Abstract
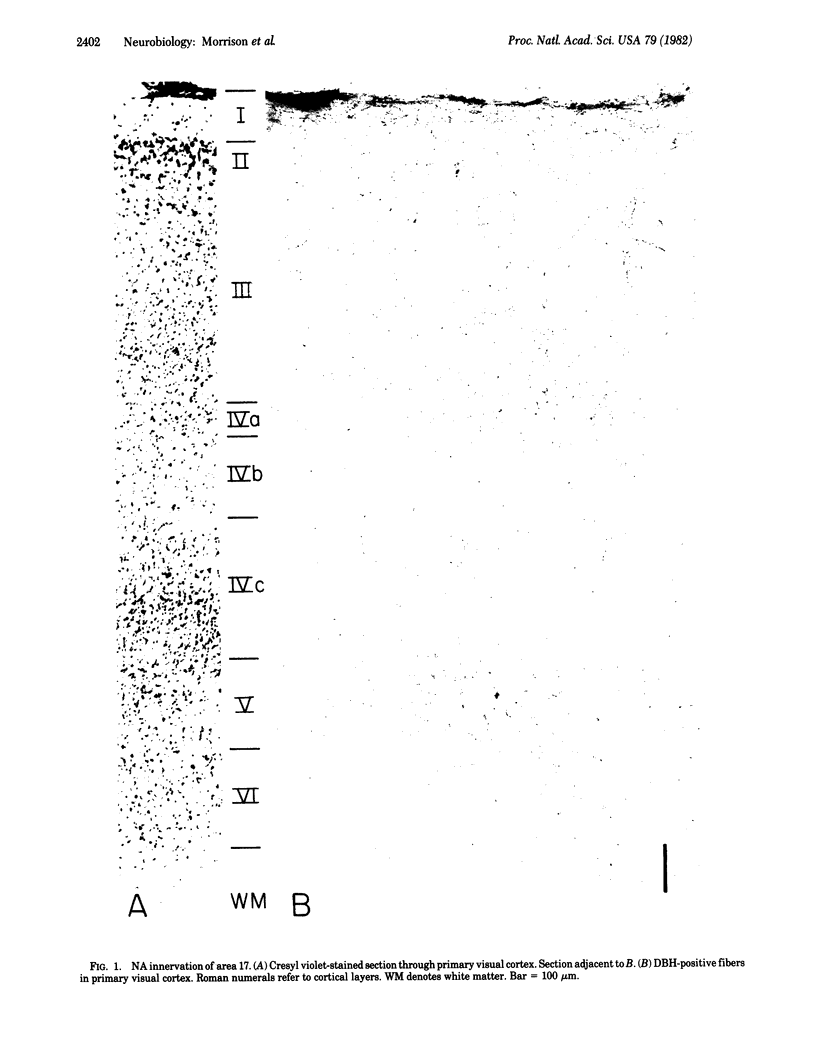
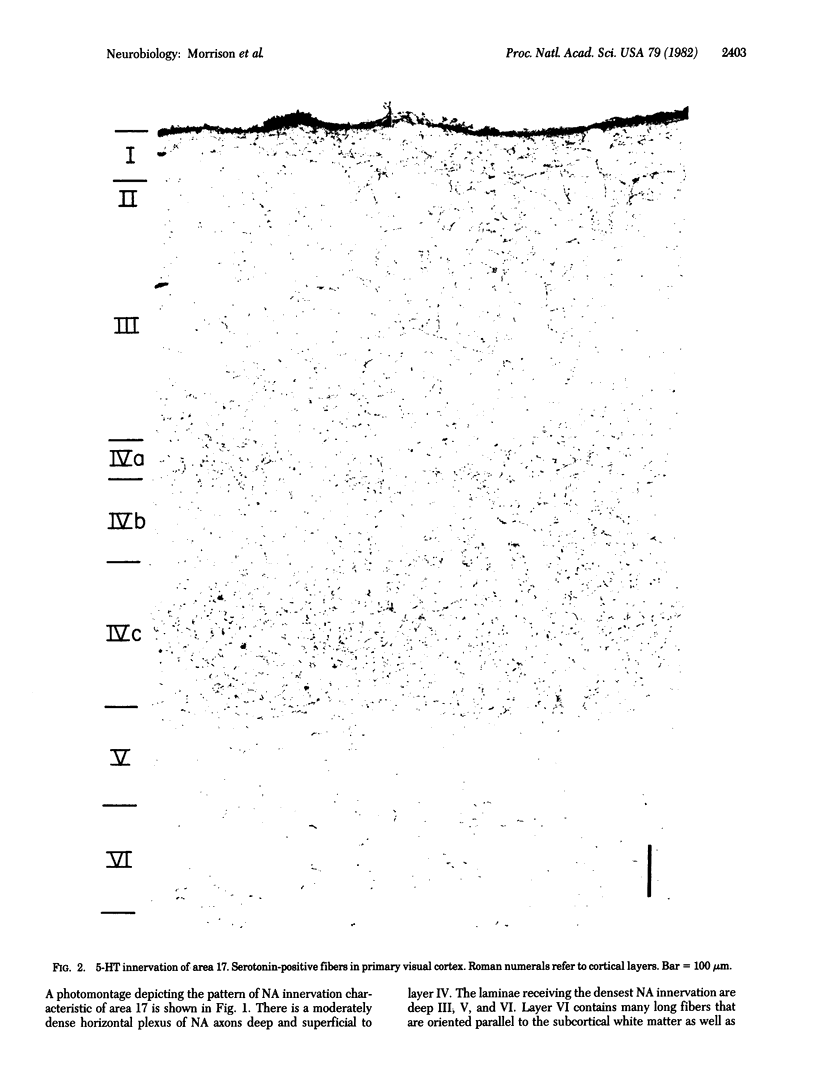
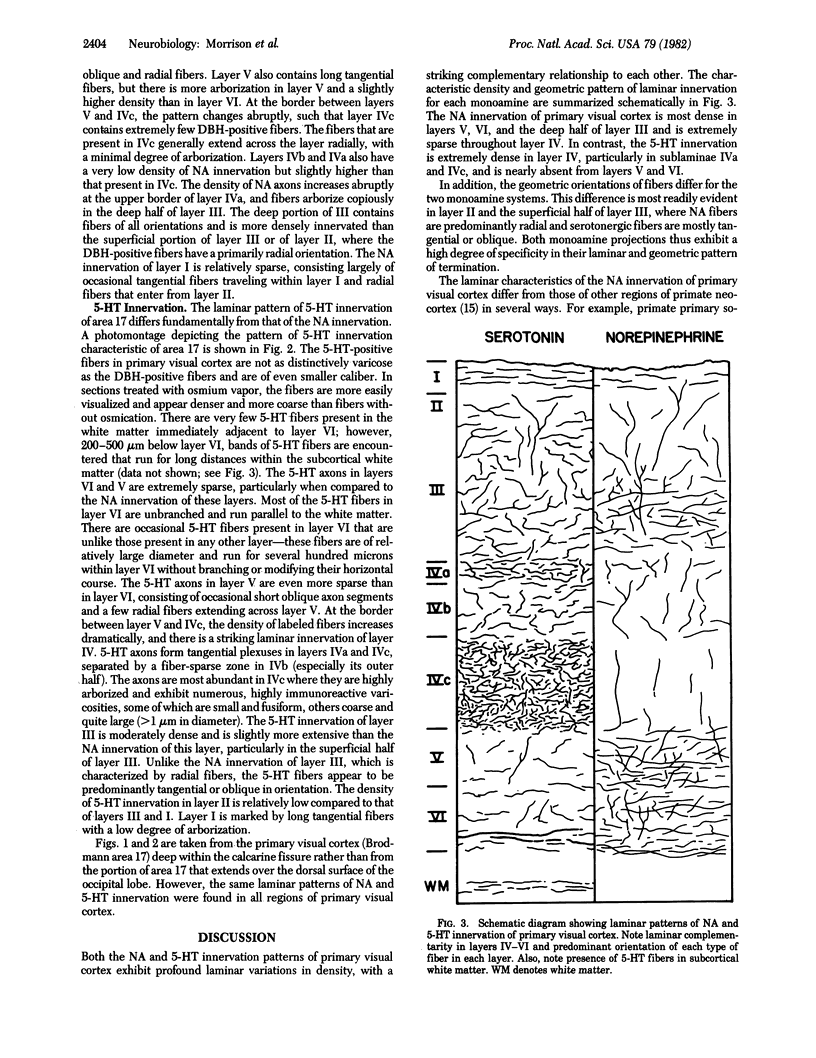
Antisera directed against human dopamine beta-hydroxylase or serotonin were used to characterize the noradrenergic and serotonergic innervation patterns within the primary visual cortex of the squirrel monkey. The noradrenergic and serotonergic projections exhibit a high degree of laminar complementarity: layers V and VI receive a dense noradrenergic projection and a very sparse serotonergic projection, whereas layer IV receives a very dense serotonergic projection and is largely devoid of noradrenergic fibers. In addition, the noradrenergic fibers manifest a geometric order that is not so readily apparent in the distribution of serotonergic fibers. These patterns of innervation imply that the two transmitter systems affect different stages of cortical information processing--the raphe-cortical serotonergic projection preferentially innervates the spiny stellate cells of layers IVa and IVc, whereas the ceruleo-cortical noradrenergic projection innervates pyramidal cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. M., Crane A. M., Goldman P. S. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 1979 May 18;168(1):133–150. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- Freedman R., Foote S. L., Bloom F. E. Histochemical characterization of a neocortical projection of the nucleus locus coeruleus in the squirrel monkey. J Comp Neurol. 1975 Nov 15;164(2):209–231. doi: 10.1002/cne.901640205. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Powell T. P. The projection of the locus coeruleus upon the neocortex in the macaque monkey. Neuroscience. 1977;2(3):441–445. doi: 10.1016/0306-4522(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura T., Kasamatsu T., Pettigrew J. D. Norepinephrine-containing terminals in kitten visual cortex: laminar distribution and ultrastructure. Neuroscience. 1981;6(2):159–175. doi: 10.1016/0306-4522(81)90052-x. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T., Pettigrew J. D., Ary M. Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J Comp Neurol. 1979 May 1;185(1):163–181. doi: 10.1002/cne.901850110. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T., Pettigrew J. D. Preservation of binocularity after monocular deprivation in the striate cortex of kittens treated with 6-hydroxydopamine. J Comp Neurol. 1979 May 1;185(1):139–161. doi: 10.1002/cne.901850109. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Molliver M. E., Morrison J. H., Lidov H. G. Complmentarity of dopaminergic and noradrenergic innervation in anterior cingulate cortex of the rat. Brain Res. 1979 Mar 23;164:328–333. doi: 10.1016/0006-8993(79)90031-3. [DOI] [PubMed] [Google Scholar]
- Lidov H. G., Grzanna R., Molliver M. E. The serotonin innervation of the cerebral cortex in the rat--an immunohistochemical analysis. Neuroscience. 1980;5(2):207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Lund R. D., Hendrickson A. E., Bunt A. H., Fuchs A. F. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975 Dec 1;164(3):287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Grzanna R., Molliver M. E., Coyle J. T. The distribution and orientation of noradrenergic fibers in neocortex of the rat: an immunofluorescence study. J Comp Neurol. 1978 Sep 1;181(1):17–39. doi: 10.1002/cne.901810103. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Molliver M. E., Grzanna R., Coyle J. T. Noradrenergic innervation patterns in three regions of medial cortex: an immunofluorescence characterization. Brain Res Bull. 1979 Nov-Dec;4(6):849–857. doi: 10.1016/0361-9230(79)90022-4. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Molliver M. E., Grzanna R., Coyle J. T. The intra-cortical trajectory of the coeruleo-cortical projection in the rat: a tangentially organized cortical afferent. Neuroscience. 1981;6(2):139–158. doi: 10.1016/0306-4522(81)90051-8. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Molliver M. E., Grzanna R. Noradrenergic innervation of cerebral cortex: widespread effects of local cortical lesions. Science. 1979 Jul 20;205(4403):313–316. doi: 10.1126/science.451605. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Frigon R. P., Stone R. A. Human pheochromocytoma dopamine-beta-hydroxylase: purification and molecular parameters of the tetramer. Mol Pharmacol. 1979 Sep;16(2):529–538. [PubMed] [Google Scholar]
- Reader T. A., Masse P., de Champlain J. The intracortical distribution of norepinephrine, dopamine and serotonin in the cerebral cortex of the cat. Brain Res. 1979 Nov 30;177(3):499–513. doi: 10.1016/0006-8993(79)90467-0. [DOI] [PubMed] [Google Scholar]
- Spatz W. B., Tigges J., Tigges M. Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri). J Comp Neurol. 1970 Oct;140(2):155–174. doi: 10.1002/cne.901400203. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W., Verhofstad A. A., Joosten H. W. Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience. 1978;3(9):811–819. doi: 10.1016/0306-4522(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Hartman B. K. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975 Oct 15;163(4):467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]





