Abstract
PURPOSE
To study the physical compatibility of ibuprofen lysine injection (NeoProfen, Ovation Pharmaceuticals Inc., Deerfield, IL) with medications commonly used in the premature neonatal population during simulated Y-site administration.
MATERIALS AND METHODS
Commonly used intravenous medications in preterm infants were evaluated for physical compatibility with ibuprofen lysine injection. A 20-mL sample of ibuprofen lysine drug product solution was mixed with a 20-mL sample of each of the 34 medications at concentrations used clinically. The mixtures were stored at room temperature and each sample was evaluated for turbidity and physical appearance at time 0 (immediately after preparation) and at 4 hours after preparation.
RESULTS
The following drugs were deemed compatible with ibuprofen lysine: ceftazidime, epinephrine, furosemide, heparin lock flush, diluted insulin, morphine sulfate, phenobarbital, potassium chloride, and sodium bicarbonate. Diluted dopamine was initially compatible at time 0 but showed a small precipitate at the 4-hour time point.
CONCLUSION
Of the 37 drug solutions tested, 14 preparations (10 medications; several with more than one diluent) showed physical compatibility with ibuprofen lysine, 1 was compatible at time 0 and incompatible at 4 hours, and 1 could not be evaluated. The remaining preparations were considered to be incompatible.
Keywords: compatibility, ductus arteriosus, ibuprofen lysine
INTRODUCTION
Patent ductus arteriosus (PDA) frequently occurs in premature infants, especially those weighing between 500 g and 1500 g. If not properly treated, PDA may increase the risk of intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, and death.1 Ibuprofen has been proven as an effective agent to close a clinically significant PDA in premature infants.
Ibuprofen lysine injection (NeoProfen, Ovation Pharmaceuticals Inc., Deerfield, IL) was recently approved by the Food and Drug Administration (FDA) to close a clinically significant PDA in premature infants weighing between 500 g and 1500 g, who are no more than 32 weeks gestational age when usual medical management (e.g., fluid restriction, diuretics, respiratory support) is ineffective.2 The dosing regimen for ibuprofen lysine is 10 mg/kg followed by 2 doses of 5 mg/kg after 24 and 48 hours. Ibuprofen lysine can be diluted to an appropriate volume with either dextrose or saline for administration within 30 minutes of preparation, and should be infused over 15 minutes. Infants are often administered multiple intravenous medications for complications associated with prematurity including, but not limited to, the prevention and treatment of infections, hemodynamic instability, respiratory distress, and nutritional supplementation. Pharmacists and nurses are challenged by the concomitant administration of drugs due to limited intravenous line access and drug-drug incompatibilities. These drug-drug incompatibilities may include, but are not limited to, the formation of gasses and/or the precipitation of solid matter. Infusing precipitate particles of sufficient size into the vasculature of neonates can cause serious adverse embolic events and even death in neonates.3 No reports on the physical compatibility of ibuprofen lysine injection with other drugs and the potential hazards of co-infusion have been published. This study was conducted to establish the physical drug-drug compatibilities of intravenous medications commonly administered in the neonatal intensive care unit (NICU) with ibuprofen lysine solution in a Y-site administration set that would foster safe and appropriate medication administration practices.4 It has been reported in the literature that mixing of an intravenous fluid in an administration set with a secondary additive from a Y injection site occurs in a 1:1 ratio, and, therefore, the same ratio was used in this study.5
MATERIALS AND METHODS
Thirty-seven drug product preparations, reflecting common admixtures of 20 medications, are shown in Table 1. After achieving the concentrations found most commonly in NICU clinical practice,4 20 mL of each drug product sample was pipetted into a 40-mL clear glass vial. The characteristics of each drug preparation were observed prior to the addition of intravenous ibuprofen lysine solution. The contents of approximately 380 vials of the 10 mg/mL commercially available ibuprofen lysine were combined into a beaker and 20 mL of this solution was added to the 20-mL drug sample previously prepared to a produce a volume determined by researchers as sufficient for turbidity quantification and visual measurement. The admixture of equal volumes of drug preparations mimics the aforementioned 1:1 Y-site mixing ratio described by Allen et al.5 Visual appearance, evaluated by the unaided human eye under fluorescent lighting, was observed for each mixture at time 0 (immediately after preparation) and at 4 hours after preparation. After each visual observation, turbidity measurements were taken. The vials were capped and stored at room temperature. A Hach 2100N turbidity meter was used to measure the turbidity of each drug product mixture.6 Turbidity is measured by the interaction between light and suspended particles in a solution. When a sample contains particulate matter, the transmittance of light is related to the size, shape, and composition of the particles. A Nephelometric Turbidity Unit (NTU) is the standard of turbidity measurement and signifies that the instrument is measuring light diffraction.7 High and low levels of Hach turbidity standard solutions were used to calibrate the turbidimeter at time 0 and at 4 hours. The standard solutions used were <0.1, 1, 20, 1000, and 4000 NTU. All specifications passed the calibration test. A compatible drug was defined as one that, upon admixture with intravenous ibuprofen lysine solution, did not form a precipitate or result in any increase in turbidity.
Table 1.
Appearance and Turbidity after Mixing Various Drug Product Samples with Ibuprofen Lysine Solution at Time 0 and at 4 Hours
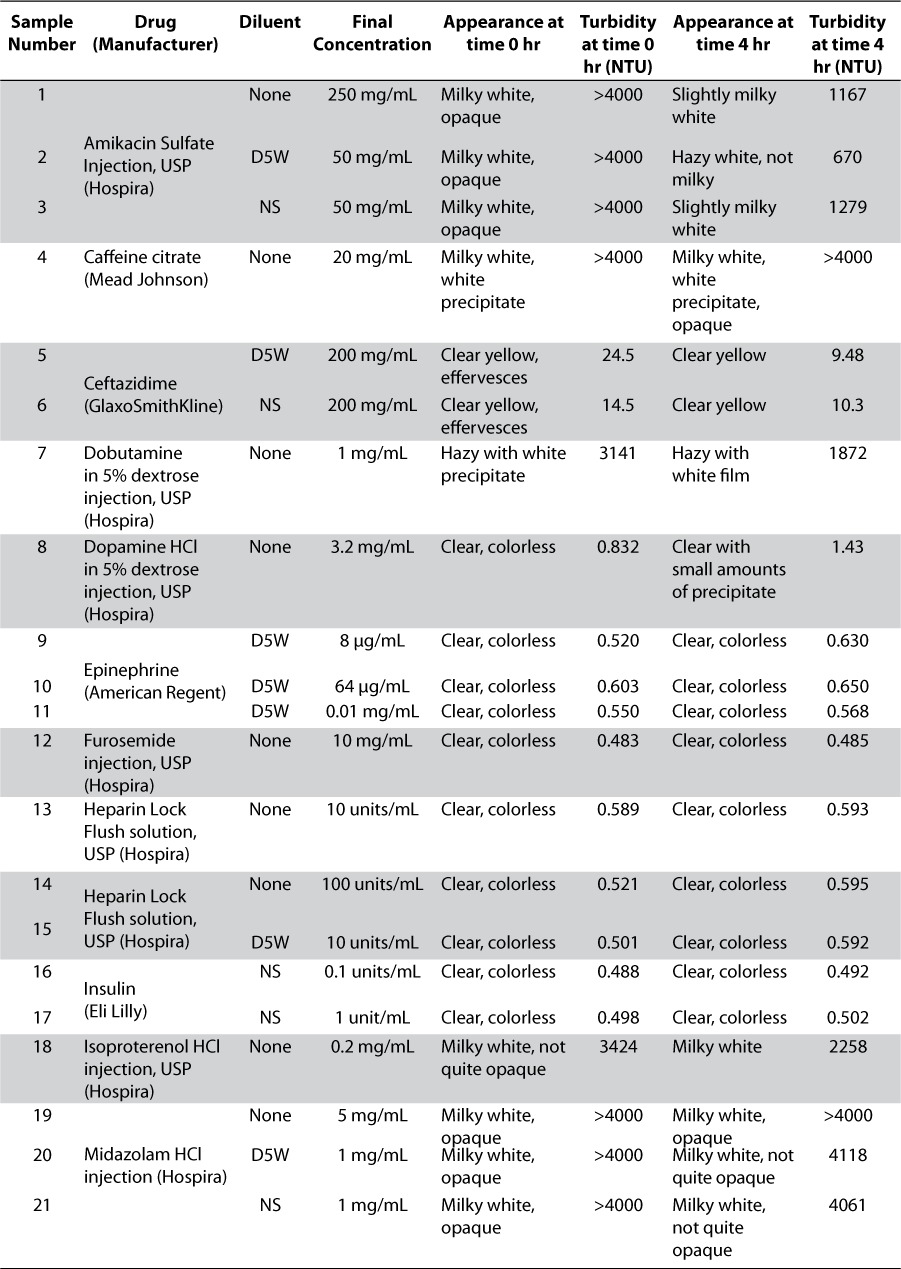
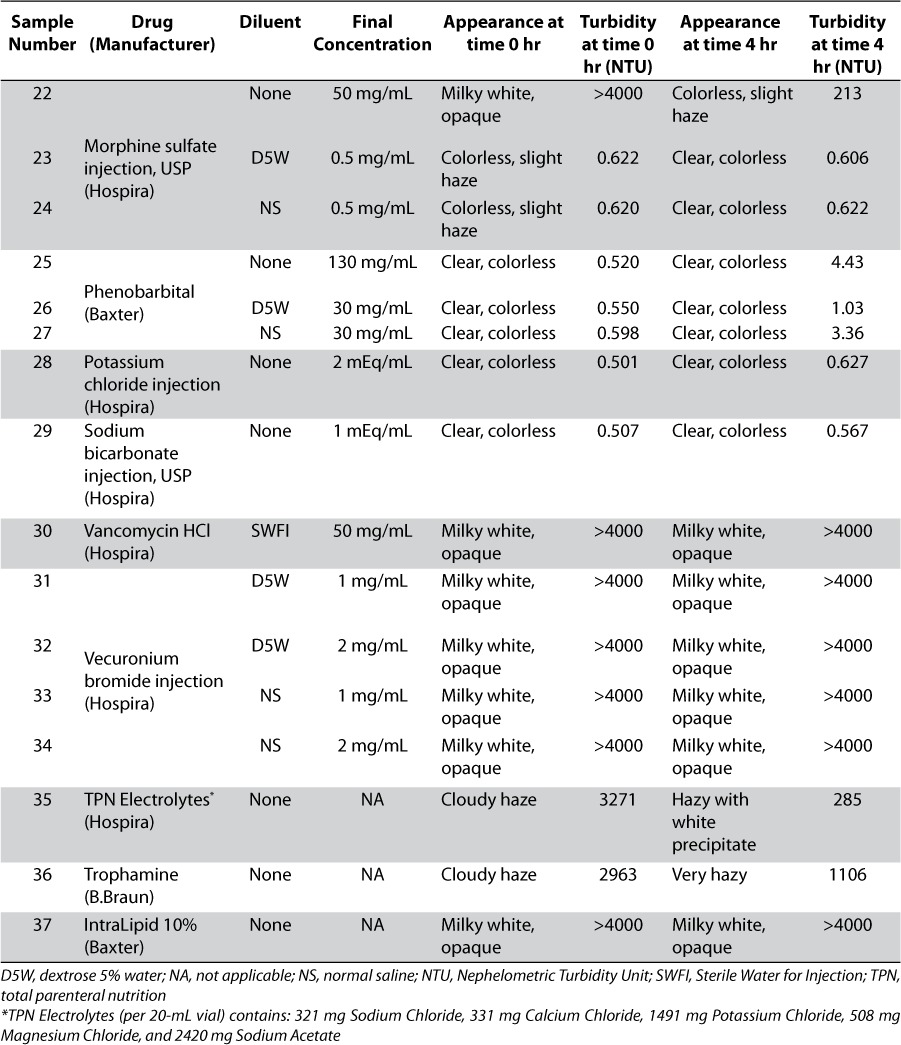
RESULTS
Prior to mixing the drug product samples with ibuprofen lysine solution, each vial was visually inspected for particles and precipitation. All solutions were clear and colorless with no visual precipitate. The exceptions included ceftazidime and vancomycin HCl, which appeared clear yellow and clear light amber in color without precipitate, respectively. IntraLipid 10% (Baxter International Inc., Deerfield, IL) was the only sample that was a milky white opaque emulsion and not a clear solution.
After mixing ibuprofen lysine solution with each drug product sample, physical appearance and turbidity measurements were observed at time 0 under the visual evaluation conditions defined above. Several mixtures immediately appeared milky white and opaque with turbidity readings exceeding the scale of the turbidimeter (NTU > 4000), as shown in Table 1. Dobutamine in dextrose 5% in water (D5W), isoproterenol HCl, TPN (total parenteral nutrition) Electrolytes, and Trophamine 10% (B. Braun Medical, Bethlehem, PA) all had turbidity readings above 2900 NTUs. Slight turbidities of 23.5 NTU and 14.5 NTU were seen with ceftazidime in D5W and normal saline (NS), respectively. These drug admixtures fulfilled the criteria defining a physical drug-drug incompatibility. All others remained clear and colorless without evidence of precipitation and the turbidity readings were less than 1 NTU.
Physical appearance and turbidity of the samples after 4 hours of storage at room temperature (20°C to 25°C) are shown in Table 1. Caffeine citrate, midazolam HCl, vancomycin HCl, and vecuronium bromide solutions, as well as IntraLipid 10% suspension, remained milky white and opaque with turbidity > 4000 NTU. The physical appearance of dobutamine in D5W became less opaque and the turbidity reading dropped from 3141 NTU to 1872 NTU. A white precipitate developed in the dopamine HCl solution. Amikacin sulfate undiluted and in NS appeared less opaque, but particulate matter was observed. Amikacin in D5W no longer displayed white particles but was hazy with a turbidity reading of 670 NTU. Undiluted morphine sulfate appeared milky white and opaque at time 0 but became colorless with a slight haze at 4 hours. All samples that were clear and colorless with low turbidity at time 0 remained unchanged; except for dopamine in D5W which formed a slight white precipitate by 4 hours.
DISCUSSION
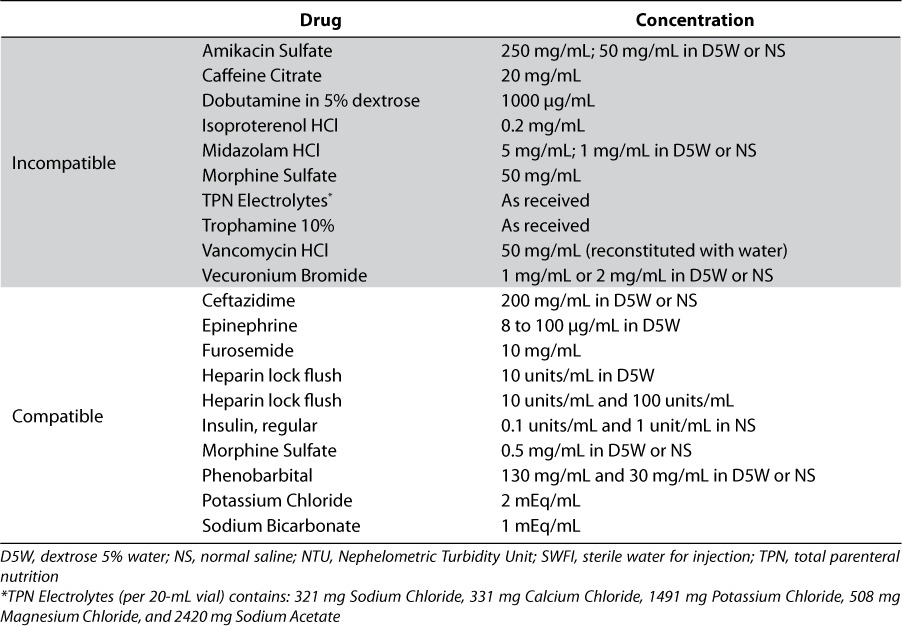
Drug product solutions considered physically compatible with ibuprofen lysine solution are those that did not produce particulate matter, color change, or increased turbidity. A summary of the results are presented in Table 2. Due to the initial hazy TPN electrolyte solution and the presence of a small amount of white precipitate, ibuprofen lysine solution cannot be considered compatible with this admixture. The admixture of IntraLipid 10% and ibuprofen lysine solution could not be evaluated due to the initial milky white opaque appearance and high turbidity value. These results support the recommendations in the ibuprofen lysine commercial labeling that TPN infusions should be stopped 15 minutes before and after the 15-minute infusion of ibuprofen lysine when administering via the same intravenous line.2
Table 2.
Summary of Physical Compatibilities of Various Drug Product Samples with Ibuprofen Lysine Solution
It is important to note that physical compatibility of 2 or more drugs does not imply in vivo or chemical compatibility. Chemical incompatibilities such as oxidation-reduction and hydrolysis reactions may alter the drug entity and not display differences in appearance or turbidity.8 Drug-disease state interactions also occur that can prohibit the concomitant administration of 2 or more drugs together. As an example, Ojala and Lehtonen proposed in a case control study that continuous exposure to heparin for maintaining catheter patency may decrease the efficacy of cyclooxygenase inhibitor therapy in closing a PDA.9
CONCLUSION
Ceftazidime 200 mg/mL in D5W and NS, epinephrine 8 mcg-0.1mg/mL in D5W, furosemide 10mg/mL, heparin lock flush solution 10 units/mL and 100 units/mL, heparin lock flush 10 units/mL in D5W, insulin 0.1–1 units/mL in NS, morphine sulfate 0.5 mg/mL in D5W or NS, phenobarbital 130 mg/mL or 30 mg/mL in D5W or NS, potassium chloride as 2 mEq/mL, and sodium bicarbonate 1 mEq/mL solution showed physical compatibility with ibuprofen lysine, dopamine HCl in D5W was compatible at time 0 and incompatible at 4 hours and IntraLipid 10% could not be evaluated. Amikacin 50 and 250 mg/nL in D5W or NS, caffeine citrate 20 mg/mL, dobutamine 5% in dextrose 1000mcg/mL, isoproterenol HCl 0.2 mg/mL, midazolam HCl 5 mg/mL and 1 mg/mL in D5W or NS, morphine sulfate 50 mg/mL, TPN electrolytes, Trophamine 10%, vancomycin HCl 50 mg/mL (in water), and vecuronium bromide 1 and 2 mg/mL in D5W or NS caffeine citrate solution, dobutamine in D5W, isoproterenol HCl, all midazolam HCl solutions, undilted morphine sulfate, vancomycin HCl, all vecuronium bromide solutions, TPN Electrolytes, and Trophamine 10% were physically incompatible.
ABBREVIATIONS
- D5W
dextrose 5% in water
- NICU
neonatal intensive care unit
- NS
normal saline
- NTU
Nephelometric Turbidity Unit
- PDA
patent ductus arteriosus
- TPN
total parenteral nutrition
Footnotes
DISCLOSURE The authors work for Ovation Pharmaceuticals, Inc., which markets ibuprofen lysine in the United States.
REFERENCES
- 1.Van Overmeire B, Smets K, Lecoutere D. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. et al. [DOI] [PubMed] [Google Scholar]
- 2.Deerfield, IL: Ovation Pharmaceuticals, Inc; 2006. NeoProfen [package insert] [Google Scholar]
- 3.Parikh MJ, Dumas G, Silvestri A. Physical compatibility of neonatal total parenteral nutrient admixtures containing organic calcium and inorganic phosphate salts. Am J Health-Syst Pharm. 2005;62:1177–1183. doi: 10.1093/ajhp/62.11.1177. et al. [DOI] [PubMed] [Google Scholar]
- 4.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 5.Allen LV, Jr, Levinson RS, Phisutsinthrop D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am J Hosp Pharm. 1977;34:939–943. [PubMed] [Google Scholar]
- 6.Hach Company. Turbidity science technical information series-booklet No. 1. Sadar MJ. 1998. Available at: http://www.hach.com/fmmimghach?/CODE%3AL7061549%7C1. Accessed June, 2008.
- 7.Dekker M. Particle Measurement Methods for Pharmaceutical Liquids. In: Meltzer T, Jornitz M, editors; Filtration in the Biopharmaceutical Industry. 2nd ed. Informa Health Care; London, UK: 2007. pp. 477–8. [Google Scholar]
- 8.Gennaro AR, editor. Remington: The Science and Practice of Pharmacy. 20th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000. p. 1697. [Google Scholar]
- 9.Ojala TH, Lehtonen L. A preliminary report - heparin counteracts indomethacin effect on ductus arteriosus in very low birth weight infants. Pediatr Crit Care Med. 2007;8:1–3. doi: 10.1097/01.pcc.0000270203.07648.8e. [DOI] [PubMed] [Google Scholar]


