Abstract
OBJECTIVE
Aminophylline is a methylxanthine with multiple physiologic actions. At low doses, aminophylline can antagonize adenosine and improve renal function via increased glomerular filtration rate. Despite its clinical use, little data exists in neonates for this indication. Therefore, the objective of this report is to describe the impact of aminophylline on renal function indices in a series of neonates with acute renal failure.
MATERIALS AND METHODS
This was a retrospective chart review of 13 neonates with acute renal failure who received aminophylline during a 15-month study period. Aminophylline was administered at 1 mg/kg intravenously or orally every twelve hours. Forty-six percent (n = 6) of the patients received a 5 mg/kg loading dose before initiation of maintenance therapy. Most patients had already received other treatments for renal failure, including diuretics and dopamine.
RESULTS
Resolution of acute renal failure (with normalization of serum creatinine and blood urea nitrogen) was documented in 10 patients (77%). Four of the thirteen patients died from complications due to their prematurity. Failure of low-dose aminophylline was observed in 3 of the 4 patients who died.
CONCLUSIONS
Low-dose aminophylline in neonates with acute renal failure is associated with an improvement in renal function indices.
Keywords: aminophylline, neonates, oliguria, renal failure, theophylline
INTRODUCTION
Acute renal failure is defined as a sudden decrease in glomerular filtration rate (GFR) that results in the progressive retention of creatinine and nitrogenous waste products and the inability to regulate fluid and electrolyte homeostasis. Acute renal failure is common in the neonatal intensive care unit (NICU), occurring in 8% to 24% of severely ill newborns. Despite significant progress in understanding the pathophysiology and management of acute renal failure, the mortality rate in neonates is still very high (33%–78%).1–4
Low-dose dopamine is commonly used in the NICU in an attempt to preserve renal function.1,3–11 According to a survey among all 19 NICUs and pediatric intensive care units in the Netherlands, dopamine is regularly used either to improve renal function (n = 7) or to enhance diuresis (n = 13).5 However, a systematic review of the literature revealed that there are few published studies in premature infants and neonates, and the approaches used in these studies differed considerably.5 Clinical trials have suggested that dopamine may actually have detrimental effects despite promoting increased urine output.6–10 In fact, based on the physiology of the kidney, dopamine may actually worsen tubular injury by impairing renal oxygen kinetics and inhibiting feedback mechanisms designed to protect the kidney from ischemia.1,6–10 Furthermore, the variability in dopamine clearance, and diminished clearance observed with renal function impairment, may predispose patients to unanticipated toxic effects.11 Despite this literature, a 2006 survey of neonatal fellowship program directors revealed that over 50% continue to use dopamine to improve renal function in neonates with elevated serum creatinine.12 Theophylline has been reported to improve renal function in neonates.13–18 Published experience with low-dose aminophylline, however, is limited, and is primarily focused on term or near-term neonates. This report presents a case series of primarily preterm neonates treated with aminophylline to reverse renal failure.
METHODS
All neonates who received aminophylline for treatment of renal failure in the NICU at Women's Hospital between January 2004 and April 2006 were included. Prior to this time, aminophylline had only been used in our NICU in larger doses with target concentrations of 5 to 16 mg/L (27.5 to 88.8 μmol/L) for treatment of apnea of prematurity or ventilator weaning. However, micromolar concentrations are needed for renal benefits in animal studies, so lower concentrations can be targeted.
This retrospective chart review was approved by the Moses Cone Health System Institutional Review Board and exempted from requiring parental informed consent. Patients were identified through an electronic database, and their charts were retrospectively reviewed. Eligibility criteria included any neonate with a diagnosis of renal impairment or acute renal failure who was treated with low-dose aminophylline for this indication. No exclusion criteria existed.
A patient was considered to have acute renal failure if either the serum creatinine (SCr) was above 1.5 mg/dL (132.6 μmol/L) or if the SCr rose by 0.3 mg/dL/day (26.5 μmol/L/day) for 2 consecutive days. Oliguria was defined as a urine output below 1 mL/kg/hr for at least 24 hours. Low-dose aminophylline was administered using a maintenance dose of 1 mg/kg every 12 hours. Doses were administered using a programmable syringe pump over 30 minutes through microbore tubing followed by 1.7 mL of flush solution. The decision to administer an initial 5 mg/kg loading dose was at the discretion of the attending neonatologist.
Demographic data included gestational age, postnatal age, sex, birth weight, Apgar scores, and umbilical cord pH. Data collected from the patient chart included the following: aminophylline dosage; markers of theophylline adverse effects (e.g., tachycardia, irritability, or feeding intolerance); renal function markers including blood urea nitrogen (BUN), SCr, and total urine output; factors that could influence renal function markers (i.e., daily protein intake and total daily fluid intake); and the dosages of other concurrent medications with renal effects (specifically indomethacin, dopamine, furosemide, glucocorticoids, antibiotics, and caffeine). These data were documented before, during, and after aminophylline therapy. Response was defined as decline in SCr to less than 1.5 mg/dL (132.6 μmol/L) or decline by 20%. In cases where BUN was elevated but serum creatinine was below 1.5 mg/dL (132.6 μmol/L), response was measured by decline of BUN to 25 mg/dL or below. Statistical analysis was not performed since this is primarily a case series with a limited number of patients.
RESULTS
During a 15-month period, 17 neonates were treated with aminophylline to increase renal perfusion. Because 4 of these neonates were treated prophylactically in anticipation of subsequent renal failure, they were not included in the analysis. Thirteen patients were eligible for inclusion and their outcomes are summarized in Tables 1 and 2. Acute renal failure resolved in 10 neonates. One of these neonates died from pulmonary hemorrhage and septic shock several weeks after resolution of renal failure. All 4 of the neonates that did not respond to aminophylline died. The causes of death included pulmonary hemorrhage, septic shock, severe respiratory distress, and necrotizing enterocolitis.
Table 1.
Individual patient information about demographics, concurrent medications, aminophylline therapy, and renal response to therapy.
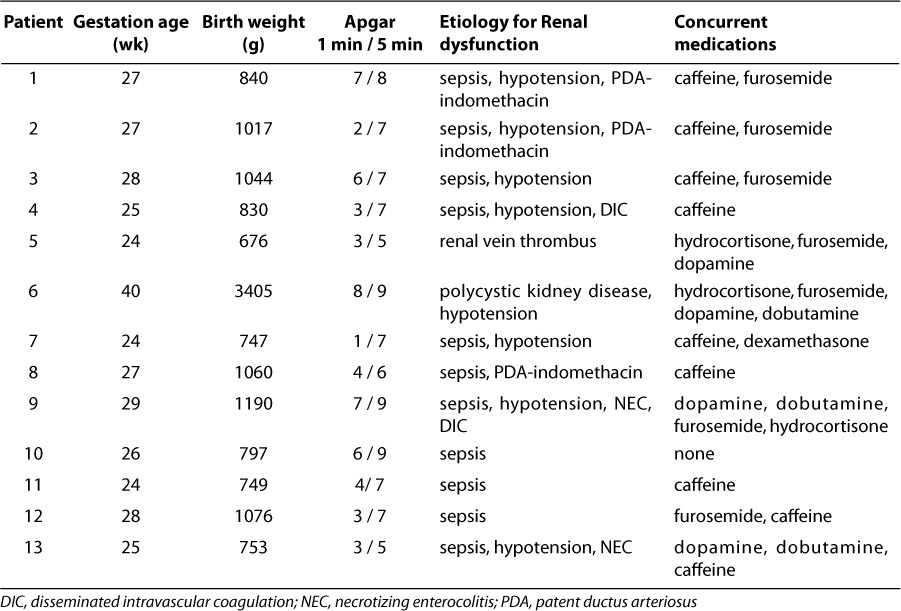
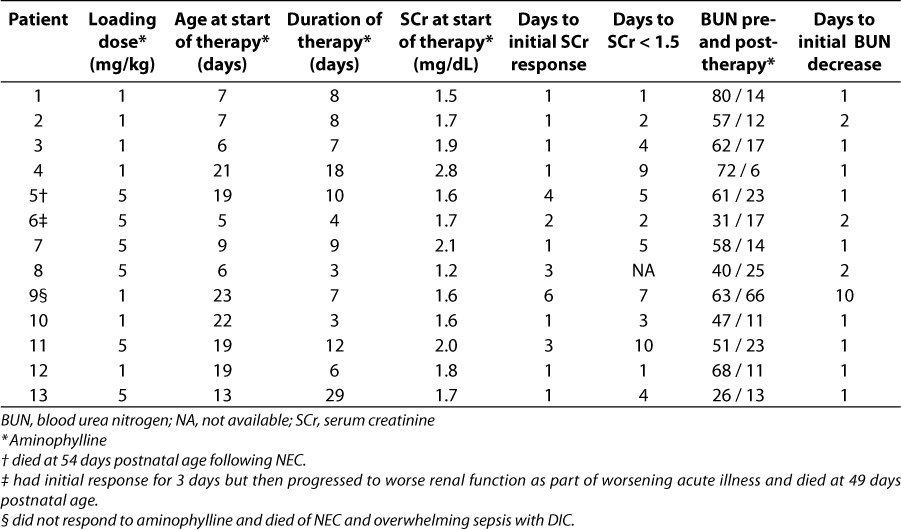
Table 2.
Summary of aminophylline treatment, response, and patient outcomes.
Patient summary data, including demographics, renal failure etiologies, and concurrent medications that could have influenced measures of renal function (SCr, BUN, urine output) can be found in Table 1. There were 2 female and 11 male neonates with gestational ages ranging from 24–40 weeks. Twelve patients were premature, with birth weight and gestational age below 1100 g and 30 weeks, respectively. Umbilical cord pH was normal in all neonates (greater than 7.2).
Table 2 summarizes details about the aminophylline treatment and response in each patient. All patients received a maintenance dose of 1 mg/kg orally or intravenously every 12 hours. Six patients received an initial loading dose of 5 mg/kg. Treatment was started between 5 and 23 days postnatal age, and lasted from 3 to 29 days. Most patients began to respond with decreasing SCr values after 1 or 2 days of treatment. Of the 12 patients with initial SCr above 1.5 mg/dL, all had SCr decrease to less than 1.5 mg/dL at some point during treatment with aminophylline. Although they were still receiving aminophylline, SCr increased again in 3 patients, and because of this increase during therapy, these patients were considered non-responders. All 3 of these patients subsequently died. BUN also decreased in 12 of 13 patients. Mean BUN for the total group decreased from 55 mg/dL (19.6 mmol/L) to 19 mg/dL (6.8 mmol/L).
To compare dosing strategies, the number of days to initial response in SCr and BUN was stratified into those patients who received a 5 mg/kg loading dose and those who did not receiving an initial loading dose. It took a mean of 1 day for the SCr to fall in responders when a 1 mg/kg initial dose was given compared to 2.3 days when a 5 mg/kg loading dose was used. Regardless of whether the initial dose was 1 mg/kg or 5 mg/kg, the BUN values began to decline after 1 or 2 days of aminophylline in all responders. Based on these results, a case cannot be made that a loading dose will accelerate response.
Concurrent medications known to produce renal effects were also given in all but one patient (Table 1). The 11 patients with sepsis were treated with a combination of vancomycin, gentamicin, and piperacillin/tazobactam before and during aminophylline therapy. Vancomycin and gentamicin were dosed using individualized pharmacokinetic parameters to optimize response and minimize toxicity. Ongoing treatment with these antibiotics did not seem to compromise renal function or reduce response to aminophylline. A total of 8 patients received caffeine concomitantly, 7 received furosemide, 3 were given hydrocortisone, 3 received dobutamine, and 1 received dexamethasone. Four patients were treated with indomethacin at some time prior to aminophylline administration, and, for 3 of these, indomethacin treatment may have played a partial role in causing renal dysfunction. Nine patients were treated with dopamine before beginning treatment with aminophylline. In all but two neonates, dopamine infusion rates were consistently above our usual infusion dose rate for “renal effects” (2 μg/kg/min). All of our patients had nonoliguric renal failure.
DISCUSSION
Premature infants are born with very low GFR, maintained by a delicate balance of intrarenal vasoconstrictor and vasodilator forces. The low GFR, although sufficient for growth and development under normal circumstances, limits renal functional adaptation to exogenous and endogenous stresses. The newborn kidney is thus very vulnerable to injury; the majority of cases of renal impairment are due to decreased renal perfusion. Such hypoperfusion may result from hypoxia, sepsis, nephrotoxic medications, or a combination of factors.1–3
The diagnosis of acute renal failure in neonates is less precise because the SCr at birth reflects maternal concentrations, which subsequently declines over 2–3 weeks in preterm infants. Measurement of creatinine clearance in neonates is cumbersome and unreliable; therefore, it is customary to follow renal function by measuring repeated SCr concentrations.1,3 Most investigators consider newborns to have acute renal failure when the SCr is 1.5 mg/dL (132.6 μmol/L) or higher, increases at least 0.3 mg/dL (26.5 μmol/L) daily for 2 days, or fails to decrease in the first week of life.2 Acute renal failure can also be defined in neonates as oliguric or nonoliguric. The definition of oliguria in a neonate is less than 1 mL/kg/hr or lack of urine output by 48 hours of age.2 Nonoliguric renal failure in neonates has a much better prognosis than oliguric renal failure.
The goals of treatment include correction of hypovolemia, hypoxia, hypotension, oliguria, and any other precipitating factors and electrolyte abnormalities. Low-dose dopamine, furosemide, and fluid boluses are often given during treatment.1,3,5–11 In hypotensive premature neonates, even relatively small doses of dopamine (2 μg/kg/minute) increase mean arterial pressure, probably because of the high sensitivity of α–receptors in these preterm infants.2 Although little is known about dopamine pharmacokinetics in critically ill infants and children, substantial interindividual variation in dopamine serum concentrations has been observed. In fact, dopamine concentrations could not be predicted accurately from dopamine infusion rates in critically ill neonates. Dopamine clearance tends to be substantially lower in critically ill patients with impaired renal function.11 If conventional treatment fails, additional or alternative therapies are needed to prevent irreversible kidney damage.
Aminophylline has been used in varying dosages for prevention or treatment of acute renal failure or for diuresis in newborn infants.13–22 These applications have been stimulated by a growing understanding of the role of adenosine in neonatal renal physiology,23–25 and supported by animal studies examining renal effects of theophylline and other methylxanthines.13,25–28 Experiments in laboratory animals clearly show that adenosine acts as a vasoconstrictive metabolite in the kidney. The kidneys produce and release adenosine during hypoxia, ischemia, and renal vasoconstriction, contributing to a fall in GFR and filtration fraction.29–31 Aminophylline improves renal function, most likely through its action as a nonspecific adenosine receptor antagonist. In various animal models, theophylline has been successfully employed to improve renal function after induction of acute renal failure.13,25–28 The protective effects of aminophylline in animal studies occurred at micromolar plasma theophylline concentrations that are not high enough to inhibit cyclic AMP phosphodiesterase.26,31 Consequently, low serum theophylline concentrations can be expected to be clinically effective, justifying the low-dose approach. Not all xanthines produce this renal arteriolar vasodilation effect seen with theophylline. Enprofylline, a xanthine derivative with low adenosine antagonistic properties, has no effect on hypoxic renal dysfunction in animal studies.13,28 In premature infants, studies have demonstrated beneficial effects of theophylline, but most focused on prevention of acute renal failure or improving diuresis rather than increased glomerular filtration rate.13,16–22 A review of the literature revealed 2 neonatal studies that could be considered similar to the purposes and endpoints of this case series.17,22 A report of 6 critically ill newborns with respiratory distress syndrome and acute renal failure documented the effects of a one-time 1 mg/kg dose of aminophylline on urinary water excretion and creatinine clearance. Five out of 6 neonates had significant improvements in both markers.22 In randomized trials using different low-dose theophylline regimens to prevent renal dysfunction following perinatal asphyxia, a marked reduction in renal involvement was observed, typically from 55% to 60% of controls to 17% to 25% of theophylline-treated patients.14–16 These patients were term or post-term newborns and aminophylline doses were administered within the first few hours of life. Prevention of vasomotor nephropathy due to respiratory distress syndrome in pre-term infants was also successful in preterm infants treated with theophylline.18 Neonates ≤ 32 weeks gestation with respiratory distress syndrome were administered theophylline 1 mg/kg/day or placebo on day 1 of life. Urine output and SCr were better in the theophylline-treated patients, and oliguria occurred in 33% of controls versus 5% of theophylline-treated patients on day 1 of life. These reports represent efforts at prevention of renal dysfunction in high risk neonates. Our case series involves treatment of patients already suffering from renal dysfunction due to multiple concurrent etiologies. The cases described by Huet et al.17 are more consistent with the application of theophylline therapy in our case series. In the 6 critically ill cases with renal dysfunction, all received only a single dose of theophylline 1 mg/ kg with improved renal function, but 5 patients subsequently died of causes unrelated to renal failure. Our case series describes a sustained benefit from theophylline. This was demonstrated by worsening renal function in several patients when theophylline was discontinued, and a rapid return of beneficial effects upon restarting theophylline (Case 3 in Figure 1, and the case in Figure 2).
Figure 1.
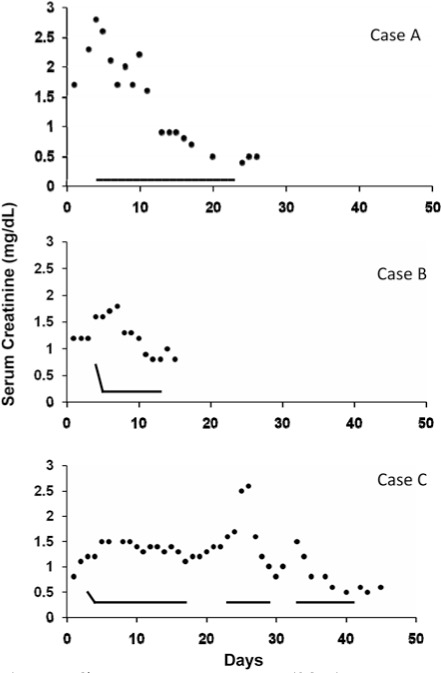
Change in serum creatinine (SCr; •) in 3 patients given aminophylline (—). Case A received 1 mg/kg every 12 hr without a loading dose. Cases B and C received a 5 mg/kg loading dose followed by a maintenance dose of 1 mg/kg every 12 hr. The onset of effect did not appear to be accelerated by a loading dose. Case C also demonstrated the rapid loss of benefit from aminophylline when discontinued prematurely, and rapid return of benefit when restarted without a loading dose. Days indicated on the x-axis are not postnatal age, but represent several days to document pretreatment serum creatinine trend and then impact during and after aminophylline treatment.
Figure 2.
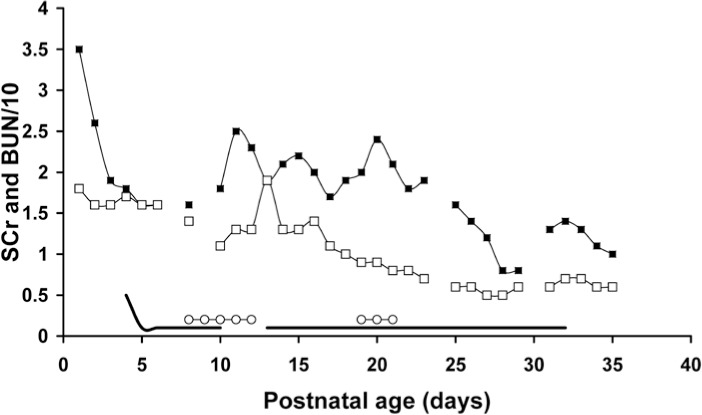
This case demonstrates the benefit of aminophylline on renal function and the apparent loss of these benefits after discontinuing aminophylline, despite treatment with caffeine for apnea at that time. Restarting aminophylline renewed the renal benefits even when caffeine was used concurrently. Days indicated on the x-axis are not postnatal age, but represent several days to document pretreatment SCr trend and then impact during and after aminophylline treatment.
▪ = BUN; □ =SCr; — = aminophylline; ○ = caffeine
A potential limitation to our case series is the variability of neonatologists in diagnosing renal failure and impairment. Six neonatologists were involved in the care of the 13 patients included in this case series. For the purposes of this report, we employed criteria that are commonly used in the literature to define neonatal renal impairment. Renal response to aminophylline can be seen by decreasing SCr, decreased BUN, and increased urine output. However, none of the patients that responded to treatment could be considered oliguric before or during treatment with aminophylline, therefore, we focused on SCr and BUN values.
While maintenance therapy was dosed consistently, almost half of the patients received a 5 mg/kg bolus before maintenance therapy. Although our sample size was small, SCr and BUN clearly did not decline more rapidly with a loading dose. It seems likely that there is no advantage to giving a loading dose before initiating aminophylline at 1 mg/kg every 12 hours. This is summarized for each patient in Table 1, and exemplified in Figure 1 with two patients who received aminophylline 5 mg/kg loading doses and one patient who did not.
Concomitant drug therapies may have also influenced the positive results seen. Furosemide and dopamine were used in several patients before and during aminophylline treatment. Clinicians often confuse an increase in diuresis with an improvement in renal function.14 Other medications that may have confounded our results include indomethacin, as it may impair renal function, and glucocorticoids, which may increase BUN.
Studies in rabbits suggested both theophylline and caffeine have beneficial renal effects.27 For this reason, we monitored caffeine use in our patients. However, no differences were seen in neonates who received caffeine concurrently compared to those who did not. Interestingly, when we replaced theophylline with caffeine, renal function markers worsened, but rapidly improved after theophylline was restarted (Figure 2). Despite our assumption that caffeine and theophylline would have similar benefits, caffeine at concentrations of 20 to 30 mg/L (103–155 μmol/L) did not produce the expected benefits.
No relationship between adverse effects and aminophylline could be identified. Patients were monitored for tachycardia, electrolyte imbalances, hypertension, feeding intolerance, irritability, and seizures. Most adverse effects reported in the literature occur at theophylline serum concentrations of 15 mg/L (88.8 μmol/L) or greater. Because our neonates received small doses of aminophylline we did not routinely monitor serum theophylline concentrations. On the rare occasion when trough theophylline concentrations were measured, the values were always less than 5 mg/L (27.8 μmol/L). Thus, the absence of toxicity was not surprising.
CONCLUSION
Given the safety and response to aminophylline in our case series, a prospective controlled trial is warranted to evaluate low-dose aminophylline maintenance therapy as a first-line therapy for neonates with compromised renal function.
ACKNOWLEDGMENTS
Presented at the Pediatric Pharmacy Advocacy Group NICU/PICU Specialty Meeting, Baltimore, Maryland, on April 1, 2006 and at the Southeastern Residency Conference, Athens, Georgia, on April 28, 2006.
ABBREVIATIONS
- BUN
blood urea nitrogen
- GFR
glomerular filtration rate
- NICU
neonatal intensive care unit
- SCr'
serum creatinine
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Moghal NE, Embleton ND. Management of acute renal failure in the newborn. Semin Fetal Neonatal Med. 2006;11:207–213. doi: 10.1016/j.siny.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Churchill PC, Bidani AK. Hypothesis: adenosine mediates hemodynamic changes in renal failure. Med Hypotheses. 1982;8:275–285. doi: 10.1016/0306-9877(82)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Chua AN, Sarwal MM. Acute renal failure management in the neonate. NeoReviews. 2005;6:e369–e375. [Google Scholar]
- 4.Andreoli SP. Acute renal failure in the newborn. Semin Perinatol. 2004;28:112–123. doi: 10.1053/j.semperi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Prins I, Plötz FB, Uiterwaal CS, van Vught HJ. Low-dose dopamine in neonatal and pediatric intensive care: a systematic review. Intensive Care Med. 2001;27:206–210. doi: 10.1007/s001340000775. [DOI] [PubMed] [Google Scholar]
- 6.Lauschke A, Teichgraber UKM, Eckardt K-U. “Low-dose” dopamine worsens renal perfusion in patients with acute renal failure. Kidney International. 2006;69:1669–1674. doi: 10.1038/sj.ki.5000310. [DOI] [PubMed] [Google Scholar]
- 7.Rice BA, Tanski MC. The case against renal dose dopamine in the pediatric intensive care unit. AACN Clinical Issues. 2005;16:246–251. doi: 10.1097/00044067-200504000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Schenarts PJ, Sagraves SG, Bard MR. Low-dose dopamine: A physiologically based review. Curr Surg. 2006;63:219–225. doi: 10.1016/j.cursur.2005.08.008. et al. [DOI] [PubMed] [Google Scholar]
- 9.Carcoana OV, Hines RL. Is renal dose dopamine protective or therapeutic? Yes. Crit Care Clin. 1996;12:677–685. doi: 10.1016/s0749-0704(05)70271-2. [DOI] [PubMed] [Google Scholar]
- 10.Jones D, Bellomo R. Renal-dose dopamine: from hypothesis to paradigm to dogma to myth and, finally, superstition? J Intensive Care Med. 2005;20:199–211. doi: 10.1177/0885066605276963. [DOI] [PubMed] [Google Scholar]
- 11.Zaritsky A, Lotze A, Stull R, Goldstein DS. Steady-state dopamine clearance in critically ill infants and children. Crit Care Med. 1988;16:217–220. doi: 10.1097/00003246-198803000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Amin SB, Handley C, Carter-Pokras O. Indomethacin use for management of patent ductus arteriosus in preterms: A web-based survey of practice attitudes among neonatal fellowship directors in the United States. Pediatr Cardiol. 2007;28:193–200. doi: 10.1007/s00246-006-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osswald H, Gleiter C, Muhlbauer B. Therapeutic use of theophylline to antagonize renal effects of adenosine. Clin Nephrol. 1995;43:S33–S37. [PubMed] [Google Scholar]
- 14.Jenik AG, Cernadas JM, Gorenstein A. A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics. 2000;105:e45. doi: 10.1542/peds.105.4.e45. et al. [DOI] [PubMed] [Google Scholar]
- 15.Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia—a study in a developing country. Pediatr Nephrol. 2005;20:1249–1252. doi: 10.1007/s00467-005-1980-z. [DOI] [PubMed] [Google Scholar]
- 16.Bhat MA, Shah ZA, Makhdoomi MS, Mufti MH. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr. 2006;149:180–184. doi: 10.1016/j.jpeds.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 17.Huet F, Semama D, Grimaldi M. Effects of theophylline on renal insufficiency in neonates with respiratory distress syndrome. Intensive Care Med. 1995;21:511–514. doi: 10.1007/BF01706205. et al. [DOI] [PubMed] [Google Scholar]
- 18.Cattarelli D, Spandrio M, Gasparoni A. A randomised, double blind, placebo controlled trial of the effect of theophylline in prevention of vasomotor nephropathy in very preterm neonates with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2006;91:80–84. doi: 10.1136/adc.2005.073650. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazkereth R, Laufer J, Jordan S. Effects of theophylline on renal function in premature neonates. Am J Perinatol. 1997;14:45–49. doi: 10.1055/s-2007-994095. et al. [DOI] [PubMed] [Google Scholar]
- 20.Lochan S, Adeniyi-Jones S, Assad FK. Coadministration of theophylline enhances diuretic response to furosemide in infants during extracorporeal membrane oxygenation: a randomized controlled pilot study. J Pediatr. 1998;133:86–89. doi: 10.1016/s0022-3476(98)70183-0. et al. [DOI] [PubMed] [Google Scholar]
- 21.Bell M, Jackson E, Mi Z. Low-dose theophylline increases urine output in diuretic-dependent critically ill children. Intensive Care Med. 1998;24:1099–1105. doi: 10.1007/s001340050723. et al. [DOI] [PubMed] [Google Scholar]
- 22.Ng GYT, Baker EH, Farrer KFM. Aminophylline as an adjunct diuretic for neonates-a case series. Pediatr Nephrol. 2005;20:220–222. doi: 10.1007/s00467-004-1692-9. [DOI] [PubMed] [Google Scholar]
- 23.Drukker A, Guignard JP. Renal aspects of the term and preterm infant: a selective update. Curr Opin Pediatr. 2002;14:175–182. doi: 10.1097/00008480-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Tóth-Heyn P, Drukker A, Guignard JP. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol. 2000;14:227–239. doi: 10.1007/s004670050048. [DOI] [PubMed] [Google Scholar]
- 25.Gouyon JB, Guignard JP. Functional renal insufficiency: role of adenosine. Biol Neonate. 1988;53:237–242. doi: 10.1159/000242796. [DOI] [PubMed] [Google Scholar]
- 26.Gouyon JB, Guignard JP. Theophylline prevents the hypoxemia-induced renal hemodynamic changes in rabbits. Kidney Int. 1988;33:1078–1083. doi: 10.1038/ki.1988.114. [DOI] [PubMed] [Google Scholar]
- 27.Gouyon JB, Guignard JP. Renal effects of theophylline and caffeine in newborn rabbits. Pediatr Res. 1987;21:615–618. doi: 10.1203/00006450-198706000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Gouyon JB, Arnaud M, Guignard JP. Renal effects of low-dose aminophylline and enprofylline in newborn rabbits. Life Sci. 1988;42:1271–1278. doi: 10.1016/0024-3205(88)90220-2. [DOI] [PubMed] [Google Scholar]
- 29.Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens. 2003;12:497–502. doi: 10.1097/00041552-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Hansen PB, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal Physiol. 2003;285:F590–F599. doi: 10.1152/ajprenal.00051.2003. [DOI] [PubMed] [Google Scholar]
- 31.Gouyon JB, Guignard JP. Adenosine in the immature kidney. Dev Pharmacol Ther. 1989;13:113–119. doi: 10.1159/000457592. [DOI] [PubMed] [Google Scholar]


