Abstract
Purpose
Long-term survivors of childhood Hodgkin lymphoma (HL) are at risk for cardiopulmonary complications and CNS stroke, although neurocognitive function has not been previously examined. The aim of this study was to examine neurocognitive and brain imaging outcomes in adult survivors of childhood HL.
Patients and Methods
In all, 62 adult survivors (mean age, 42.2 years; standard deviation [SD], 4.77; mean age at diagnosis, 15.1 years; SD, 3.30) were identified by stratified random selection from a large cohort treated with either high-dose (≥ 30 Gy) thoracic radiation (n = 38) or lower-dose (< 30 Gy) thoracic radiation combined with anthracycline (n = 24). Patients underwent neurocognitive evaluations, brain magnetic resonance imaging (MRI), echocardiograms, pulmonary function tests, and physical examinations.
Results
Compared with national age-adjusted norms, HL survivors demonstrated lower performance on sustained attention (P = .004), short-term memory (P = .001), long-term memory (P = .006), working memory (P < .001), naming speed (P < .001), and cognitive fluency (P = .007). MRI revealed leukoencephalopathy in 53% of survivors, and 37% had evidence of cerebrovascular injury. Higher thoracic radiation dose was associated with impaired cardiac diastolic function (E/E′; ratio of peak mitral flow velocity of early rapid filling [E] to early diastolic velocity of the mitral annulus [E′]; P = .003), impaired pulmonary function (diffusing capacity of lungs for carbon monoxide [DLcocorr; P = .04), and leukoencephalopathy (P = .02). Survivors with leukoencephalopathy demonstrated reduced cognitive fluency (P = .001). Working memory impairment was associated with E/E′, although impaired sustained attention and naming speed were associated with DLcocorr. Neurocognitive performance was associated with academic and vocational functioning.
Conclusion
These results suggest that adult long-term survivors of childhood HL are at risk for neurocognitive impairment, which is associated with radiologic indices suggestive of reduced brain integrity and which occurs in the presence of symptoms of cardiopulmonary dysfunction.
INTRODUCTION
Survivors of childhood Hodgkin lymphoma (HL) are not considered to be at risk for neurocognitive dysfunction. Compared with siblings, adult survivors of childhood HL self-report higher rates of cerebrovascular accident/stroke,1 although little data exist on brain imaging outcomes. Children diagnosed with HL are treated with thoracic radiation and/or anthracyclines and thus are at risk for cardiovascular morbidity.2 Many HL survivors are also treated with bleomycin which, similar to thoracic radiation, has been associated with pulmonary dysfunction.3 Literature in noncancer populations demonstrates an association between cardiovascular and pulmonary health and CNS integrity. Adults with cardiovascular disease more frequently demonstrate multifocal white matter hyperintensities (ie, leukoencephalopathy) on magnetic resonance imaging (MRI) of the brain than those without disease.4 Patients with chronic heart disease demonstrate problems with attention, processing speed, memory, and executive functions,5 and those with vascular complications, such as hypertension, demonstrate increased risk for cognitive impairment and future dementia.6 In the Framingham Heart Study,7 a 10% increase in cardiovascular disease risk was associated with a significant decline in cognitive function over a 10-year interval. Chronic pulmonary disease also increases the risk of stroke,8 leukoencephalopathy,9 and neurocognitive impairment in noncancer populations.9
Although children diagnosed with HL do not usually receive cranial radiation or chemotherapeutic agents known to have an impact on CNS integrity (eg, methotrexate), the factors outlined in the previous paragraph suggest that long-term survivors may be at increased risk for neurocognitive impairment and CNS morbidity. Although only 2% of cancers diagnosed before the age of 20 years are HL, survival rates have historically been high.10 By using population-based survivor prevalence,11 we can predict that there are approximately 38,000 survivors of pediatric HL in the US population today; thus, the potential risk has significant societal impact. The purpose of this investigation was to examine neurocognitive function and CNS integrity, as reflected through brain MRI, in adult long-term survivors of childhood HL. These outcomes were correlated to indices of cardiac and pulmonary health, as well as HL treatment characteristics. We hypothesized that survivors treated with higher doses of mantle field radiation would be at increased risk for cardiac and/or pulmonary morbidity, and such morbidity would be associated with abnormal brain MRI findings and neurocognitive dysfunction. Although modern protocols involve combined-modality therapy with reduced thoracic radiation doses, this study aims to evaluate CNS integrity in patients who received more intensive therapy and have now reached middle adulthood. Future studies will be needed to address similar issues in survivors treated on modern protocols once they reach middle adulthood.
PATIENTS AND METHODS
Patients
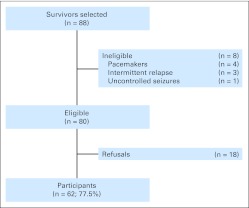
Participants were randomly selected from a cohort of more than 400 adult survivors of childhood HL registered in the St Jude Lifetime Cohort Study (SJLIFE).12 Eligibility criteria for our sample included age at diagnosis ≤ 21 years, current age 18 to 55 years, ≥ 15 years from diagnosis, and treatment with thoracic radiation with or without anthracycline chemotherapy. Patients were excluded if they had a history of a developmental disorder or neurologic event unrelated to their cancer history, or if they relapsed or were diagnosed with a second cancer. From perceived eligible survivors, 88 were randomly selected and invited to participate, eight were subsequently deemed ineligible, and of the remaining 80 truly eligible patients, 62 (77.5%) agreed to participate (Figure 1). No differences between participants and nonparticipants were identified in sex, race, age at diagnosis, current age, or treatment characteristics. All survivors provided informed written consent, and the study protocol was approved by the St Jude institutional review board.
Fig 1.
Patient flow diagram. Eighty-eight survivors were randomly selected from a cohort of more than 400 adult survivors of Hodgkin lymphoma, on the basis of original treatment characteristics.
Procedure
Per the SJLIFE study protocol, all survivors completed a risk-based medical examination consistent with the Children's Oncology Group (COG) Long-Term Follow-Up Guidelines.13 These examinations included heart rate, blood pressure, and anthropometrics, as well as laboratory evaluations and other diagnostic procedures. Survivors underwent echocardiography and pulmonary function tests. 2D echocardiograms with Doppler and M-mode were performed by using a GE Vivid 7 machine (General Electric, Milwaukee, WI) and following the American Society of Echocardiography guidelines.14 Full systolic and diastolic assessments, including peak mitral flow velocity of early rapid filling (E), early diastolic velocity of the mitral annulus (E′) and their ratio (E/E′), evaluation of the pulmonary veins, and estimation of right atrial and right ventricular systolic pressure, were performed. Aortic valve (AV) and mitral valve (MV) regurgitation were coded by a board certified cardiologist (A.R.A.), blinded to treatment history and neurocognitive/brain imaging outcomes. Pulmonary function tests were performed in a single laboratory according to the American Thoracic Society task force guidelines.15 Pulmonary function tests included spirometry, lung volume measurements by body plethysmography, and single-breath diffusing capacity of lungs for carbon monoxide (DLCO). Spirometry was performed by pneumotachograph, and lung volumes were determined by using plethysmography (Medical Graphics Platinum Elite, St. Paul, MN). DLCO was measured by using the single-breath technique and corrected for hemoglobin concentration to yield DLcocorr. The observed values of each patient for forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), total lung capacity, and DLcocorr were compared with those predicted for the patient's age, race, sex, and height on the basis of reference equations.16
Neurocognitive testing was conducted during a single 2-hour visit within 3 days of the medical examinations. Assessed neurocognitive domains (and instruments) included intelligence (Wechsler Abbreviated Scale of Intelligence),17 attention (Trail Making Test Part A; Conners' Continuous Performance Test-II [CPT-II]; Digit Span Forward subtest of the Wechsler Adult Intelligence Scale-III [WAIS-III]),18–20 memory (California Verbal Learning Test-II),21 processing speed (Grooved Pegboard; Stroop Color Word Test; CPT-II),19,20 and executive function (Trail Making Test Part B; Controlled Oral Word Test; Digit Span Backward subtest of the WAIS-III).18,19 Order of testing and survivors' schedules were controlled to limit impact from fatigue and extraneous factors on neurocognitive testing. Survivors also completed self-report behavior ratings to assess symptoms of executive dysfunction (Behavior Rating Inventory of Executive Function-Adult version).22 Fatigue is a frequent concern in survivors of HL23 and is commonly reported as a source of functional limitations in noncancer patients with cardiopulmonary disease.24 Thus, survivors completed a self-report of fatigue symptoms (Functional Assessment of Chronic Illness Therapy [FACIT] Fatigue Scale).25
Brain MRI examinations were obtained within 3 days of the httphhhneurocognitive testing and cardiac and pulmonary evaluations. MRI was conducted on a 3T Siemens Trio platform (Siemens, Iselin, NJ). T1-weighted fast low angle shot (FLASH) images were acquired with 3D T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE), and 3D T2 fluid attenuated inversion recovery (FLAIR). Proton density and T2-weighted images were acquired simultaneously with a dual spin-echo sequence. Susceptibility weighted images were obtained to detect evidence of prior intracranial hemorrhage (ie, hemosiderin deposits) and mineralization. 3D time-of-flight magnetic resonance angiography of the Circle of Willis and large arteries of the neck was obtained to evaluate for stenoses and aneurysms. All brain images were clinically examined and systematically coded for pathology by a board-certified neuroradiologist (N.D.S.), blinded to treatment history and medical examinations and neurocognitive outcomes. Coding was conducted for evidence of vasculopathy, aneurysms, white matter disease, lacunes, enlarged perivascular spaces, macrocysts, volume loss, and foci of susceptibility change, including hemosiderin deposits. The high-resolution T1-weighted MRI set was processed with FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) to assess brain cortical thickness, after registration to a common stereotactic space (Talairach) by using a modified version of the statistical parametric mapping 2 (SPM2) implementation of affine image coregistration. FreeSurfer software was used to align images, to correct radio-frequency inhomogeneities, to conduct brain extraction, and to segment cortical tissue. Segmentation was used to create pial and gray/white matter surfaces to calculate the perpendicular distance or thickness. Automated parcellation of the brain was conducted to extract thickness for specific frontal, parietal, temporal, and occipital regions. This process was also used to calculate total volume of right and left hippocampi.
Statistical Analyses
Descriptive measures were obtained for demographic and treatment characteristics, as well as cardiac, pulmonary, brain imaging, and neurocognitive outcomes. For brain imaging outcomes, leukoencephalopathy and hemosiderin deposits were defined as normal/abnormal on the basis of neuroradiologist coding, although cortical thickness values (focused on frontal, parietal, and temporal regions) obtained from the FreeSurfer program were used as continuous variables. For cardiovascular functions, hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or currently taking medication for hypertension; abnormal ejection fraction (EF) and diastolic function were defined as EF ≤ 55% and E/E′ ≥ 12; AV/MV regurgitation was defined as present/absent on the basis of cardiologist rating. For pulmonary functions, all variables (FVC, FEV1, total lung capacity, and DLcocorr) were classified as abnormal if they fell below 60% of the predicted value. Because this was a pilot study, no adjustment for multiple comparisons was made, and the findings should be interpreted only as exploratory and hypothesis generating.
The impact of radiation on brain imaging outcomes and cardiovascular/pulmonary functions was assessed by classifying survivors into two groups (those treated with less than 30 Gy and those treated with ≥ 30 Gy thoracic radiation) and comparing outcomes of interest by using the χ2 test for categorical variables and the two-sample t test for continuous measures. Performance on neurocognitive measures was transformed in age-adjusted z scores by using national normative data, and one-sample t tests were conducted to identify those measures that differed from an expected z = 0. Subsequent analyses were conducted only on those neurocognitive measures that demonstrated significantly lower values.
Associations between leukoencephalopathy/hemosiderin and neurocognitive measures were evaluated by using a two-sample t test in which neurocognitive measures were taken as continuous variables. Because no validated cutoffs exist for cortical thinning, we assessed the association by dichotomizing relevant neurocognitive measures as impaired (z < −1.0) or not and by using a two-sample t test to test whether mean cortical thickness differed between groups. Data for attention (span) and memory (short-term recall) were examined with respect to specific brain regions associated with those neurocognitive processes. In a similar manner, cardiovascular/pulmonary functions and relevant neurocognitive measures were evaluated by using two-sample t tests in which neurocognitive z scores were compared between the groups of survivors classified as normal/abnormal on cardiac and pulmonary measure. Finally, the functional implications of neurocognitive measures on college graduation and employment were examined by comparing the neurocognitive measures between survivors with versus without a college degree and survivors who were employed full time versus less than full time.
RESULTS
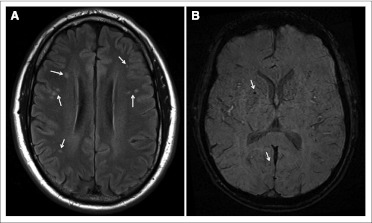
Table 1 presents demographic characteristics, treatment history, and medical examination results from the 62 survivors. Survivors demonstrated high rates of neuropathology on structural brain imaging, with leukoencephalopathy identified in 53% of survivors; common locations comprised frontal (35%) and parietal (13%) regions, including the centrum semiovale (30%). Hemosiderin deposits were identified in 37% of survivors; 34.5% of patients with leukoencephalopathy had hemosiderin deposits compared with 40% of those without leukoencephalopathy. Figure 2presents MRI images of two typical patients who demonstrated leukoencephalopathy and hemosiderin deposits. Of note is the pattern of multifocal leukoencephalopathy, suggestive of microvascular insults. Survivors with hemosiderin deposits had thinner cortices in dorsolateral frontal regions compared with those without cerebrovascular pathology (mean, 2.53 mm; standard deviation [SD], 0.12 v 2.64 mm; SD, 0.09, respectively; P = .006).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. of Patients | % | Mean | SD | Range |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex | |||||
| Male | 29 | 46.8 | |||
| Female | 33 | 53.2 | |||
| Race/ethnicity | |||||
| White | 55 | 88.7 | |||
| African American | 7 | 11.3 | |||
| Employed full time | |||||
| Yes | 52 | 83.9 | |||
| No | 10 | 16.1 | |||
| Current age, years | 62 | 42.2 | 4.77 | 34.4-55.4 | |
| Survivors' education, years | 62 | 14.4 | 2.52 | 9-20 | |
| Treatment characteristics | |||||
| Age at diagnosis, years | 62 | 15.1 | 3.30 | 5.9-19.0 | |
| Time since diagnosis, years | 62 | 27.1 | 5.65 | 18.3-39.6 | |
| Mantle field radiation dose, Gy | |||||
| < 30 | 24 | 20.6 | 1.70 | 19.2-26.3 | |
| ≥ 30 | 38 | 36.7 | 2.03 | 34.1-45.0 | |
| Doxorubicin, mg/m2 | |||||
| < 30 Gy group | 24 | 187.8 | 72.15 | 0-316 | |
| ≥ 30 Gy group | 38 | 19.1 | 52.88 | 0-205 | |
| Bleomycin, mg/m2 | |||||
| < 30 Gy group | 24 | 49.9 | 31.12 | 0-112 | |
| ≥ 30 Gy group | 38 | 4.5 | 14.57 | 0-70 | |
| Vital signs and measures | |||||
| BMI, kg/m2 | 62 | 27.3 | 5.67 | 17-41 | |
| Cholesterol, mg/dL | 62 | 198.1 | 31.80 | 131-279 | |
| HDL, mg/dL | 62 | 52.8 | 16.37 | 24-108 | |
| LDL, mg/dL | 62 | 118.3 | 29.55 | 54-180 | |
| Systolic blood pressure, mmHg | 62 | 123.0 | 12.71 | 94-158 | |
| Diastolic blood pressure, mmHg | 62 | 71.6 | 10.65 | 53-103 | |
| Cardiac functions | |||||
| Heart rate, bpm | 62 | 85.5 | 13.68 | 61-115 | |
| Left ventricular EF, % | 62 | 61.7 | 8.62 | 35-83 | |
| E/E′ | 62 | 10.8 | 4.67 | 4.8-33.9 | |
| Pulmonary functions | |||||
| FVC, % predicted | 62 | 79.8 | 15.52 | 40-109 | |
| FEV, % predicted | 62 | 78.9 | 16.53 | 28-111 | |
| TLC, % predicted | 62 | 81.6 | 13.19 | 56-112 | |
| DLCO, % predicted | 62 | 78.1 | 15.01 | 49-115 |
Abbreviations: BMI, body mass index; DLCO, diffusing capacity of lungs for carbon monoxide; E/E′, ratio of peak mitral flow velocity of early rapid filling (E) to early diastolic velocity of the mitral annulus (E′); EF, ejection fraction; FEV, forced expiratory volume; FVC, forced vital capacity; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation: TLC, total lung capacity.
Fig 2.
(A) Axial flair brain image of a 50-year-old white male diagnosed with Hodgkin lymphoma at age 18 years and treated with 45 Gy radiation to the mantle field. Image demonstrates multiple sites of leukoencephalopathy (arrows). At time of imaging, patient demonstrated pulmonary hypertension and left ventricular diastolic dysfunction by echocardiography. (B) Susceptibility-weighted image of 38-year-old white male diagnosed with Hodgkin lymphoma at age 6 years and treated with 35 Gy radiation to the mantle field. Image demonstrates foci of susceptibility (arrows). Phase filtering (not shown) confirms sites to be consistent with hemosiderin deposits. At time of imaging, patient demonstrated pulmonary hypertension and left ventricular diastolic dysfunction by echocardiography. No history of overt stroke was present in either patient's medical record.
Outcomes on clinical imaging and medical examinations were associated with radiation dose (Table 2). Survivors treated with ≥ 30 Gy mantle field radiation demonstrated higher rates of leukoencephalopathy (P = .02), higher E/E′ (P = .003), more prevalent AV and MV regurgitation (P = .04 and P = .001, respectively), lower FVC and FEV1 (P = .003 and P = .002, respectively), and lower DLcocorr (P = .04). Cardiac and pulmonary morbidities were associated with lower doses of doxorubicin (P = .005 for E/E′; P = .02 for DLcocorr), likely due to confounding between low doxorubicin dose and high mantle field radiation dose. Bleomycin dose was not related to any outcomes.
Table 2.
Clinical Imaging and Medical Outcome Data by Mantle Field Radiation
| Outcome | < 30 Gy |
≥ 30 Gy |
P* | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | % Abnormal | Mean | SD | % Abnormal | ||
| Brain imaging | |||||||
| Leukoencephalopathy | — | 34.8 | — | 64.5 | .02 | ||
| Hemosiderin deposits | — | 26.1 | — | 45.2 | .07 | ||
| Cardiovascular functions | |||||||
| Heart rate, bpm | 81.4 | 11.58 | 87.9 | 14.39 | .01 | ||
| Systolic blood pressure, mmHg | 120.8 | 11.38 | 124.3 | 13.42 | .30 | ||
| Diastolic blood pressure, mmHg | 71.9 | 9.89 | 71.4 | 11.21 | .86 | ||
| Hypertension | — | 33.3 | — | 26.3 | .55 | ||
| EF, % | 62.0 | 6.86 | 21.7 | 59.9 | 7.79 | 26.3 | .28 |
| E/E′ | 9.1 | 2.72 | 21.7 | 11.9 | 5.28 | 6.5 | .003 |
| AV regurgitation | — | 8.7 | — | 31.6 | .04 | ||
| MV regurgitation | — | 13.0 | — | 55·3 | .001 | ||
| Pulmonary functions | |||||||
| FVC, % predicted | 86.6 | 13.34 | 8.3 | 74.8 | 15.02 | 13.2 | .003 |
| FEV, % predicted | 86.4 | 13.27 | 8.3 | 73.5 | 16.56 | 18.9 | .002 |
| TLC, % predicted | 84.5 | 12.91 | 4.2 | 79.2 | 12.76 | 5.3 | .12 |
| DLCO, % predicted | 82.3 | 16.96 | 12.5 | 74.3 | 12.67 | 1.5 | .04 |
NOTE. % abnormal is the percent of the sample demonstrating abnormal outcomes on the following medical tests: brain imaging, present or absent; hypertension, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or currently taking medication for hypertension; ejection fraction (EF) ≤ 55 %; E/E′, ratio of peak mitral flow velocity of early rapid filling (E) to early diastolic velocity of the mitral annulus (E′) ≥ 11; aortic valve (AV) and mitral valve (MV) regurgitation, present or absent; and pulmonary function tests ≤ 60% predicted. (—), variables that were coded dichotomously; thus, means and standard deviations (SDs) are not available.
Abbreviations: DLCO, diffusing capacity of lungs for carbon monoxide; FEV, forced expiratory volume; FVC, forced vital capacity; TLC, total lung capacity.
P values ≤ .05 identified in bold.
Neurocognitive assessments revealed increased rates of impairment in multiple areas (Table 3). Survivors demonstrated poor sustained attention (P = .004), variability of attention (P = .01), and attention span (P = .01). Short-term memory recall was below expectations (P = .001), as was long-term recall (P = .006). Survivors demonstrated low performance on fine motor speed (P < .001) and naming speed (P < .001), and cognitive fluency was also reduced (P = .007). On standardized behavioral ratings, survivors reported problems with working memory (P < .001), task completion (P = .01), and fatigue (P < .001). Neurocognitive performance was associated with academic and vocational functioning. Compared with college graduates, survivors who did not graduate from college demonstrated lower attention span (P = .01), worse short-term memory (P = .001), and worse long-term memory (P = .004). Ten survivors (16.1%) reported being currently unemployed and, compared with employed survivors, demonstrated slower motor speed (P = .01) and more problems with working memory (P = .02), task efficiency (P = .03), and fatigue (P = .001).
Table 3.
Performance and Impairment Rates on Neurocognitive and Behavioral Outcomes
| Functional Outcome | Mean | SD | Range | % Impaired* | P† |
|---|---|---|---|---|---|
| Intelligence | 0.05 | 0.99 | −2.67-1.733 | 14.8 | .76 |
| Attention | |||||
| Selective | 0.43 | 0.89 | −2.47-1.67 | 8.2 | 1.00 |
| Sustained | −0.04 | 1.41 | −6.00-0.80 | 12.1 | .004 |
| Variability | −0.42 | 1.19 | −3.70-1.70 | 29.3 | .01 |
| Span | −0.18 | 0.91 | −1.86-1.81 | 27.9 | .01 |
| Memory | |||||
| New learning | −0.27 | 1.04 | −2.00-1.60 | 29.5 | .03 |
| Short-term recall | −0.41 | 0.97 | −3.00-1.50 | 45.9 | .001 |
| Long-term recall | −0.30 | 0.93 | −2.00-1.50 | 34.4 | .006 |
| Speed | |||||
| Fine motor dexterity | −0.59 | 1.02 | −4.00-1.33 | 33.3 | < .001 |
| Naming | −0.48 | 0.99 | −2.80-2.10 | 23.0 | < .001 |
| Reaction time | −0.28 | 1.60 | −3.40-3.00 | 43.1 | .10 |
| Executive function | |||||
| Cognitive flexibility | 0.09 | 1.02 | −3.80-1.47 | 14.8 | .96 |
| Cognitive fluency | −0.32 | 0.91 | −2.67-1.33 | 24.6 | .007 |
| Working memory | −0.25 | 0.73 | −1.97-1.56 | 13.1 | .03 |
| Behavior | |||||
| Inhibition | −0.22 | 0.95 | −2.80-1.30 | 21.0 | .06 |
| Flexibility | −0.31 | 1.04 | −3.40-1.10 | 24.2 | .02 |
| Emotional control | −0.30 | 1.27 | −3.90-1.20 | 32.3 | .07 |
| Self-monitor | 0.01 | 1.18 | −3.20-1.30 | 30.7 | .57 |
| Initiation | −0.22 | 1.27 | −3.70-1.30 | 27.4 | .26 |
| Working memory | −0.70 | 1.35 | −4.40-1.10 | 33.9 | < .001 |
| Planning | −0.27 | 1.26 | −4.10-1.10 | 27.4 | .10 |
| Task completion | −0.43 | 1.17 | −3.50-1.30 | 30.7 | .01 |
| Organization | 0.02 | 1.00 | −3.60-1.30 | 17.7 | .82 |
| Fatigue | 35.58 | 11.39 | 8.00-50.00 | 29.0 | < .001 |
Impairment defined as scores falling at least one standard deviation (SD) below the expected mean. P values based on one-sample t test of mean performance referenced to expected mean of 0 and SD of 1.
P values ≤ .05 identified in bold and correspond to functional outcomes used in subsequent analyses with brain imaging and cardiopulmonary functions.
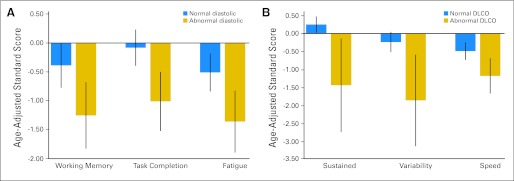
Neurocognitive function was not directly associated with mantle field radiation intensity (all P > .10), although it was associated with brain imaging as well as cardiac and pulmonary morbidity. Lower cognitive fluency was demonstrated in survivors with leukoencephalopathy in the centrum semiovale (mean z, −0.92; SD, 0.97) compared with those without leukoencephalopathy (mean z, −0.05; SD, 0.81; P < .002). Attention span and short-term memory were positively correlated with cortical thickness in frontal and temporal lobes, respectively (P = .03 for attention; P = .001 for memory). Survivors with impaired attention had reduced cortical thickness in dorsolateral frontal brain regions (P = .005), although survivors with impaired memory had reduced thickness in lateral temporal brain regions (P = .006; Appendix Fig A1, online only). E/E′ was significantly related to problems with working memory (P = .01), task completion (P = .002), and fatigue (P = .007; Fig 3). DLcocorr was associated with attention problems (P = .001 for sustained and variability) and slowed processing speed (P = .03; Fig 3).
Fig 3.
Neurocognitive performance in age-adjusted z scores (mean, 0; standard deviation, 1.0) as a function of cardiac and pulmonary morbidity. (A) Mean performance (and 95% CIs) for survivors with normal and abnormal cardiac diastolic function. (B) Mean neurocognitive performance (and 95% CIs) for survivors with normal and abnormal diffusing capacity of the lungs for carbon monoxide (DLCO). Significant group differences are identified for working memory (P = .01), task completion (P = .002), fatigue (P = .007), sustained and variability of attention (P = .001 for each), and processing speed (P = .03).
DISCUSSION
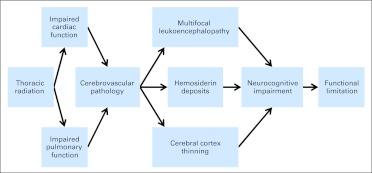
We report a new finding that long-term survivors of childhood HL are at risk for neurocognitive impairment and CNS pathology. HL survivors treated with thoracic radiation are at risk for cardiac and pulmonary morbidity, and such morbidity was related to dose of mantle field radiation exposure. Cardiac and pulmonary morbidity increased risk for cerebrovascular pathology, evidenced by increased prevalence of multifocal leukoencephalopathy and hemosiderin deposits. The rates of cardiac morbidity and leukoencephalopathy in this study are more than six-fold higher than those reported in community samples of comparable age.26,27 These previous studies in the Framingham cohort identified echocardiographic evidence of systolic dysfunction (ie, EF) in 3% and diastolic dysfunction in 4.8% compared with respective rates of 24% and 40% in this study. In terms of neuropathology, the Framingham report found MRI hyperintensities (ie, leukoencephalopathy) in just under 8% compared with 48% in this study. CNS pathology was associated with neurocognitive impairment. Neurocognitive impairment was associated with functional limitations, including lower educational attainment and unemployment. A theoretical model of the associations between these variables and neurocognitive outcomes is illustrated in Figure 4. Although chemotherapy agents may have a direct impact on brain function, doxorubicin and bleomycin doses used in our cohort did not increase risk, likely because of their inverse association with radiation intensity. To the best of our knowledge, these long-term functional deficits and CNS pathology have not been previously reported in this population.
Fig 4.
Theoretical model of process resulting in neurocognitive impairment and functional limitations in survivors of Hodgkin lymphoma who were treated with thoracic radiation. Although chemotherapy used to treat Hodgkin lymphoma agents may have a direct impact on brain function, the doses of doxorubicin and bleomycin used in our cohort did not increase such risk, likely because higher mantle field radiation intensity was associated with lower doxorubicin and bleomycin exposure. Direct CNS impact of chemotherapy agents should be investigated in survivors treated using modern protocols.
Cardiac and pulmonary morbidity may be related to cerebrovascular pathology through altered cerebral perfusion or reduced blood oxygenation. Impaired diastolic function, in conjunction with MV and AV regurgitation places individuals at increased risk for pulmonary hypertension, which can lead to restricted blood flow through the lungs and reduced blood oxygenation. Another indication of the association between decreased cardiac function and neurocognitive impairment is suggested by literature on brain natriuretic peptide (BNP). Plasma BNP is increased among patients with MV regurgitation.28 A recent study in 950 community-dwelling older adults demonstrated associations between BNP and neurocognitive function, controlling for age, sex, education, body mass index, cholesterol, and previous cardiac disease.29 In addition to impaired cardiac function, we found that reduced DLcocorr was associated with neurocognitive impairment. DLcocorr reflects the extent to which blood is oxygenated in the lungs.30 Reduced blood oxygenation or hypoxia has been demonstrated to increase cerebrovascular pressure,31 which may result in multifocal lesions in small cerebral arteries. This multifocal lesion pattern is consistent with the leukoencephalopathy observed in this cohort. Furthermore, the distribution of frontoparietal and centrum semiovale as predominant sites for leukoencephalopathy in this cohort is consistent with the pattern of vascular dementia seen in elderly hypertensive cohorts.32
The process of neurocognitive impairment in HL survivors is likely to occur at a later stage of development compared with that seen in survivors of CNS tumors and acute lymphoblastic leukemia. The later onset stems from the older age at diagnosis for most patients with childhood HL combined with the delayed onset of pathology, a factor that will reduce the impact on core academic skills because most survivors will have completed their primary and secondary education before the delayed onset of cardiac and pulmonary morbidity. Thus, neurocognitive impairment in HL is likely to have a negative impact during postsecondary education and employment, as was demonstrated in this study. This pattern may account for the fact that the neurocognitive deficits in long-term survivors of HL have gone unreported before this study.
This study is not without limitations. The relatively small sample size limited opportunities for multivariable modeling. The fact that all survivors received thoracic radiation did not permit examination of unique contributions from chemotherapeutic agents like anthracyclines or bleomycin. The mantle radiation treatment fields varied only in dose in this cohort, which did not allow the study of the effect of radiation to differing volumes of the heart, great vessels, and the carotids. The diversity in presence and location of neuropathology outcomes (ie, leukoencephalopathy and hemosiderin) limited the degree to which location-based specificity in neurocognitive impairment could be examined. In addition, an age- and sex-matched control group was not used, requiring rates of neurocognitive impairment to be determined on the basis of reference to age-adjusted national norms. Notwithstanding these limitations, this report includes the only sample of adult survivors of childhood HL comprehensively evaluated for CNS integrity to date, and it raises numerous questions that require larger cross-sectional and prospective studies to answer. Further recruitment of our cohort is planned to address some of these questions and permit more systemic examination of treatment factors and localization of brain pathology.
Survivors of HL treated with increasing doses of mantle field radiation are at risk for neurocognitive impairment that requires therapeutic interventions and, given the delayed onset, preventive interventions should be considered. Primary prevention has begun with the transition from mantle field radiation to modern protocols that incorporate combined-modality therapy with reduced dose, limited volume radiation, and polychemotherapy (eg, anthracyclines and bleomycin). Although this approach may reduce long-term morbidity, significant risk is likely to remain, given the known associations between anthracyclines and cardiac toxicity and bleomycin and pulmonary toxicity. Longitudinal follow-up of such cohorts will be important to fully evaluate long-term neurocognitive and CNS pathology outcomes.
Appendix
Fig A1.
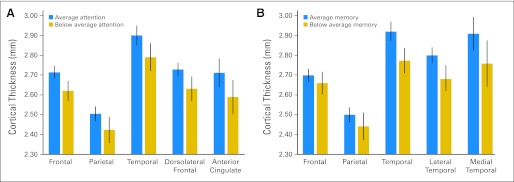
Cortical thickness in frontal, parietal, and temporal brain regions as a function of neurocognitive performance. (A) Mean thickness values and 95% CIs for survivors with average v below average attention span. (B) Mean thickness values and 95% CIs for survivors with average v below average short-term memory free recall. Significant group differences are identified in attention span for frontal (P = .005), parietal (P = .05), temporal (P = .02), and dorsolateral frontal (P = .005) regions. Significant differences in short-term memory were identified for temporal (P = .001), lateral temporal (P = .009), and medial temporal P = .05) regions.
Footnotes
Supported by Cancer Center Support (CORE) Grant No. CA21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
Presented, in part, at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00760656.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin R. Krull, Leslie L. Robison, Melissa M. Hudson
Financial support: Leslie L. Robison, Melissa M. Hudson
Administrative support: Leslie L. Robison, Melissa M. Hudson
Provision of study materials or patients: Melissa M. Hudson
Collection and assembly of data: Kevin R. Krull, Noah D. Sabin, Wilburn E. Reddick, Melissa M. Hudson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of Hodgkin's disease: A report from the Childhood Cancer Survivor Study. J Clin Oncol . 2005;23:6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer . 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 4.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: The Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 5.Debette S, Bauters C, Leys D, et al. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail . 2007;13:205–208. doi: 10.1111/j.1527-5299.2007.06612.x. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke . 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaffashian S, Dugravot A, Nabi H, et al. Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: Evidence from the Whitehall II study. Eur Heart J . 2011;32:2326–2332. doi: 10.1093/eurheartj/ehr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Reuck J, Proot P, Van Maele G. Chronic obstructive pulmonary disease as a risk factor for stroke-related seizures. Eur J Neurol . 2007;14:989–992. doi: 10.1111/j.1468-1331.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 9.Sachdev PS, Anstey KJ, Parslow RA, et al. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord . 2006;21:300–308. doi: 10.1159/000091438. [DOI] [PubMed] [Google Scholar]
- 10.Howlander N, Noone AM, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2011. SEER Cancer Statistics Review, 1975-2008. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 11.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev . 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 12.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol . 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr . 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J . 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med . 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. San Antonio, TX: Psychological Corporation; 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- 18.Wechsler D. San Antonio, TX: Psychological Corporation; 1997. Wechsler Adult Intelligence Scale-Third Edition. [Google Scholar]
- 19.Strauss E, Sherman EM, Spreen O. New York, NY: Oxford University Press; 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary (ed 3) [Google Scholar]
- 20.Conners CK. North Tonawanda, NY: Multi-Health Systems; 2001. Conners' Continuous Performance Test II. [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, et al. San Antonio, TX: Psychological Corporation; 2000. California Verbal Learning Test-Second Edition. [Google Scholar]
- 22.Roth RM, Isquith PK, Gioia GA. Lutz, FL: Psychological Assessment Resources; 2005. Behavior Rating Inventory of Executive Function-Adult Version. [Google Scholar]
- 23.Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS) Sleep . 2008;31:271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith OR, Michielsen HJ, Pelle AJ, et al. Symptoms of fatigue in chronic heart failure patients: Clinical and psychological predictors. Eur J Heart Fail . 2007;9:922–927. doi: 10.1016/j.ejheart.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA . 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 27.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke . 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kargın R, Esen O, Pala S, et al. The relationship between echocardiographic parameters and brain natriuretic peptide levels in acute and chronic mitral regurgitation. Turk Kardiyol Dern Ars . 2011;39:191–197. doi: 10.5543/tkda.2011.01273. [DOI] [PubMed] [Google Scholar]
- 29.Daniels LB, Laughlin GA, Kritz-Silverstein D, et al. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. Am J Med. 2011;124:670.e1–8. doi: 10.1016/j.amjmed.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes JM, Bates DV. Historical review: The carbon monoxide diffusing capacity (DLCO) and its membrane (DM) and red cell (Theta. Vc) components. Respir Physiol Neurobiol. 2003;138:115–142. doi: 10.1016/j.resp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Ursino M, Magosso E. Role of tissue hypoxia in cerebrovascular regulation: A mathematical modeling study. Ann Biomed Eng . 2001;29:563–574. doi: 10.1114/1.1380423. [DOI] [PubMed] [Google Scholar]
- 32.Jellinger KA. The pathology of “vascular dementia”: A critical update. J Alzheimers Dis. 2008;14:107–123. doi: 10.3233/jad-2008-14110. [DOI] [PubMed] [Google Scholar]