Abstract
Purpose
In acute myeloid leukemia (AML), initial treatment response by morphologic analysis of bone marrow predicts long-term outcome. Response can now be assessed by minimal residual disease (MRD) monitoring with flow cytometry or polymerase chain reaction (PCR). We determined the relation among the results of these approaches and their prognostic value.
Patients and Methods
In the multicenter AML02 study, follow-up bone marrow samples from 203 children and adolescents with newly diagnosed AML were examined by flow cytometry (n = 1,514), morphology (n = 1,382), and PCR amplification of fusion transcripts (n = 508). Results were correlated with treatment outcome.
Results
Among 1,215 samples with less than 5% leukemic myeloblasts by morphology, 100 (8.2%) were MRD positive (≥ 0.1%) by flow cytometry, whereas 96 (57.5%) of the 167 samples with ≥ 5% blasts were MRD negative. Virtually all (308 of 311; 99.0%) MRD-negative samples by PCR were also MRD negative by flow cytometry. However, only 19 (9.6%) of the 197 PCR-positive samples were flow cytometry positive, with analyses of AML1-ETO and CBFβ-MYH11 accounting for most discrepancies, whereas eight of 13 MLL-positive samples had detectable MRD by flow cytometry. MRD by flow cytometry after induction 1 or 2 predicted lower event-free survival and higher relapse rate (P < .001) and was an independent prognostic factor in a multivariable analysis; prediction was not improved by morphologic information or molecular findings.
Conclusion
In childhood AML, morphologic assessment of treatment response has limited value if MRD is measured by flow cytometry. MLL fusion transcripts can provide prognostic information in some patients, whereas monitoring of AML1-ETO and CBFβ-MYH11 transcripts is largely uninformative.
INTRODUCTION
In patients with acute leukemia, assessment of early treatment response predicts relapse hazard and is used for risk-directed therapy.1–6 Residual leukemic cells are traditionally identified morphologically, but this task is difficult owing to their similarity to normal hematopoietic cells. Methods to track leukemic cells based on flow cytometry or polymerase chain reaction (PCR) have the potential for superior sensitivity and accuracy; they allow the recognition of leukemic cells present at levels well below those detectable by morphology (ie, minimal residual disease [MRD]).1–6 In acute myeloid leukemia (AML), flow cytometric detection of MRD predicts outcome.7–13 Likewise, increases in levels of oncogenic fusion transcripts measured by PCR are reportedly associated with a higher probability of relapse in patients with specific genetic subtypes of AML.14–23 Our recent multi-institutional study of children and adolescents with AML (AML02) used flow cytometric assessments of MRD to guide therapy and resulted in high overall cure rates, suggesting that this approach can contribute to improving clinical management.24
The clinical utility of morphologic assessment of treatment response in the MRD era is unclear. Moreover, virtually all MRD studies in AML reported to date have relied on one single technique; hence, the relation between MRD results obtained by flow cytometry and those obtained by PCR is unknown. A better understanding of the relative prognostic strength of each approach is critical to the interpretation of the prognostic significance of early treatment response and designing of risk assignment strategies. We report here the results of flow cytometric monitoring of MRD in 1,514 bone marrow samples obtained from 203 patients enrolled onto the AML02 study during and after completion of therapy. We compared the results with those of morphology and PCR and correlated the results with treatment outcome.
PATIENTS AND METHODS
Patients and Treatment Protocol
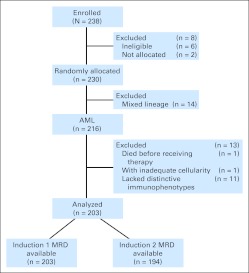
From October 2002 to June 2008, 216 patients with AML were enrolled onto the AML02 study at seven centers.24 Of these patients, one died before receiving therapy. Among the remaining 215, 11 (5.1%) lacked distinctive immunophenotypes at diagnosis for an MRD assay with at least 0.1% sensitivity, and one had follow-up samples with inadequate cellularity. Thus, the data reported are from 203 patients (Fig 1). The study was approved by the participating institutional review boards; informed consent was obtained from the patients or their parents or legal guardians, and assent was given by the patients, as appropriate.
Fig 1.
Outline of patient enrollment onto the study. AML, acute myeloid leukemia; MRD, minimal residual disease.
Cytogenetic analysis and reverse transcriptase-PCR to detect the presence of fusion transcripts were performed at diagnosis.8,25 The AML02 treatment protocol was previously reported24 and is summarized in the Data Supplement.
Morphologic Assessment of Treatment Response and Studies of MRD
The first bone marrow evaluation was performed on day 22 of induction 1. If more than 7 days passed between day 22 and blood count recovery (absolute neutrophils ≥ 300/μL; platelets ≥ 30,000/μL), a second evaluation was performed to determine response to induction 1. Additional evaluations were performed after each phase of treatment.
Bone marrow aspirate smears were examined for morphologic indication of disease. For flow cytometric detection of MRD, bone marrow aspirates were processed as previously described (Data Supplement).24 Patients with a fusion transcript at diagnosis were monitored by PCR (Data Supplement).
Statistical Analysis
Event-free survival (EFS) was defined as the time elapsed from study enrollment to induction failure, withdrawal, relapse, secondary malignancy, or death. The association between presenting features and MRD was assessed using Fisher's exact test with Monte Carlo simulation. The Kaplan-Meier method was used to estimate the probability of EFS, and SEs were determined by the method of Peto et al.26 Survival comparisons were performed by Mantel-Haenszel log-rank test.27 A multivariable analysis of EFS was performed using Cox proportional hazards regression, stratified by treatment arm.
RESULTS
Relation Between MRD by Flow Cytometry, Presenting Features, and Morphologic Assessment of Residual Disease
Among the 1,514 bone marrow samples studied, 202 (13.3%) had ≥ 0.1% mononucleated cells expressing leukemia-associated immunophenotypes determined at diagnosis. MRD was more prevalent during the early phases of treatment (Data Supplement). The proportion of samples with MRD ≥ 1% was also higher after induction 1 (54 of 86 samples) than after induction 2 (23 of 56; P = .016), After induction 1, MRD (≥ 0.1%) was more prevalent in samples with French-American-British M1 morphology (P = .001), normal karyotype (P < .001), and FLT3–internal tandem duplication (ITD; P < .001) and less prevalent in patients with inv(16) (P < .001); after induction 2, it was more prevalent in patients with FLT3-ITD (P < .001) and less frequent in patients with inv(16) (P = .003; Data Supplement).
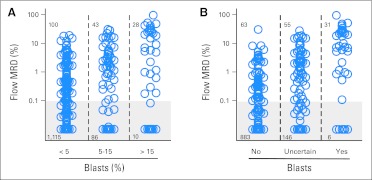
Data on cell morphology were available for 1,382 (91.3%) of the 1,514 samples from 202 (99.5%) of the 203 patients. We plotted flow cytometric MRD data against morphologic blast cell counts (Fig 2A). Twenty-eight (73.7%) of the 38 samples with > 15% myeloblasts were MRD positive by flow cytometry (median, 17.1%; range, 0.18% to 97.0%), and one had 0.08% of cells with an aberrant immunophenotype. MRD was positive in 43 (33.3%) of the 129 samples with 5% to 15% myeloblasts (median, 2.58%; range, 0.11% to 31%), with five additional samples containing 0.01% to 0.09% abnormal cells. Finally, 100 (8.2%) of the 1,215 samples with < 5% myeloblasts were also MRD positive (median, 0.66%; range, 0.1%–19.0%); an additional 44 samples had cells expressing abnormal immunophenotypes at < 0.1%, the threshold used to define MRD positivity by flow cytometry in this study (median, 0.03%; range, 0.01% to 0.09%).
Fig 2.
Relation between morphologic and flow cytometric detection of residual disease during and after treatment in childhood acute myeloid leukemia. (A) Percentage of bone marrow mononucleated cells expressing leukemia-associated immunophenotypes as measured by flow cytometry within groups defined by the percentage of myeloblasts counted by morphology. Shaded area encloses data points below the 0.1% threshold used to define positive minimal residual disease (MRD) in this study. (B) Flow cytometric MRD data within groups defined by hemopathologist judgment regarding the presence of leukemic myeloblasts.
We also compared hemopathologist assessments, available for 1,184 samples from 185 patients, regarding the presence of leukemic myeloblasts with flow cytometric results (Fig 2B). Of the 37 samples deemed to contain leukemic myeloblasts by morphology, 31 were MRD positive by flow cytometry (median, 17.9%; range, 0.11% to 97.0%), whereas six samples contained only immunophenotypically normal maturing myeloid cells. Of the 946 samples with no detectable leukemic myeloblasts by morphology, 63 (6.7%) were MRD positive by flow cytometry (median, 0.49%; range, 0.1% to 31%), and an additional 27 samples had immunophenotypically abnormal cells at < 0.1% (median, 0.04%; range, 0.01% to 0.09%). Finally, a substantial proportion of samples (n = 201; 17.0%) were difficult to interpret morphologically, and no conclusion could be made with regard to the presence of leukemic blasts; 55 (27.4%) of these were MRD positive by flow cytometry (median, 2.1%; range, 0.1% to 28.4%); an additional 12 samples contained immunophenotypically abnormal cells at < 0.1% (median, 0.04%; range, 0.01% to 0.09%). Thus, a substantial number of samples with no morphologic evidence of disease or with uncertain morphology contained leukemic cells, whereas some samples with cells appearing morphologically as myeloblasts lacked immunophenotypic abnormalities and were most likely normal myeloid progeniors.
MRD by PCR and Comparison With Flow Cytometry
We evaluated MRD by PCR in patients whose leukemic cells at diagnosis contained AML1-ETO (n = 31), CBFβ-MYH11 (n = 24), MLL fusion transcripts (n = 22: MLL-AF9, n = 12; MLL-AF10, n = 5; MLL-ENL, n = 4, MLL-ELL, n = 1), and RBM15-MKL1 (n = 3; Data Supplement). Of the 1,514 samples studied by flow cytometry, 508 (33.6%) were also studied by PCR.
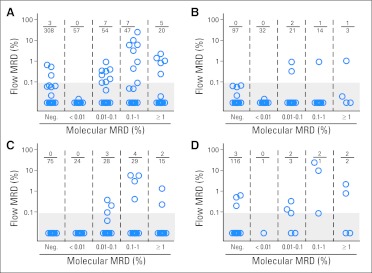
Of the 311 samples classified as MRD negative by PCR, 308 (99.0%) were also MRD negative by flow cytometry (Fig 3A; Data Supplement). However, in three samples from two patients (one with MLL-AF9; one with MLL-AF10), flow cytometry detected 0.21%, 0.53%, and 0.66% mononucleated cells with an aberrant immunophenotype identical to the one determined at diagnosis. In an additional five MRD-negative samples by PCR, MRD was detected by flow cytometry but < 0.1% (median, 0.06%; range, 0.02% to 0.07%). Surprisingly, only 19 (9.6%) of the 197 samples regarded as MRD positive by PCR were also MRD positive by flow cytometry (median, 0.92%; range, 0.1% to 25.1%); an additional eight samples were positive by flow cytometry < 0.1% (median, 0.04%; range, 0.01% to 0.09%). Thus, 170 (86.3%) of 197 samples with detectable fusion transcripts by PCR did not have detectable leukemic cells by flow cytometry. Even when we restricted the analysis to samples with ≥ 0.1% MRD by PCR (79 of 197), the proportion of those containing AML cells by flow cytometry (including at < 0.1%) remained low: 16 (20.3%) of 79.
Fig 3.
Relation between molecular and flow cytometric detection of residual disease during and after treatment in childhood acute myeloid leukemia. Percentage of bone marrow mononucleated cells expressing leukemia-associated immunophenotypes as measured by flow cytometry within groups of samples with different levels of minimal residual disease (MRD) according to analysis of (A) fusion transcripts (AML1-ETO, CBFβ-MYH11, MLL-AF9, MLL-AF10, MLL-ENL, MLL-ELL, or RBM15-MKL1) by polymerase chain reaction. Results obtained in subgroups defined by (B) AML1-ETO, (C) CBFβ-MYH11, and (D) MLL transcripts are also shown. Shaded areas enclose data points below the 0.1% threshold used to define positive MRD by flow cytometry in this study. Samples collected at any time point during and after treatment were included.
Analyses of AML1-ETO or CBFβ-MYH11 transcripts accounted for most discrepancies between PCR and flow cytometric results. Of the 179 samples that were MRD positive by PCR in this group, only 13 (7.3%) were MRD positive by flow cytometry, and four also had abnormal cells < 0.1% (Figs 3B and 3C); among the 69 that were ≥ 0.1% by PCR, only eight (11.6%) had detectable AML cells by flow cytometry. There was a better correlation in samples positive for the presence of MLL fusion transcripts (Fig 3D); six of these 13 samples were MRD positive by flow cytometry, including four of the seven that were ≥ 0.1% by PCR. In analyses limited to samples collected at the end of induction 1, there was agreement between flow cytometry and PCR on MLL fusion transcripts (all four positive samples had abnormal cells detected by flow cytometry) but not with PCR on the other transcripts.
Relation Between Treatment Response by Different Approaches and Outcome
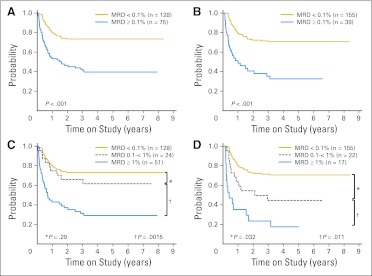
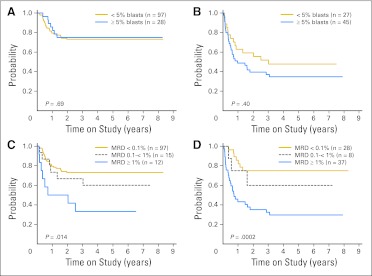
Table 1 shows the results of univariate analyses relating EFS with treatment response after induction 1 or 2 as measured by the three methods (ie, flow cytometry, morphology, and PCR). In line with a previous report,24 this updated analysis confirmed the prognostic importance of flow cytometric measurements of MRD; EFS rates were significantly worse in patients with MRD ≥ 1% after induction 1 and/or ≥ 0.1% after induction 2 (Table 1; Figs 4A to 4D). A majority of events were leukemia relapses (Data Supplement).
Table 1.
Univariate Analysis of EFS According to Flow Cytometric, Morphologic, and Molecular Analysis of Residual Disease
| Residual Disease Measurement | Induction 1 |
Induction 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | 5-Year EFS | ± SD | P | No. of Patients | 5-Year EFS | ± SD | P | |
| Flow cytometry, % | < .001 | < .001 | ||||||
| ≥ 1 | 51 | 29.3 | 8.2 | 17 | 17.6 | 16.0 | ||
| 0.1 to < 1 | 24 | 61.8 | 14.4 | 22 | 44.6 | 13.6 | ||
| < 0.1 | 128 | 73.4 | 6.1 | 155 | 70.7 | 5.6 | ||
| Blasts, % | .016 | .058 | ||||||
| ≥ 5 | 73 | 50.2 | 9.5 | 23 | 47.8 | 14.1 | ||
| < 5 | 124 | 67.4 | 6.1 | 168 | 65.6 | 5.7 | ||
| Morphology | < .001 | .001 | ||||||
| Yes | 23 | 29.0 | 14.1 | 7 | 14.3 | 13.2 | ||
| Uncertain | 76 | 59.8 | 8.3 | 19 | 66.3 | 14.5 | ||
| No | 81 | 74.0 | 7.1 | 120 | 75.4 | 5.8 | ||
| PCR (all transcripts), % | .75 | .67 | ||||||
| ≥ 1 | 7 | 71.4* | 38.2 | 4 | 100 | 0 | ||
| 0.1 to < 1 | 13 | 84.6 | 19.2 | 11 | 81.8 | 15.6 | ||
| < 0.1 | 35 | 85.7 | 8.4 | 51 | 82.3 | 8.7 | ||
| CBF, % | .23 | .78 | ||||||
| ≥ 1 | 7 | 71.4* | 38.2 | 4 | 100 | 0 | ||
| 0.1 to < 1 | 9 | 100 | 0 | 10 | 90 | 12.7 | ||
| < 0.1 | 20 | 90 | 9.0 | 31 | 87.1 | 9.9 | ||
| MLL, % | .009 | .059 | ||||||
| ≥ 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0.1 to < 1 | 2 | 0† | 0 | 1 | 0† | 0 | ||
| < 0.1 | 14 | 78.6 | 16.3 | 17 | 70.6 | 17.1 | ||
Abbreviations: CBF, AML1-ETO and CBFβ-MYH11 transcripts; EFS, event-free survival; PCR, polymerase chain reaction; SD, standard deviation.
Four-year EFS is shown, because no patient in this group was observed for 5 years.
Patients died within 1 year.
Fig 4.
Event-free survival (EFS) for patients with acute myeloid leukemia included in this study according to minimal residual disease (MRD) by flow cytometry. EFS according to presence (≥ 0.1%) or absence (< 0.1%) of MRD (A) after induction 1 and (B) after induction 2. EFS according to levels of MRD (C) after induction 1 and (D) after induction 2.
Detection of ≥ 5% myeloblasts in bone marrow smears by morphology was generally associated with EFS (P = .016 after induction 1; P = .058 after induction 2; Table 1). Qualitative assessment based on pathologist impression regarding the presence or absence of residual disease was significantly correlated with EFS (P < .001 after induction 1; P = .001 after induction 2; Table 1). We determined whether morphologic assessment could refine the prognostic value of flow cytometric measurements of MRD. Percentage of myeloblasts by morphology did not affect the relation between MRD by flow cytometry and treatment outcome (Figs 5A and 5B), nor did assessment of the presence of blasts (Data Supplement). By contrast, MRD determined by flow cytometry was a significant predictor of relapse regardless of the morphologic result (Figs 5C and 5D).
Fig 5.
Relation between event-free survival (EFS) for patients with acute myeloid leukemia included in this study according to flow cytometry and morphology results after induction 1. EFS of patients (A) who were minimal residual disease (MRD) negative (< 0.1%) by flow cytometry according to percentage of blasts by morphology, (B) who were MRD positive (≥ 0.1%) by flow cytometry according to percentage of blasts by morphology, (C) with < 5% blasts by morphology according to MRD levels by flow cytometry, and (D) with ≥ 5% blasts by morphology according to MRD levels by flow cytometry.
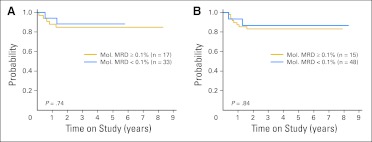
MRD measurements by PCR, when considered collectively using the same MRD cutoff levels used for flow cytometry, were not significantly related to EFS (Table 1). Likewise, a lower cutoff level (0.01%) did not yield significant correlations with outcome (P = .63 after induction 1; P = .73 after induction 2). Moreover, detection of MRD by PCR did not identify a subset of patients with worse outcome among patients for whom MRD negativity was identified through flow cytometry, whether using the 0.1% (Figs 6A and 6B) or 0.01% cutoff (Data Supplement). Only three of the 55 patients with core-binding factor abnormalities (31 with AML1-ETO; 24 with CBFβ-MYH11) in this series relapsed (Data Supplement). One patient with AML1-ETO relapsed 1 year after diagnosis; MRD had been negative by both PCR and flow cytometry up to 3 months before relapse. In another patient with the CBFβ-MYH11 gene fusion, PCR MRD levels rose from 0.005% during follow-up to 1% 10 months after the end of therapy; at this time, MRD also became detectable by flow cytometry (0.24%), and morphologic relapse occurred 2 months later. The third patient with CBFβ-MYH11 had an isolated CNS relapse before the third consolidation; at the last time point at which MRD was measured in the bone marrow (6 weeks before relapse), it was undetectable by flow cytometry and was 0.1% by PCR. Thirty-five of the 55 patients (all in complete remission at the time of this analysis) had detectable transcripts (estimated MRD levels: 0.0005% to 10%) beyond completion of induction 2, which persisted after completion of therapy in 24 patients. One additional patient with CBFβ-MYH11 had persistent molecular MRD after initiation of treatment; MRD became detectable by flow cytometry (3.2%) 8 months after the end of therapy, and the patient underwent allogeneic hematopoietic cell transplantation, after which MRD became undetectable by both PCR and flow cytometry.
Fig 6.
Event-free survival (EFS) among patients who were minimal residual disease (MRD) negative (< 0.1%) by flow cytometry at the end of (A) induction 1 or (B) induction 2 according to levels of fusion transcripts (AML1-ETO, CBFβ-MYH11, MLL-AF9, MLL-AF10, MLL-ENL, MLL-ELL, or RBM15-MKL1) above or below the 0.1% threshold. Analyses using the 0.01% threshold are shown in the Data Supplement. Mol., molecular.
The relation between MLL transcript detection and relapse was different than that observed for AML1-ETO and CBFβ-MYH11. Of 16 patients studied after induction 1, two had MRD > 0.1%, and both relapsed within 1 year, whereas relapse occurred in three of the 14 patients with MRD lower or undetectable at this time point (P = .009). The cutoff level with the highest predictive power for MLL fusion transcripts was 0.05% after induction 1 (log-rank P = .012) and after induction 2 (P = .063). Of note, one MLL-AF10 patient had detectable MRD by flow cytometry (0.53%) after induction 2 but no detectable MLL-AF10 transcripts at that time point and relapsed 3 months after the end of therapy. None of the three patients with RBM15-MKL1 relapsed, despite positive PCR findings in two patients at the end of induction 1 (0.1% and 0.5%) and in one patient at the end induction 2 (0.05%).
We performed a multivariable analysis including measurements of treatment response by flow cytometry and by morphology as well as all presenting features previously shown to be associated with adverse outcome in this cohort: absence of AML1-ETO or CBFβ-MYH11 abnormalities, M7 AML without t(1;22), and FLT3-ITD.24 MRD ≥ 0.1% by flow cytometry remained a significant predictor of outcome (P = .014 after induction 1; P = .013 after induction 2). Presence of ≥ 5% blasts by morphology after induction 1 was not a significant predictor (P = .39), whereas it was after induction 2 (P = .042). Of the other factors, only absence of AML1-ETO or CBFβ-MYH11 abnormalities remained significant (P < .01; Data Supplement).
DISCUSSION
In patients with AML, response to initial treatment is used to determine intensity of subsequent therapy and to select candidates for allogeneic stem-cell transplantation.24,28–33 Therefore, uncertainties regarding the relative predictive value of different approaches to measure response can affect clinical management, particularly when the results are apparently contradictory. In this study, we compared data obtained by morphologic examination of bone marrow smears, flow cytometric analysis of aberrant immunophenotypes, and PCR amplification of oncogenic fusion transcripts. We found a poor correlation among the three methods overall. A considerable proportion of samples with no clear blasts by morphology or uncertain morphology had leukemic cells by flow cytometry, whereas most samples with residual AML1-ETO or CBFβ-MYH11 transcripts had flow cytometry–negative results. MRD levels by flow cytometry strongly correlated with clinical outcome. Most patients with leukemic blasts identifiable unequivocally by morphology also had high levels of MRD by flow cytometry and had a high relapse hazard. Outcome prediction by PCR MRD was mixed; detection of MLL fusion transcripts predicted relapse, whereas AML1-ETO and CBFβ-MYH11 transcripts persisted in many patients who remained in long-term remission.
Our results illustrate the limitations of assessing treatment response by morphology; 17% of the samples had uninterpretable morphology, and 9.5% of those considered to be leukemia free had leukemic cells by flow cytometry, exceeding 1% in several cases. Grouping by percentage of myeloblasts using the 5% cutoff, a strategy used for risk classification in many studies,34,35 was significantly related to EFS when assessed after induction 1 but did not add useful prognostic information to flow cytometric MRD classification. Conventional cytogenetic analysis may help clarifying the nature of myeloblasts, particularly when these are present in relatively high percentages, and it has been shown to provide prognostic information.36 However, the sensitivity of this approach (approximately 5%) is limited, as compared with that of flow cytometry.
Previous studies of adult patients with AML have shown that quantitative PCR studies of AML1-ETO and CBFβ-MYH11 can identify patients at a higher risk of relapse, particularly when high transcript levels are detected during consolidation therapy or later.14–23 However, Lane et al37 observed that levels of these transcripts after induction and consolidation chemotherapy were not predictive of outcome, but a ≥ 1 log10 rise correlated with inferior leukemia-free survival, suggesting that MRD monitoring by this approach may be informative only if sequential measurements are performed. The basis for the poor relation between detection of AML1-ETO and CBFβ-MYH11 transcripts, flow cytometric results, and relapse in our study is unclear. Conceivably, low levels of MRD by PCR (undetectable by flow cytometry) could be controlled by subsequent chemotherapy or immune reconstitution and hence fail to predict relapse. Alternatively, the presence of fusion transcripts could result from the persistence of preleukemic cells or from partially differentiated leukemic cells that have lost not only their aberrant immunophenotypic features but also their clonogenic potential.
The prognostic value of MRD monitoring targeting MLL-rearranged transcripts in AML has not been extensively studied. Scholl et al38 studied 19 patients and observed that the 11 with consistently negative PCR findings had lower relapse rates than the remaining eight. Among the 16 patients with MLL transcripts whom we studied at induction 1, the two who had MRD > 0.1% relapsed within 1 year. By contrast, 13 of the 18 patients with MLL-AML with persistently negative PCR MRD after induction 2 are still in remission at the time of this report. Although there was a much better correlation between flow cytometric results and detection of MLL transcripts as compared with AML1-ETO and CBFβ-MYH11 transcripts, this was not absolute; six of 13 MLL-positive samples were MRD positive by flow cytometry, whereas two additional samples had cells just below the 0.1% threshold; conversely, three of the 119 PCR-negative samples were positive by flow cytometry.
There are other potential targets for molecular studies of MRD in AML that were not used in this study. The Wilms tumor gene (WT1) is highly expressed in most acute leukemias, and its detection in bone marrow has been associated with relapse,39–42 but the background of normal bone marrow cells with WT1 expression limits the applicability of this assay.43 Another potential target for molecular studies is FLT3-ITD, which, in line with a previous study,44 was present in 13% of patients in our cohort. However, it has been reported that this marker may not be stable during the course of disease,45,46 reducing its value in MRD monitoring. Finally, nucleophosmin mutations are a promising target for MRD monitoring,47 but less than 10% of children with AML have this abnormality.48
In sum, our study shows that measurements of treatment response by flow cytometry are widely applicable and provide strong prognostic information; in the subset of patients with MLL gene abnormalities, monitoring of MLL fusion transcripts is also clinically useful. By contrast, the value of morphologic monitoring is limited, and PCR results of AML1-ETO and CBFβ-MYH11 are difficult to interpret. We would argue that the use of such tests to select treatment strategies should be undertaken with caution or could be abandoned altogether, particularly if a reliable flow cytometric assay is available.
Supplementary Material
Acknowledgment
We thank Soheil Meshinchi, MD, PhD (Fred Hutchinson Cancer Research Center, Seattle, WA), and Gladstone Airewele, MD (Texas Children's Cancer Center, Houston, TX), for entering patients in AML02; Christopher Clark, Laura Key, Peixin Liu, and Mo Mehrpooya for technical assistance with flow cytometric studies; and Kathy Jackson (St Jude Children's Research Hospital, Memphis, TN), Christopher Brawley and Alyson Falwell (Stanford Cancer Center, Stanford, CA), and Kathy Contreras (Cook Children's Medical Center, Fort Worth, TX) for help in the collection of morphology data.
Footnotes
Supported by Grants No. R01 CA115422, R25 CA23944, and P30 CA21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00136084.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Hiroto Inaba, Elaine Coustan-Smith, Stanley B. Pounds, Jeffrey E. Rubnitz, Dario Campana
Financial support: Hiroto Inaba, James R. Downing, Ching-Hon Pui, Dario Campana
Administrative support: Raul C. Ribeiro, James R. Downing, Ching-Hon Pui
Provision of study materials or patients: Hiroto Inaba, Elaine Coustan-Smith, Sheila A. Shurtleff, Susana C. Raimondi, Mihaela Onciu, Raul C. Ribeiro, Gary V. Dahl, W. Paul Bowman, Jeffrey W. Taub, Barbara Degar, Wing Leung, James R. Downing, Jeffrey E. Rubnitz
Collection and assembly of data: Hiroto Inaba, Elaine Coustan-Smith, Sheila A. Shurtleff, Kathleen Y. Wang, Dario Campana
Data analysis and interpretation: Hiroto Inaba, Elaine Coustan-Smith, Xueyuan Cao, Stanley B. Pounds, Dario Campana
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Brüggemann M, Schrauder A, Raff T, et al. Standardized MRD quantification in European ALL trials: Proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010;24:521–535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- 3.Buccisano F, Maurillo L, Spagnoli A, et al. Monitoring of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol. 2009;21:582–588. doi: 10.1097/CCO.0b013e3283311856. [DOI] [PubMed] [Google Scholar]
- 4.Kern W, Bacher U, Haferlach C, et al. The role of multiparameter flow cytometry for disease monitoring in AML. Best Pract Res Clin Haematol. 2010;23:379–390. doi: 10.1016/j.beha.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Shook D, Coustan-Smith E, Ribeiro RC, et al. Minimal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma. 2009;9(suppl 3):S281–S285. doi: 10.3816/CLM.2009.s.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimwade D, Vyas P, Freeman S. Assessment of minimal residual disease in acute myeloid leukemia. Curr Opin Oncol. 2010;22:656–663. doi: 10.1097/CCO.0b013e32833ed831. [DOI] [PubMed] [Google Scholar]
- 7.Sievers EL, Lange BJ, Alonzo TA, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: Results from a prospective Children's Cancer Group study of 252 acute myeloid leukemia patients. Blood. 2003;101:3398–3406. doi: 10.1182/blood-2002-10-3064. [DOI] [PubMed] [Google Scholar]
- 8.Coustan-Smith E, Ribeiro RC, Rubnitz JE, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- 9.Langebrake C, Creutzig U, Dworzak M, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: The MRD-AML-BFM Study Group. J Clin Oncol. 2006;24:3686–3692. doi: 10.1200/JCO.2005.05.4312. [DOI] [PubMed] [Google Scholar]
- 10.van der Velden VH, Sluijs-Geling A, Gibson BE, et al. Clinical significance of flow cytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–1606. doi: 10.1038/leu.2010.153. [DOI] [PubMed] [Google Scholar]
- 11.San Miguel JF, Vidriales MB, López-Berges C, et al. Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood. 2001;98:1746–1751. doi: 10.1182/blood.v98.6.1746. [DOI] [PubMed] [Google Scholar]
- 12.Kern W, Voskova D, Schoch C, et al. Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood. 2004;104:3078–3085. doi: 10.1182/blood-2004-03-1036. [DOI] [PubMed] [Google Scholar]
- 13.Maurillo L, Buccisano F, Del Principe MI, et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol. 2008;26:4944–4951. doi: 10.1200/JCO.2007.15.9814. [DOI] [PubMed] [Google Scholar]
- 14.Marcucci G, Livak KJ, Bi W, et al. Detection of minimal residual disease in patients with AML1/ETO-associated acute myeloid leukemia using a novel quantitative reverse transcription polymerase chain reaction assay. Leukemia. 1998;12:1482–1489. doi: 10.1038/sj.leu.2401128. [DOI] [PubMed] [Google Scholar]
- 15.Tobal K, Newton J, Macheta M, et al. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood. 2000;95:815–819. [PubMed] [Google Scholar]
- 16.Guerrasio A, Pilatrino C, De Micheli D, et al. Assessment of minimal residual disease (MRD) in CBFbeta/MYH11-positive acute myeloid leukemias by qualitative and quantitative RT-PCR amplification of fusion transcripts. Leukemia. 2002;16:1176–1181. doi: 10.1038/sj.leu.2402478. [DOI] [PubMed] [Google Scholar]
- 17.Viehmann S, Teigler-Schlegel A, Bruch J, et al. Monitoring of minimal residual disease (MRD) by real-time quantitative reverse transcription PCR (RQ-RT-PCR) in childhood acute myeloid leukemia with AML1/ETO rearrangement. Leukemia. 2003;17:1130–1136. doi: 10.1038/sj.leu.2402959. [DOI] [PubMed] [Google Scholar]
- 18.Marcucci G, Caligiuri MA, Döhner H, et al. Quantification of CBFbeta/MYH11 fusion transcript by real time RT-PCR in patients with INV(16) acute myeloid leukemia. Leukemia. 2001;15:1072–1080. doi: 10.1038/sj.leu.2402159. [DOI] [PubMed] [Google Scholar]
- 19.Buonamici S, Ottaviani E, Testoni N, et al. Real-time quantitation of minimal residual disease in inv(16)-positive acute myeloid leukemia may indicate risk for clinical relapse and may identify patients in a curable state. Blood. 2002;99:443–449. doi: 10.1182/blood.v99.2.443. [DOI] [PubMed] [Google Scholar]
- 20.van der Reijden BA, Simons A, Luiten E, et al. Minimal residual disease quantification in patients with acute myeloid leukaemia and inv(16)/CBFB-MYH11 gene fusion. Br J Haematol. 2002;118:411–418. doi: 10.1046/j.1365-2141.2002.03738.x. [DOI] [PubMed] [Google Scholar]
- 21.Leroy H, de Botton S, Grardel-Duflos N, et al. Prognostic value of real-time quantitative PCR (RQ-PCR) in AML with t(8;21) Leukemia. 2005;19:367–372. doi: 10.1038/sj.leu.2403627. [DOI] [PubMed] [Google Scholar]
- 22.Perea G, Lasa A, Aventín A, et al. Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [t(8;21) and inv(16)] Leukemia. 2006;20:87–94. doi: 10.1038/sj.leu.2404015. [DOI] [PubMed] [Google Scholar]
- 23.Corbacioglu A, Scholl C, Schlenk RF, et al. Prognostic impact of minimal residual disease in CBFB-MYH11–positive acute myeloid leukemia. J Clin Oncol. 2010;28:3724–3729. doi: 10.1200/JCO.2010.28.6468. [DOI] [PubMed] [Google Scholar]
- 24.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukemia: Results of the AML02 multicenter trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raimondi SC, Chang MN, Ravindranath Y, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: Clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999;94:3707–3716. [PubMed] [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 28.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: Results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 29.Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: The AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- 30.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children's Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: Results of the MRC AML12 trial. J Clin Oncol. 2010;28:586–595. doi: 10.1200/JCO.2009.22.9088. [DOI] [PubMed] [Google Scholar]
- 33.Löwenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364:1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 34.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial: United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 35.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Cortes J, Estrov Z, et al. Persistence of cytogenetic abnormalities at complete remission after induction in patients with acute myeloid leukemia: Prognostic significance and the potential role of allogeneic stem-cell transplantation. J Clin Oncol. 2011;29:2507–2513. doi: 10.1200/JCO.2010.34.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane S, Saal R, Mollee P, et al. A ≥ 1 log rise in RQ-PCR transcript levels defines molecular relapse in core binding factor acute myeloid leukemia and predicts subsequent morphologic relapse. Leuk Lymphoma. 2008;49:517–523. doi: 10.1080/10428190701817266. [DOI] [PubMed] [Google Scholar]
- 38.Scholl C, Schlenk RF, Eiwen K, et al. The prognostic value of MLL-AF9 detection in patients with t(9;11)(p22;q23)-positive acute myeloid leukemia. Haematologica. 2005;90:1626–1634. [PubMed] [Google Scholar]
- 39.Bergmann L, Miething C, Maurer U, et al. High levels of Wilms' tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217–1225. [PubMed] [Google Scholar]
- 40.Cilloni D, Gottardi E, Fava M, et al. Usefulness of quantitative assessment of the WT1 gene transcript as a marker for minimal residual disease detection. Blood. 2003;102:773–774. doi: 10.1182/blood-2003-03-0980. [DOI] [PubMed] [Google Scholar]
- 41.Weisser M, Kern W, Rauhut S, et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia. 2005;19:1416–1423. doi: 10.1038/sj.leu.2403809. [DOI] [PubMed] [Google Scholar]
- 42.Lapillonne H, Renneville A, Auvrignon A, et al. High WT1 expression after induction therapy predicts high risk of relapse and death in pediatric acute myeloid leukemia. J Clin Oncol. 2006;24:1507–1515. doi: 10.1200/JCO.2005.03.5303. [DOI] [PubMed] [Google Scholar]
- 43.Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: A European LeukemiaNet study. J Clin Oncol. 2009;27:5195–5201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 44.Meshinchi S, Stirewalt DL, Alonzo TA, et al. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood. 2008;111:4930–4933. doi: 10.1182/blood-2008-01-117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kottaridis PD, Gale RE, Langabeer SE, et al. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: Implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100:2393–2398. doi: 10.1182/blood-2002-02-0420. [DOI] [PubMed] [Google Scholar]
- 46.Shih LY, Huang CF, Wu JH, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: A comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 47.Krönke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: A study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 48.Brown P, McIntyre E, Rau R, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–985. doi: 10.1182/blood-2007-02-076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.