Abstract
OBJECTIVE
The purpose of this study is to retrospectively assess the incremental value of contrast-enhanced MRI (CE-MRI) to T2-weighted MRI in the detection of postsurgical local recurrence of prostate cancer by readers of different experience levels, using biopsy as the reference standard.
MATERIALS AND METHODS
Fifty-two men with biochemical recurrence after prostatectomy underwent 1.5-T endorectal MRI with multiphase contrast-enhanced imaging and had biopsy within 3 months of MRI. Two radiologists (reader 1 had 1 year and reader 2 had 6 years of experience) independently reviewed each MRI study and classified the likelihood of recurrent cancer on a 5-point scale. Areas under receiver operating characteristic curves (Az) were calculated to assess readers’ diagnostic performance with T2-weighted MRI alone and combined with CE-MRI. Interobserver agreement was assessed using Cohen kappa statistics.
RESULTS
Thirty-three patients (63%) had biopsy-proven local recurrence of prostate cancer. With the addition of CE-MRI to T2-weighted imaging, the Az for cancer detection increased significantly for reader 1 (0.77 vs 0.85; p = 0.0435) but not for reader 2 (0.86 vs 0.88; p = 0.7294). The use of CE-MRI improved interobserver agreement from fair (κ = 0.39) to moderate (κ = 0.58).
CONCLUSION
CE-MRI increased interobserver agreement and offered incremental value to T2-weighted MRI in the detection of locally recurrent prostate cancer for the relatively inexperienced reader.
Keywords: contrast-enhanced MRI, locally recurrent prostate cancer, MRI, prostate cancer, prostatectomy
Radical prostatectomy (RP) is a common form of treatment of localized prostate cancer [1–3]. Studies with long-term follow-up have shown that up to 40% of men who undergo RP will experience recurrent disease manifesting initially as an increasing serum prostate-specific antigen (PSA) level [4, 5]. Detecting the site of prostate cancer recurrence after RP is critical for developing an appropriate treatment strategy. Local recurrence can potentially be cured by radiotherapy, whereas distant metastasis requires systemic therapy [6].
Although PSA is not specific for the diagnosis of prostate cancer, after RP surgery, PSA should decrease to an undetectable level (< 0.1 ng/mL) [7]. A detectable PSA level after RP can be due to residual benign glands, extraprostatic PSA production in epithelial cells, or recurrent cancer [8–10]. The most commonly used definition for biochemical recurrence after RP is a PSA value of 0.2 ng/mL or greater followed by a further increase in PSA [11–13]. In clinical practice, if a patient’s PSA level increases after RP, prostate cancer recurrence is suspected and a clinical workup is initiated. Imaging plays a critical role in distinguishing between local recurrence and distant spread of disease [14, 15]. Although CT and bone scintigraphy are used to identify distant metastases in the lymph nodes and bones, respectively, transrectal ultrasound or endorectal MRI is used to identify local recurrence [16]. Biopsy of the prostatic fossa is not routinely recommended, especially during early phase relapse with low PSA values (< 0.5 ng/mL), because of a low detection rate [17–19].
The use of endorectal MRI has gained clinical acceptance and plays an important role in the detection of local recurrence in the prostatectomy bed [20–24]. Studies have shown that detection of local recurrence of prostate cancer on MRI can be improved by the addition of contrast-enhanced MRI (CE-MRI) [20, 21, 23, 24]. However, the studies were performed using single or consensus readings with various reference standards. As a result, little is known about the impact of reader experience on the value of combining T2-weighted imaging with CE-MRI or about the effect of such a combined protocol on interobserver variability. In addition, the optimal way of integrating findings from CE-MRI and T2-weighted imaging and the diagnostic performance of protocols that combine the two techniques need further evaluation. Thus, the purpose of our study was to assess the incremental value of CE-MRI to T2-weighted MRI in the detection of postsurgical local recurrence of prostate cancer by readers of different experience levels, using biopsy as the reference standard.
Materials and Methods
Patient Population
This retrospective study was approved by our institutional review board with a waiver of informed consent. We reviewed the medical records and radiologic databases for the period from March 2005 to December 2008 to identify all patients who met the following inclusion criteria: 1.5-T endorectal CE-MRI of the prostatectomy bed performed after postsurgical biochemical recurrence of prostate cancer (PSA level of ≥ 0.10 ng/mL after RP and a confirmatory increase of PSA level ≥ 0.2 ng/mL). One hundred forty-seven patients met the inclusion criteria. We excluded patients who did not undergo biopsy (n = 75), who were not biopsied within 3 months before or after the MRI (n = 18), or who had other primary tumors on biopsy (n = 2). Thus, 52 patients were included in the study. Table 1 summarizes the patients’ clinical and surgicopathologic characteristics at inclusion.
TABLE 1.
Patients’ Clinical and Surgicopathologic Characteristics
| Characteristic | Value |
|---|---|
| No. of patients | 52 |
| Age at MRI (y), median (range) | 66 (43–80) |
| PSA level at MRI (ng/mL), mean (95% CI) | 2.2 (1.3–3.2) |
| Time between RP and MRI (mo), mean (95% CI) | 85 (75.6–107.2) |
| Time between MRI and biopsy (d), mean (95% CI) | 15 (6.2–24.9) |
| Gleason score at prostatectomy (no. of patients) | |
| G6 (3 + 3) | 8 |
| G7 (3 + 4, n = 13; 4 + 3, n = 11) | 24 |
| G8 (4 + 4, n = 5; 5 + 3, n = 2) | 7 |
| G9 (4 + 5, n = 8; 5 + 4, n = 2) | 10 |
| Unknown | 3a |
| Positive surgical margins (no. of patients) | 17 |
| Extracapsular extension (no. of patients) | 24 |
| Seminal vesicle invasion (no. of patients) | 11 |
Note—PSA = prostate-specific antigen, RP = radical prostatectomy.
Histopathologic reports listing pathologic stage were not available for three patients included in the study because they underwent RP at a different institution.
MRI Technique
Images were obtained with a 1.5-T whole-body MRI system (Signa HD, GE Healthcare). The patients were examined in the supine position. A body coil was used for excitation, and a pelvic four-channel phased-array coil combined with a balloon-covered expandable endorectal coil (Prostate eCoil, Medrad) was used for signal reception. First, transverse, coronal, and sagittal T2-weighted fast spin-echo images were obtained (TR/TE, 4000–6000/120; echo-train length, 16; section thickness, 3 mm with no intersection gap; FOV, 12–14 cm; and matrix, 256 × 192). Subsequently, a transverse T1-weighted 3D fast spoiled gradient-recalled sequence (TR/TE, 4.5–6.5/1.5–2.2; flip angle, 12°; bandwidth, 62.5 kHz; section thickness, 3 mm with no intersection gap; FOV, 12–14 cm; and matrix, 256 × 192) with fat suppression was performed before and at a minimum of three time points (25–30 seconds, 1 minute, and 3 minutes) after IV injection of 0.1 mmol/kg body weight dose of paramagnetic contrast medium (gadopentetate dimeglumine; Magnevist, Bayer HealthCare) by means of a power injector with an injection rate of 2 mL/s, followed by a 20-mL saline flush.
MRI Interpretation
All MRI studies were retrospectively and independently reviewed by two readers who were aware that the patients had post-RP biochemical recurrence of prostate cancer but were blinded to the patients’ other clinical and histopathologic findings. Each reader independently scored each case according to his or her estimate of the likelihood that local recurrence was present on a 1–5 index scale (1, definitely absent; 2, probably absent; 3, indeterminate; 4, probably present; and 5, definitely present). Both readers first interpreted and assigned a score on the basis of T2-weighted imaging alone (in the axial, sagittal, and coronal planes). In the same sitting, the readers then recorded a second score on the basis of both T2-weighted imaging and CE-MRI (the CE-MRI was performed in the axial plane only). Reader 1 was a board-certified radiologist and had 1 year of experience interpreting endorectal MRI; reader 2 was a board-certified radiologist and genitourinary specialist with 6 years of experience interpreting endorectal MRI.
Local recurrence was suspected if an area of slight hyperintensity to surrounding muscles was seen on T2-weighted imaging, particularly if the area had a nodular appearance and if it showed greater enhancement than the surrounding muscles on CE-MRI. Each reader recorded the number, location, and largest transverse diameter of recurrent tumors; tumor margins (well-defined or ill-defined); presence of invasion of adjacent structures; and the type of MRI sequence on which the lesion was best seen (T2-weighted MRI or CE-MRI). According to published literature [20–24], locations of suspected recurrence were classified as perianastomotic (around the urinary bladder or membranous urethra), retrovesical or bladder wall (between the urinary bladder and rectum and within the urinary or bladder wall), within retained seminal vesicles, or at the anterior or lateral surgical margins of the prostatectomy bed.
Standard of Reference
For all patients, the reference standard was histopathologic findings of tissue specimens obtained by biopsy (transrectal ultrasound–guided biopsy in 46 patients and transurethral bladder biopsy in six patients). A patient was considered to have local recurrence if the biopsy result showed malignant cells consistent with prostate cancer. To avoid potential bias based on a false-negative biopsy result, a patient was considered free of local recurrence only when the biopsy showed benign tissue and 1-year follow-up showed stable PSA, stable PSA and no change on follow-up endorectal MRI, or negative repeat biopsy.
Statistical Analysis
The Fisher exact test was used to examine the association between the initial surgical pathology Gleason score and the recurrent pathology Gleason score. The area under the receiver operating characteristic curve (Az) was calculated to measure diagnostic accuracy for T2-weighted MRI alone as well as T2-weighted MRI plus CE-MRI and was graphically plotted for each reader. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated (scores 1–3, no suspicion of tumor; scores 4–5, suspicion of tumor) for T2-weighted MRI alone and T2-weighted plus CE-MRI for each reader. A p value of 0.05 or less was considered to indicate statistical significance.
To assess interobserver agreement regarding the presence or absence of local recurrence on a perlesion level, Cohen kappa statistics were calculated for T2-weighted MRI as well as T2-weighted MRI plus CE-MRI findings. Kappa values were interpreted as follows: less than 0.20 indicates poor agreement, 0.20–0.39 indicates fair agreement, 0.40–0.59 indicates moderate agreement, 0.60–0.79 indicates substantial agreement, and 0.80 or higher indicates excellent agreement. A kappa value of 1 would indicate perfect agreement, and kappa of 0 or less would indicate no agreement other than what would be expected by chance.
To further assess the impact of CE-MRI on a per-lesion basis, increases or decreases in the readers’ 5-point scale for the likelihood of recurrent cancer were recorded, and the numbers of lesions correctly and incorrectly reclassified after inspection of CE-MRI were determined.
Results
Clinical Results
Of the 52 patients in the study, 33 (63%) had biopsy-proven local recurrence of prostate cancer. In 19 patients (37%), biopsy was negative for local recurrence (benign tissue or postoperative fibrosis); at least 1 year of follow-up of each of these 19 patients showed stable PSA (n = 12), stable PSA and no change on follow-up endorectal MRI studies (n = 5), or negative repeat biopsy (n = 2). In the latter two cases, repeat biopsies were performed 13 and 69 days after the initial biopsy.
Table 2 shows the Gleason scores from initial surgical pathology and postsurgical biopsy for patients with local recurrence. Gleason scores were provided in the biopsy reports of 23 patients, of whom 15 (65%) had a higher Gleason score on biopsy than at RP (p = 0.04).
TABLE 2.
Gleason Scores From Surgical Pathology and Postoperative Biopsy Results From the 33 Patients for Whom Biopsy Results Were Positive for Local Recurrence of Prostate Cancer
| Radical Prostatectomy Histopathology Gleason Score, Recurrent Carcinoma Gleason Score |
No. of Patients |
|---|---|
| G6 | 3 |
| G7 | 1 |
| G8 | 2 |
| G7 | 16 |
| G7 | 8 |
| G8 | 4 |
| G9 | 4 |
| G8, G9 | 3 |
| G9, G9 | 1 |
| G6–9, poorly differentiated prostate cancer | 4 |
| G6–9, prostate adenocarcinoma, not further specified | 6 |
MRI Results
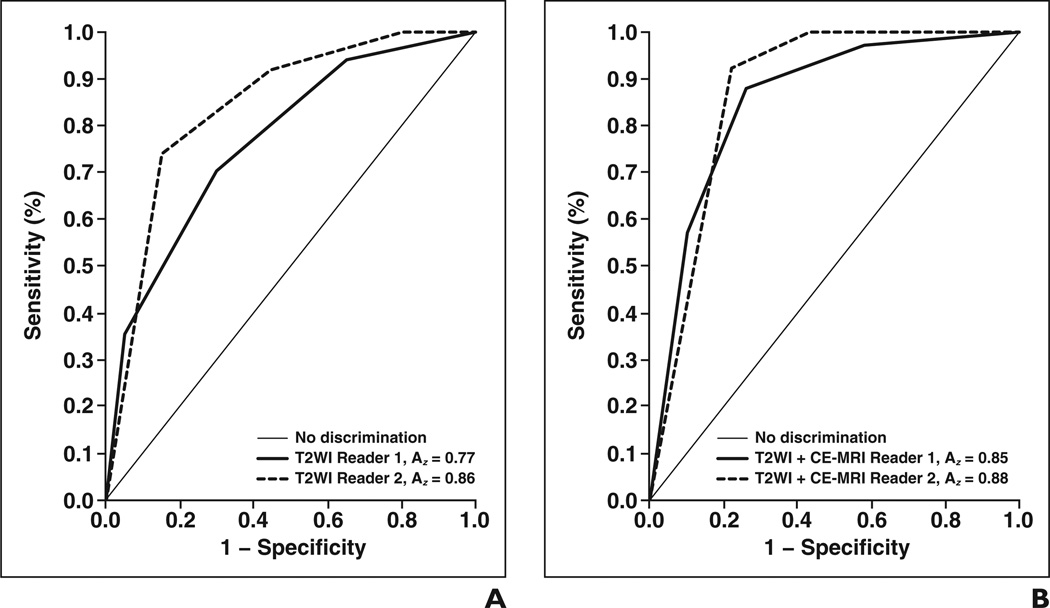
Receiver operating characteristic analysis (Fig. 1) showed that adding CE-MRI to T2-weighted imaging significantly (p = 0.0435) improved diagnostic accuracy for reader 1 (the less-experienced reader), increasing the Az from 0.77 (95% CI, 0.65–0.90) to 0.85 (95% CI, 0.74–0.96); for reader 2, the Az increased from 0.86 (95% CI, 0.76–0.96) to 0.88 (95% CI, 0.77–0.98), but the difference was not statistically significant (p = 0.7294). When MRI scores of 1–3 were considered negative and scores of 4–5 were considered positive, sensitivity in the detection of local recurrence increased with the addition of CE-MRI from 73% (95% CI, 55–87%) to 88% (95% CI, 72–97%) for reader 1 and from 91% (95% CI, 76–98%) to 100% (95% CI, 89–100%) for reader 2, whereas specificity increased from 68% (95% CI, 43–87%) to 74% (95% CI, 49–91%) for reader 1 and remained at 58% (95% CI, 34–80%) for reader 2. Tables 3 and 4 show the readers’ sensitivity, specificity, positive predictive value, and negative predictive value for using T2-weighted MRI alone and T2-weighted MRI plus CE-MRI together at different cutoff points of the MRI suspicion scale.
Fig. 1.
Receiver operating characteristic (ROC) curves.
A and B, Graphs show accuracy of reader 1 and reader 2 for T2-weighted imaging (T2WI) alone (A) and T2WI plus contrast-enhanced MRI (CE-MRI) (B). With addition of CE-MRI, area under ROC curve (Az) increased significantly for reader 1 (p = 0.0435) but not for reader 2 (p = 0.7294). Curves reflect that adding CE-MRI improves interobserver variability. Sensitivity is defined as true-positive rate, and 1 − specificity is defined as false-positive rate.
TABLE 3.
Diagnostic Performance of Endorectal T2-Weighted Imaging (T2WI) Alone and T2WI Plus Contrast-Enhanced MRI (CE-MRI) for Detection of Local Recurrence of Prostate Cancer With 5-Point Scoring System Dichotomized as Scores 1–3 (No Suspicion of Tumor) and Scores 4–5 (Suspicion of Tumor)
| Reader, Imaging Techniques | Sensitivity, % (No./Total) [95% CI] |
Specificity, % (No./Total) [95% CI] |
Positive Predictive Value, % (No./Total) [95% CI] |
Negative Predictive Value, % (No./Total) [95% CI] |
|---|---|---|---|---|
| Reader 1 | ||||
| T2WI | 73 (24/33) [55–87] | 68 (13/19) [43–87] | 80 (24/30) [61–92] | 59 (13/22) [36–79] |
| T2WI plus CE-MRI | 88 (29/33) [72–97] | 74 (14/19) [49–91] | 85 (29/34) [69–95] | 78 (14/18) [52–94] |
| Reader 2 | ||||
| T2WI | 91 (30/33) [76–98] | 58 (11/19) [34–80] | 79 (30/38) [63–90] | 79 (11/14) [49–95] |
| T2WI plus CE-MRI | 100 (33/33) [89–100] | 58 (11/19) [34–80] | 80 (33/41) [65–91] | 100 (11/11) [72–100] |
TABLE 4.
Diagnostic Performance of Endorectal T2-Weighted Imaging (T2WI) Alone and T2WI Plus Contrast-Enhanced MRI (CE-MRI) for Detection of Local Recurrence of Prostate Cancer With 5-Point Scoring System Dichotomized as Scores 1–2 (No Suspicion of Tumor) and Scores 3–5 (Suspicion of Tumor)
| Reader, Imaging Techniques | Sensitivity, % (No./Total) [95% CI] |
Specificity, % (No. Total) [95% CI] |
Positive Predictive Value, % (No. Total) [95% CI] |
Negative Predictive Value, % (No. Total) [95% CI] |
|---|---|---|---|---|
| Reader 1 | ||||
| T2WI | 94 (31/33) [80–99] | 37 (7/19) [16–62] | 72 (31/43) [56–85] | 78 (7/9) [40–97] |
| T2WI plus CE-MRI | 97 (32/33) [84–100] | 42 (8/19) [20–67] | 74 (32/43) [59–86] | 89 (8/9) [52–100] |
| Reader 2 | ||||
| T2WI | 100 (33/33) [89–100] | 21 (4/19) [6–46] | 69 (33/48) [54–81] | 100 (4/4) [40–100] |
| T2WI plus CE-MRI | 100 (33/33) [89–100] | 37 (7/19) [16–62] | 73 (33/45) [58–85] | 100 (7/7) [59–100] |
In determining the presence or absence of local recurrence with T2-weighted MRI alone, readers agreed in 71% of cases (κ = 0.39, indicating fair agreement). With the addition of CE-MRI to T2-weighted MRI, the readers agreed in 83% of cases (κ = 0.58, indicating moderate agreement).
Descriptive Tumor Statistics
The readings of the more-experienced radiologist were used to calculate descriptive statistics in cases where the radiologists disagreed. There was substantial agreement regarding the location of recurrent tumors (κ = 0.87). There was a discrepancy between the readers in the location of recurrence in six of 312 (1.9%) possible locations (six locations in each of the 52 patients). The locations for the biopsied recurrent tumors in the prostatic fossa were perianastomotic in 16 patients (48%) (Fig. 2), retrovesical or bladder wall in 10 patients (30%) (Fig. 3), within retained seminal vesicles in five patients (15%), anterior or lateral surgical margins in one patient (3%), and other (pelvic sidewall or perirectal) in one patient (3%). The median recurrent lesion size (i.e., maximal transverse dimension) was 20 mm (range, 9–69 mm). Thirty-one of the 33 biopsy-proven recurrences (94%) were well defined in relation to surrounding tissue, whereas the other two (6%) were ill defined. Invasion of adjacent structures was noted in the rectum in 11 (33%) recurrent lesions, in the bladder wall in 10 (30%) recurrent lesions, in the levator muscle in five (15%) recurrent lesions, and in the pelvic sidewall in three (9%) lesions.
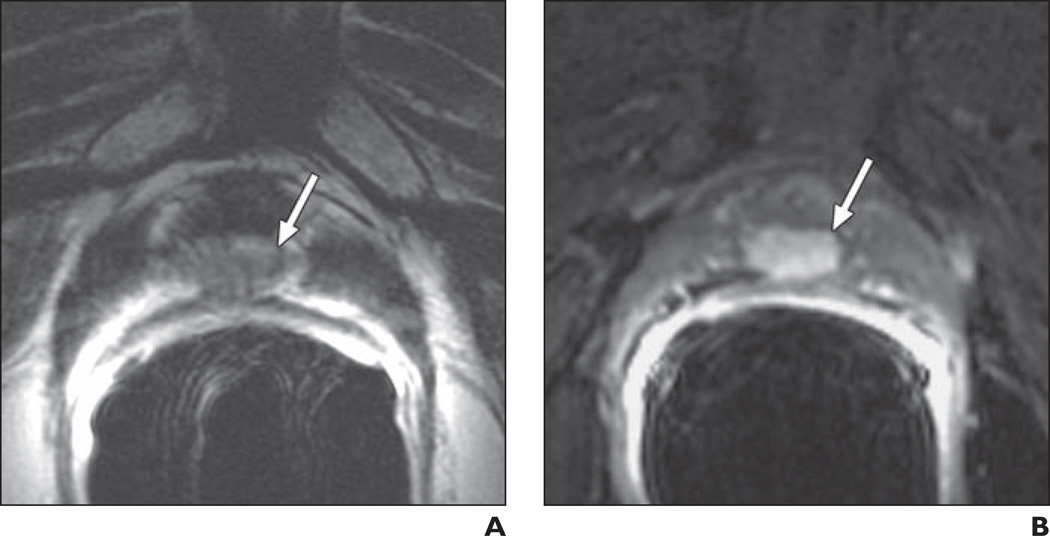
Fig. 2.
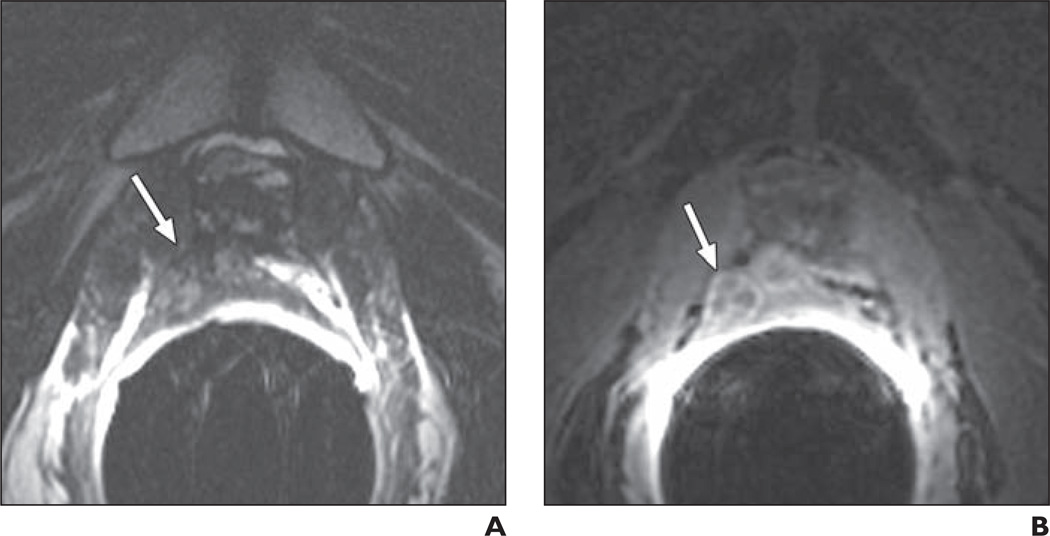
71-year-old man 15 years after radical prostatectomy for Gleason 7 (4 + 3) tumor. Follow-up showed biochemical recurrence with elevated prostate-specific antigen level of 0.52 ng/mL. A, Transverse T2-weighted image shows 12-mm nodule (arrow) suggestive of local recurrence in posterior part of vesicourethral anastomosis. B, With addition of contrast-enhanced MRI, less-experienced reader correctly upgraded lesion (arrow) from 4 to 5 on 5-point likelihood of recurrence scale.
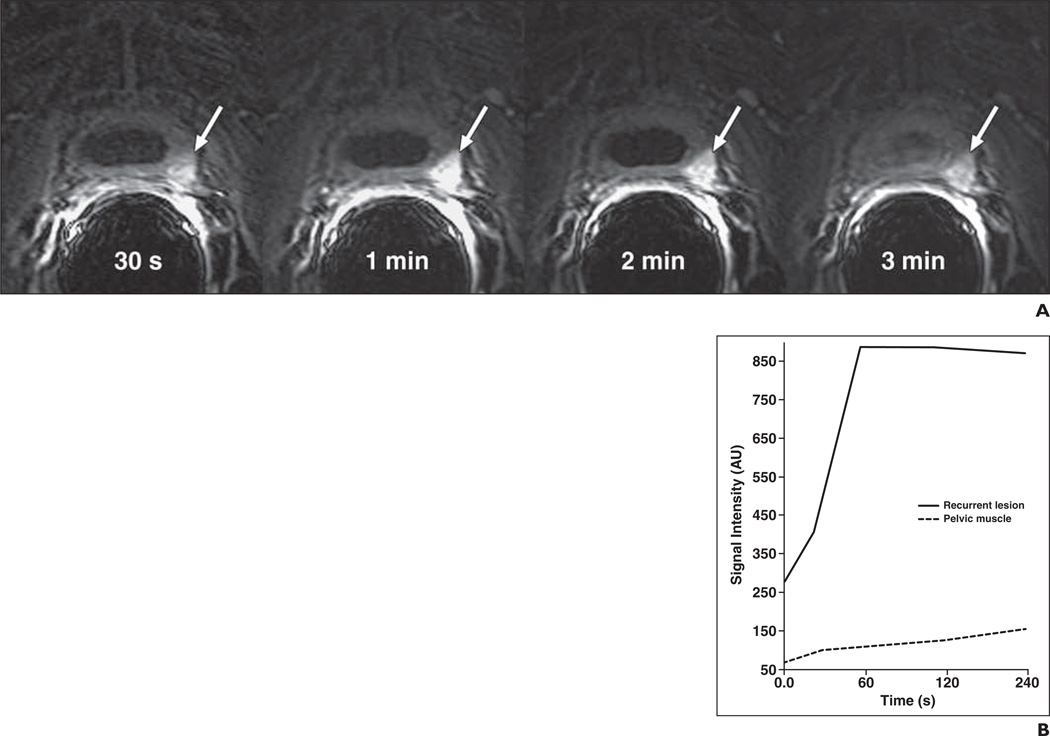
Fig. 3.
77-year-old man 12 years after radical prostatectomy for Gleason 7 (4 + 3) tumor. Follow-up showed biochemical recurrence with elevated prostate-specific antigen of 0.2 ng/mL. Transverse contrast-enhanced MRI (CE-MRI) was performed.
A, CE-MRI at four time points (30 s, 1 min, 2 min, and 3 min) in this patient shows enhancement in 20-mm local recurrence (arrows) in left bladder wall, with highest signal intensity at 1 min after injection.
B, Corresponding signal enhancement–time curve shows difference in contrast enhancement between recurrent carcinoma and pelvic muscle.
The readers agreed that 82% (27/33) of the lesions were better defined in relation to surrounding organs with the addition of CE-MRI to T2-weighted imaging. Adding CE-MRI to T2-weighted imaging allowed correct reclassification and increased certainty (i.e., correct upgrading or downgrading of the likelihood of recurrence) in 13 patients (25%) for reader 1 and six patients (12%) for reader 2 (Figs. 4 and 5).
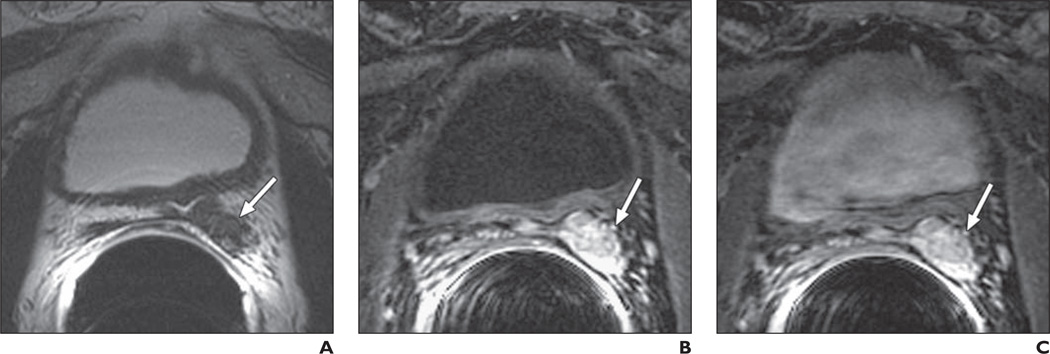
Fig. 4.
73-year-old man 10 years after radical prostatectomy for Gleason 7 (4 + 3) tumor of pathologic stage pT3A, with left extracapsular extension but no seminal vesicle invasion. Follow-up showed biochemical recurrence with elevated prostate-specific antigen level of 2.17 ng/mL.
A, Transverse T2-weighted image shows 20-mm soft-tissue mass (arrow) suggestive of local recurrence located retrovesically in residual tissue of left seminal vesicle.
B and C, On contrast-enhanced MRI 1 minute (B) and 3 minutes (C) after injection, clear enhancement (arrows) could be seen. Transrectal ultrasound–guided biopsy verified Gleason score 7 local recurrence.
Fig. 5.
66-year-old man 4 years after radical prostatectomy for Gleason 7 (3 + 4) tumor. Follow-up showed biochemical recurrence with elevated prostate-specific antigen of 0.19 ng/mL and palpable nodule at digital rectal examination.
A, Transverse T2-weighted image shows 22-mm nodule (arrow) suggestive of local recurrence in right posterior part of vesicourethral anastomosis.
B, With addition of contrast-enhanced MRI (CE-MRI), level of suspicion did not increase for either of the two readers, both of whom had assigned a score of 5 according to T2-weighted imaging; however, CE-MRI allowed both readers to better demarcate local recurrence (arrow) in relation to surrounding organs. Transrectal ultrasound–guided biopsy verified Gleason score 7 local recurrence.
Discussion
Up to 40% of patients who undergo RP for localized prostate cancer will have biochemical recurrence. Localization of recurrent prostate cancer is crucial for appropriate patient management, and MRI is gaining acceptance as the most accurate imaging method for identifying sites of local recurrence after RP [20–26]. Our findings indicate that adding CE-MRI to T2-weighted endorectal MRI can facilitate postoperative detection of local recurrence by relatively inexperienced readers. Specifically, we found that, for a radiologist with 1 year of experience reading endorectal MRI, the addition of CE-MRI significantly improved recurrent tumor detection, resulting in a level of diagnostic accuracy similar to that of a radiologist with 6 years of experience reading endorectal MRI. Furthermore, adding CE-MRI to T2-weighted imaging markedly improved interreader agreement.
Our results are in accordance with those of two previous studies on the detection of postoperative local recurrence, in which the addition of CE-MRI to T2-weighted imaging significantly increased sensitivities (from 48–61% to 84–88%) and specificities (from 52–82% to 89–100%) [20, 21]. Another study [24] compared the accuracy of CE-MRI alone and in combination with MR spectroscopic imaging in patients at high risk of local recurrence after RP; in the detection of local recurrence, the use of CE-MRI alone yielded an Az of 0.93, whereas the use of CE-MRI combined with MR spectroscopic imaging yielded an Az of 0.96.
Substantial variability in accuracy due to differing levels of reader experience has been reported as an important concern in studies of MRI of the prostate [27, 28]. However, to our knowledge, the impact of reader experience on the value of CE-MRI for detecting postoperative local recurrence of prostate cancer has not previously been analyzed. Our results are in agreement with those of a prior study, which found that adding CE-MRI to T2-weighted MRI significantly improved the performance of two relatively inexperienced readers in staging primary prostate cancer but did not significantly benefit a more experienced reader [29]. Research has also shown that a dedicated interactive training curriculum in endorectal MRI can significantly improve inexperienced readers’ accuracy in the detection and staging of primary prostate cancer [30].
In our study, the most common location for local recurrence was perianastomotic (48%); this finding was in concordance with earlier studies, in which 45–52% of recurrent lesions were perianastomotic [20, 24]. The second most common location of local recurrence in our study was retrovesical or bladder wall (30%). Conversely, Sella et al. [22] found that retrovesical or bladder wall was the most common location for local recurrence, with 40% of lesions, whereas perianastomotic was the second most common, with 29% of lesions. The rate of recurrence in retained seminal vesicles was 15% in our study, as compared with 22% and 6% in studies by Sella et al. [22] and Sciarra et al. [24], respectively.
Prior studies have used a variety of reference standards for local recurrence after RP, most often biopsy, a significant PSA decrease after external-beam radiation therapy, or follow-up imaging with an increase of at least 20% in recurrent tumor size [20–22, 24].
Interestingly, we found a trend toward higher Gleason scores at the time of local recurrence than at surgery, reflecting histopathologic progression. More than half the patients with recurrent cancer who received a Gleason score at RP had a higher Gleason score on postsurgical biopsy. To our knowledge, only one previous study has specifically investigated upgrading of prostate cancer at local recurrence after surgery, and it found a similar, though not statistically significant, trend [31].
Our study had a number of limitations, including its retrospective design. Because it was performed at a tertiary care cancer center, the results may not be generalizable to other settings, because patient demographics, image acquisition, and interpretation expertise may vary across institutions. There is also a sample selection bias because we included included only patients who underwent biopsy; this selection bias may be reflected in the relatively large mean diameter of the recurrent lesions and relatively high sensitivities found at MRI. Furthermore, some patients were excluded because they had a biopsy performed more than 3 months before or after the MRI. We chose 3 months as a cutoff to avoid the clinical scenario in which disease progression or recurrence would develop in the interval between the MRI and the biopsy, interfering with our measurements of diagnostic accuracy. In addition, the number of patients studied was relatively small, and the standard of reference used was not perfect, because a negative biopsy result could not rule out recurrence (though follow-up was at least 1 year for all patients with benign biopsy results to minimize this bias).
In conclusion, our results show that adding CE-MRI to T2-weighted MRI increases accuracy in the detection of postoperative local recurrence of prostate cancer by relatively inexperienced readers and improves agreement between readers of differing levels of experience. Furthermore, the enhancement observed in recurrent tumors on CE-MRI is useful for defining the relationship of the tumor to surrounding organs—information that may be crucial for planning further local treatment.
Acknowledgment
We thank Ada Mueller for editing the manuscript
The study was funded by National Institutes of Health grant R01-CA076423. H. A. Vargas was supported by the Peter Michael Foundation.
References
- 1.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu-Yao GL, Yao SL. Population-based study of long-term survival in patients with clinically localised prostate cancer. Lancet. 1997;349:906–910. doi: 10.1016/S0140-6736(96)09380-4. [DOI] [PubMed] [Google Scholar]
- 3.Yan Y, Carvalhal GF, Catalona WJ, et al. Primary treatment choices for men with clinically localized prostate carcinoma detected by screening. Cancer. 2000;88:1122–1130. [PubMed] [Google Scholar]
- 4.Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2:174–182. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 5.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy: the 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 7.Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369–375. doi: 10.1016/s0360-3016(00)00645-3. [DOI] [PubMed] [Google Scholar]
- 8.Scattoni V, Montorsi F, Picchio M, et al. Diagnosis of local recurrence after radical prostatectomy. BJU Int. 2004;93:680–688. doi: 10.1111/j.1464-410x.2003.04692.x. [DOI] [PubMed] [Google Scholar]
- 9.Olsson AY, Bjartell A, Lilja H, et al. Expression of prostate-specific antigen (PSA) and human glandular kallikrein 2 (hK2) in ileum and other extraprostatic tissues. Int J Cancer. 2005;113:290–297. doi: 10.1002/ijc.20605. [DOI] [PubMed] [Google Scholar]
- 10.Cohen RJ, Garrett K, Golding JL, et al. Epithelial differentiation of the lower urinary tract with recognition of the minor prostatic glands. Hum Pathol. 2002;33:905–909. doi: 10.1053/hupa.2002.127440. [DOI] [PubMed] [Google Scholar]
- 11.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 12.Freedland SJ, Sutter ME, Dorey F, et al. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy: prostate-specific antigen. Urology. 2003;61:365–369. doi: 10.1016/s0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–3978. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 14.Aus G, Abbou CC, Bolla M, et al. European Association of Urology. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546–551. doi: 10.1016/j.eururo.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 16.Pucar D, Sella T, Schoder H. The role of imaging in the detection of prostate cancer local recurrence after radiation therapy and surgery. Curr Opin Urol. 2008;18:87–97. doi: 10.1097/MOU.0b013e3282f13ac3. [DOI] [PubMed] [Google Scholar]
- 17.Leventis AK, Shariat SF, Slawin KM. Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology. 2001;219:432–439. doi: 10.1148/radiology.219.2.r01ma20432. [DOI] [PubMed] [Google Scholar]
- 18.Koppie TM, Grossfeld GD, Nudell DM, et al. Is anastomotic biopsy necessary before radiotherapy after radical prostatectomy? J Urol. 2001;166:111–115. [PubMed] [Google Scholar]
- 19.Saleem MD, Sanders H, Abu El Naser M, et al. Factors predicting cancer detection in biopsy of the prostatic fossa after radical prostatectomy. Urology. 1998;51:283–286. doi: 10.1016/s0090-4295(97)00509-8. [DOI] [PubMed] [Google Scholar]
- 20.Casciani E, Polettini E, Carmenini E, et al. Endorectal and dynamic contrast-enhanced MRI for detection of local recurrence after radical prostatectomy. AJR. 2008;190:1187–1192. doi: 10.2214/AJR.07.3032. [DOI] [PubMed] [Google Scholar]
- 21.Cirillo S, Petracchini M, Scotti L, et al. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur Radiol. 2009;19:761–769. doi: 10.1007/s00330-008-1174-8. [DOI] [PubMed] [Google Scholar]
- 22.Sella T, Schwartz LH, Swindle PW, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;231:379–385. doi: 10.1148/radiol.2312030011. [DOI] [PubMed] [Google Scholar]
- 23.Silverman JM, Krebs TL. MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. AJR. 1997;168:379–385. doi: 10.2214/ajr.168.2.9016212. [DOI] [PubMed] [Google Scholar]
- 24.Sciarra A, Panebianco V, Salciccia S, et al. Role of dynamic contrast-enhanced magnetic resonance (MR) imaging and proton MR spectroscopic imaging in the detection of local recurrence after radical prostatectomy for prostate cancer. Eur Urol. 2008;54:589–600. doi: 10.1016/j.eururo.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Beresford MJ, Gillatt D, Benson RJ, et al. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) 2010;22:46–55. doi: 10.1016/j.clon.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol. 2010;20:1254–1266. doi: 10.1007/s00330-009-1647-4. [DOI] [PubMed] [Google Scholar]
- 27.Mullerad M, Hricak H, Wang L, et al. Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology. 2004;232:140–146. doi: 10.1148/radiol.2321031254. [DOI] [PubMed] [Google Scholar]
- 28.Yu KK, Hricak H, Alagappan R, et al. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology. 1997;202:697–702. doi: 10.1148/radiology.202.3.9051019. [DOI] [PubMed] [Google Scholar]
- 29.Fütterer JJ, Engelbrecht MR, Huisman HJ, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005;237:541–549. doi: 10.1148/radiol.2372041724. [DOI] [PubMed] [Google Scholar]
- 30.Akin O, Riedl CC, Ishill NM, et al. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol. 2010;20:995–1002. doi: 10.1007/s00330-009-1625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo R, Sugar L, Hong E, et al. Is there a progression of histologic grade from radical prostatectomy to local recurrence in patients with clinically isolated local recurrence following surgery? Can J Urol. 2003;10:1981–1985. [PubMed] [Google Scholar]