled by a tension-only struss element (type T3D2). Systolic loading was accomplished via application of an 80 mmHg pressure gradient across the MV; patient-specific transvalvular pressure loads were not utilized to ensure consistent experimental conditions with regard to factors not being examined in this research. Stress, strain and displacement were recorded as output variables.
Figure 1.

Representative geometry of the human MV baseline model. Anterior leaflet (light grey), posterior leaflet (dark grey), and 32 chordae tendinae are shown in oblique view. Mid-AA: mid-anterior annulus; Mid-PA: mid-posterior annulus; AC: anterior commissure; PC: posterior commissure. Leaflet coaptation length (h) was altered to test the contribution of coaptation length to leaflet stress in Experiment (1).
Table 1.
Mitral valve material properties used in FE model (from reference 15).
| Parameter | Anterior Leaflet | Posterior Leaflet | Chordae |
|---|---|---|---|
| Thickness (mm) | 1.4 | 1.1 | - |
| Cross-sectional Area (mm2) | - | - | primary 0.4 secondary 0.7 |
| Ecirc (Pa) | 6.20 ×106 | 2.35×106 | 2.20×107 |
| Erad (Pa) | 2.10 ×106 | 1.89×106 | - |
| Poisson’s Ratio | 0.45 | 0.45 | 0.45 |
| Density (kg/m3) | 1.04×103 | 1.04×103 | 1.04×103 |
Ecirc = circumferential modulus of elasticity. Erad = radial modulus of elasticity.
Model Perturbations
Valve geometries and model constraints were altered minimally to examine the effects on FEA-predicted stresses of: (1) varying coaptation area, (2) varying non-coapted leaflet tissue area, and (3) varying interleaflet coefficient of friction μ in the coaptation zone. In the first experiment (1), leaflet coaptation length was set to 10%, 50%, or 100% of actual coaptation length to test the influence of coaptation length or area on stress distribution. In the second experiment (2), leaflet surfaces were augmented by 1% overall and by 2% in the noncoapted “belly” regions to test whether additional noncoapted leaflet tissue decreased predicted stresses. In the third experiment (3), the coefficient of friction between the anterior and posterior coapted leaflets was set to μ = 0, 0.05, or 0.3 to test the influence of leaflet coaptation interaction on leaflet and chordal stresses.
Statistics
Repeated measures ANOVA and paired t-tests were used as appropriate to test statistical significance. All results are given as mean ± standard deviation.
Results
Experiment (1)
Changes in leaflet coaptation length did not alter the von Mises stress distribution in either the coapted or noncoapted leaflet regions: peak leaflet stress was 0.36±0.17 MPa at 100% (actual), 0.35±0.14 MPa at 50%, and 0.35±0.15 MPa at 10% coaptation lengths (F=0.19 and P=0.85 by ANOVA). In both base model and models with altered coaptation length, anterior leaflet stresses were significantly higher than those in the posterior leaflet. Leaflet stress distribution is depicted in Figure 2 for one of the n=3 normal human MV’s subject to deformed (reduced) coaptation lengths. Similarly, coaptation length did not affect chordal tension: peak chordal tension was 0.77±0.34 Newtons (N) at 100% (actual), 0.82±0.45 N at 50%, and 0.80±0.29 N at 10% coaptation lengths (F=0.41 and P=0.74).
Figure 2.

Leaflet stress computed in one representative normal human MV in experiment (1). Results are presented in oblique (top) and left atrial views (bottom). (A) baseline 100% coaptation length model, (B) 50% coaptation length model and (C), 10% coaptation length model. Notice the consistent distribution and magnitude of leaflet stresses in all three cases.
Experiment (2)
Increasing the noncoapted leaflet area decreased the peak valvular stresses by 5±2% (P=0.02), consistent with increased billowing and therefore decreased local leaflet radius of curvature, resulting in lower predicted leaflet stresses. Leaflet stress distribution is depicted in Figure 3 for one of the n=3 normal human MV’s subject to enhanced non-coapted area.
Figure 3.
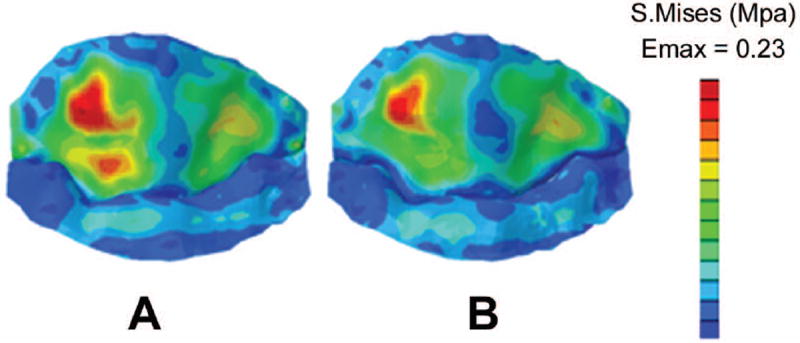
Leaflet stress computed in one representative normal human MV in experiment (2), in which the noncoapted area of the leaflet tissue was expanded by 2%. (A) baseline MV model, and (B) expanded leaflet tissue model. Notice the decreased leaflet stresses in (B).
Experiment (3)
Varying the coefficient of friction between leaflets did not alter leaflet stress distribution: peak leaflet stress at μ=0 was 0.34±0.15 MPa, at μ=0.05 was 0.34±0.15 MPa, and at μ=0.30 was 0.35±0.15 MPa (F=0.75 and P=0.63). Similarly, frictional forces did not affect chordal tension: peak chordal tension was 0.82±0.38 Newtons (N) at μ=0, 0.81±0.39 N at μ=0.05, and 0.77±0.34 N at μ=0.3 (F=15.0 and P=0.18).
Comment
Recurrent MR after MV repair for either degenerative MR is a significant problem. [3-9] Repair failure from disruption at leaflet, chordal, or annular suture lines suggests mechanical stress as one possible etiologic factor in recurrence. [6] It has been proposed that leaflet billowing and more extensive coaptation length or area act to reduce mechanical stresses on the leaflets and chordae tendinae, and as a result may improve repair durability. [17-21] The current study was undertaken to better understand how increased leaflet coaptation, increased leaflet tissue, and friction might affect mechanical stresses and loads to the MV apparatus.
Experiment (1) demonstrates that the changes of von Mises leaflet stress distribution (both peak stress and stress pattern) are minimal in either the coapted or noncoapted regions with alterations in coaptation length. This implies that – although ensuring adequate leaflet coaptation during MV repair may improve durability, the effect is mediated through mechanisms independent of mechanical transvalvular stress modification. The increased coapting leaflet length may simply make the valve more resistant to the deleterious geometric and anatomic effects of later ventricular remodeling and annular dilatation: more coaptation implies more leaflet area for the same mitral orifice, and therefore more leaflet redundancy.
Experiment (2) examines the contribution of extra non-coapting leaflet tissue in reducing MV mechanical stresses. The predicted peak stress is reduced 5% by expanding the noncoapted leaflet area 2%. A small percent increase in mesh element area was imposed to test the influence of increased leaflet billowing without changing the gross geometry of the MV. This experiment indicates that the reduction in leaflet stress after valve repair may be achieved by preservation or augmentation of leaflet tissue. Increased leaflet area provides additional amounts of non-coapted leaflet tissue that can billow towards the left atrium during systole, thereby increasing leaflet curvature and favorably altering leaflet stress distribution.
Experiment (3) demonstrates that predicted computational MV apparatus stresses are independent of the degree of frictional interaction between the coapting AL and PL in the coaptation region.
Drawbacks of the current study include a somewhat simplified MV model. While the leaflet surface profile is accurately determined by rt-3DE, chordae tendinae and papillary muscles were not reliably imaged and so their incorporation in the model was standardized based on prior anatomic results from Kunzelman et. al. [15,16] and prior FEA results from our laboratory. [14] In addition, the material properties utilized are likewise simplified as linear orthotropic. However, these drawbacks are irrelevant to the stated aims and robust conclusions of the study: the goal was not to create a religiously accurate MV FEA model, but rather to combine real-time 3D echocardiography and computational methods to examine one isolated aspect of the multifactorial problem. The combined advances in both imaging and computation help us to better understand mechanisms of valve repair failure and provide guidelines to refine the current MV repair techniques. Furthermore, given the complicated 3D structure and geometry of the mitral valve apparatus, veritable in vivo geometry is essential to computational stress modeling of the mitral valve; the current results implement the techniques validated in prior work done in our laboratory laying the groundwork for bioengineering analyses of the MV using geometric models derived from noninvasive three-dimensional imaging methods.
The correspondence between leaflet redundancy and reduced leaflet stresses – demonstrated in experiment (2) – has been shown previously. For example, using FEA, Kunzelman et. al. discovered that annular dilatation causes decreased coaptation and increased leaflet stresses. [22] However, that work made no attempt to distinguish the etiology of increased leaflet stress; the current research clearly demonstrates that reduced coaptation length in and of itself does not increase pressure-load induced noncoapted leaflet stresses nor chordal tensions. Rather, the geometric alterations in noncoapted leaflet configuration are likely responsible for increases in leaflet mechanical stress with decreased leaflet surface area or increased annular dimension.
A number of studies – including studies from our laboratory – have demonstrated biomechanical advantages to saddle ring annuloplasty as opposed to flat ring annuloplasty for mitral regurgitation. [23-25] These benefits may in part be derived from increased leaflet curvature (convex towards the left atrium) resulting from more physiologic annular contour imparted by saddle-shaped rings. [26] Similarly, posterior leaflet augmentation during MV repair would presumably augment leaflet curvature. [27,28] The current study reinforces the rationale for imposing increased leaflet curvature: increased noncoapting leaflet area would tend to decrease mitral leaflet stresses, as demonstrated in experiment (2).
Conclusions
Redundant MV leaflet tissue reduces pressure load-induced mechanical stress on the noncoapted leaflets; the extent of coaptation or frictional inter-leaflet interaction does not independently influence leaflet stresses. Operative or interventional strategies for MV repair that increase coaptation length in isolation are not expected to be beneficial with respect to altering the mechanical stresses on the MV. Repair techniques which increase or preserve noncoapted leaflet area, thereby augmenting leaflet billowing, may decrease leaflet pressure-load-derived mechanical stresses and thereby enhance repair durability.
Acknowledgments
This work was supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD (HL63954, HL73021 and HL103723). R. Gorman and J. Gorman are supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX. A. Jassar was funded by postdoctoral research grant from the American Heart Association. M. Vergnat was supported by a French Federation of Cardiology Research Grant. C. Xu was supported by a Ruth L. Kirschstein National Research Award (HL099172).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics 2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2006;114:e84–231. [Google Scholar]
- 3.Gillinov AM, Cosgrove DM, III, Shiota Takahiro, et al. Cosgrove-Edwards Annuloplasty System: Midterm Results. Ann Thorac Surg. 2000;69:717–21. doi: 10.1016/s0003-4975(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 4.Flameng W, Herijgers P, Bogaerts K. Recurrence of Mitral Valve Regurgitation After Mitral Valve Repair in Degenerative Valve Disease. Circulation. 2003;107:1609–13. doi: 10.1161/01.CIR.0000058703.26715.9D. [DOI] [PubMed] [Google Scholar]
- 5.Flameng W, Meuris B, Herijgers P, et al. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg. 2008;135(2):274–282. doi: 10.1016/j.jtcvs.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Gillinov AM, Cosgrove DM, Blackstone EH, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg. 1998;116(5):734–743. doi: 10.1016/S0022-5223(98)00450-4. [DOI] [PubMed] [Google Scholar]
- 7.Newcomb AE, David TE, Lad VS, et al. Mitral Valve Repair for Advanced Myxomatous Degeneration with Posterior Displacement of the Mitral Annulus. J Thorac Cardiovasc Surg. 2008;136(6):1503–1509. doi: 10.1016/j.jtcvs.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 8.Chang BC, Youn YN, Ha JW, et al. Long Term Clinical Results of Mitral Valvuloplasty Using Flexible and Rigid Rings: A Prospective and Randomized Study. J Thorac Cardiovasc Surg. 133(4):995–99. doi: 10.1016/j.jtcvs.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Zegdi R, Ghassan S, Letremouille C, Berrebi A, Carpentier A. Reoperation for Failure of Mitral Valve Repair in Degenerative Disease: A single Center Experience. Ann Thorac Surg. 2008;86:1480–4. doi: 10.1016/j.athoracsur.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ryan LP, Jackson BM, Enomoto Y, et al. Description of regional mitral annular nonplanarity in healthy human subjects: a novel methodology. J Thorac Cardiovasc Surg. 2007;134(3):644–648. doi: 10.1016/j.jtcvs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Ryan LP, Jackson BM, Eperjesi TJ, et al. A methodology for assessing human mitral leaflet curvature using real-time 3-dimensional echocardiography. J Thorac Cardiovasc Surg. 2008;136(3):726–734. doi: 10.1016/j.jtcvs.2008.02.073. Epub 2008 Jul 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jassar AS, Brinster CJ, Vergnat M, et al. Quantitative mitral valve modeling using real-time three-dimensional echocardiography: technique and repeatability. Ann Thorac Cardiovasc Surg. 2011;91(1):165–171. doi: 10.1016/j.athoracsur.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vergnat M, Jassar AS, Jackson BM, et al. Ischemic mitral regurgitation: a quantitative three-dimensional echocardiographic analysis. Ann Thorac Cardiovasc Surg. 2011;91(1):157–164. doi: 10.1016/j.athoracsur.2010.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Brinster CJ, Jassar AS, et al. A novel approach to in vivo mitral valve stress analysis. Am J Physiol Heart Circ Physiol. 2010;299(6):1790–1794. doi: 10.1152/ajpheart.00370.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunzelman KS, Cochran RP, Chuong C, et al. Finite element analysis of the mitral valve. J Heart Valve Dis. 1993;2(3):326–340. [PubMed] [Google Scholar]
- 16.Kunzelman KS, Cochran RP. Mechanical properties of basal and marginal mitral valve chordae tendineae. ASAIO Trans. 1990;36(3):M405–408. [PubMed] [Google Scholar]
- 17.Chikwe J, Adams DH. State of the art: degenerative mitral valve disease. Heart Lung Circ. 2009;18(5):319–329. doi: 10.1016/j.hlc.2009.02.005. Epub 2009 May 2017. [DOI] [PubMed] [Google Scholar]
- 18.Spiegelstein D, Moshkovitz Y, Sternik L, et al. Midterm results of mitral valve repair: closed versus open annuloplasty ring. Ann Thorac Cardiovasc Surg. 2010;90(2):489–495. doi: 10.1016/j.athoracsur.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Taniguchi K, Kuki S, et al. Evaluation of the mitral valve leaflet morphology after mitral valve reconstruction with a concept “coaptation length index”. J Card Surg. 2005;20(5):432–435. doi: 10.1111/j.1540-8191.2005.200329.x. [DOI] [PubMed] [Google Scholar]
- 20.Adams DH, Anyanwu AC, Sugeng L, et al. Degenerative mitral valve regurgitation: surgical echocardiography. Curr Cardiol Rep. 2008;10(3):226–232. doi: 10.1007/s11886-008-0038-9. [DOI] [PubMed] [Google Scholar]
- 21.Perier P, Hohenberger W, Lakew F, et al. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the “respect rather than resect” approach. Ann Thorac Surg. 2008;86(3):718–725. doi: 10.1016/j.athoracsur.2008.05.015. discussion 718-725. [DOI] [PubMed] [Google Scholar]
- 22.Kunzelman KS, Reimink MS, Cochran RP. Annular dilatation increases stress in the mitral valve and delays coaptation: a finite element computer model. Cardiovasc Surg. 1997;5(4):427–434. doi: 10.1016/s0967-2109(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 23.Salgo IS, Gorman JH, 3rd, Gorman RC, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106(6):711–717. doi: 10.1161/01.cir.0000025426.39426.83. [DOI] [PubMed] [Google Scholar]
- 24.Padala M, Hutchison RA, Croft LR, et al. Saddle shape of the mitral annulus reduces systolic strains on the P2 segment of the posterior mitral leaflet. Ann Thorac Surg. 2009;88(5):1499–1504. doi: 10.1016/j.athoracsur.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez JH, Liou SW, Padala M, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J Thorac Cardiovasc Surg. 2007;134(6):1562–1568. doi: 10.1016/j.jtcvs.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Ryan LP, Jackson BM, Hamamoto H, et al. The influence of annuloplasty ring geometry on mitral leaflet curvature. Ann Thorac Surg. 2008;86(3):749–760. doi: 10.1016/j.athoracsur.2008.03.079. discussion 749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robb JD, Minakawa M, Koomalsingh KJ, et al. Posterior leaflet augmentation improves leaflet tethering in repair of ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2011;40:1501–7. doi: 10.1016/j.ejcts.2011.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Varennes B, Chaturvedi R, Sidhu S, et al. Initial results of posterior leaflet extension for severe type IIIb ischemic mitral regurgitation. Circulation. 2009;119:2837–43. doi: 10.1161/CIRCULATIONAHA.108.831412. [DOI] [PubMed] [Google Scholar]


