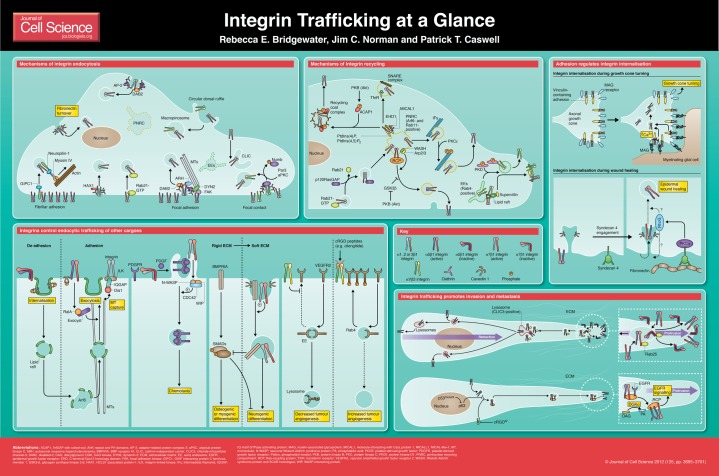
Integrins are heterodimeric transmembrane receptors for extracellular matrix (ECM) components and they link the intracellular actin cytoskeleton to the cellular environment (Humphries et al., 2006; Hynes, 2002). The α- and β-subunits of integrin heterodimers are type I membrane proteins that typically have a large extracellular domain and a short intracellular tail. Through different combinations of the 18 α- and eight β-subunits, 24 distinct integrin heterodimers exist in mammals. The integrin family thus comprises an array of cell surface receptors, which are expressed in a cell- and tissue-specific manner, for a plethora of soluble and insoluble ECM ligands, including collagens, laminins, fibronectin and vitronectin (Humphries et al., 2006). As a result, integrins are able to control diverse cellular processes, including proliferation, apoptosis, differentiation and cell migration and thus have key roles in development, immune responses and the progression of diseases such as cancer (Legate et al., 2009). Integrin heterodimers can adopt a bent or closed conformation that has a low affinity for ligand (‘inactive’) or an extended or open conformation that has a high affinity for ligand (‘active’). As a consequence of this conformational switching, integrins are able to signal bidirectionally across the membrane: ligand binding elicits signalling responses within the cell (‘outside-in’ signalling), but binding of intracellular proteins such as talin and kindlins to integrins regulates the activation of integrins to promote ligand-binding (‘inside-out’ signalling) (Legate et al., 2009).
In addition, the control of integrin availability at the plasma membrane is key to their function. However, although we have known for over 20 years that integrins undergo an exocytic–endocytic cycle, these pathways have only been studied in detail relatively recently. In this Cell Science at a Glance article, we will describe the mechanisms that control integrin endocytosis and recycling, and discuss the insight that understanding these mechanisms has provided into the role of trafficking in integrin function and vice versa. Furthermore, we draw attention to two emerging features of integrin trafficking: first, the specific nature of the mechanisms that control aspects of integrin trafficking, and, second, the role that integrin trafficking has in intracellular signalling.
Mechanisms of integrin endocytosis
Integrin endocytosis has been studied extensively in the context of viral entry (Pellinen and Ivaska, 2006), but mechanisms that regulate integrin internalisation in the absence of pathogens have been identified. Integrins can be internalised through the major endocytic routes (Box 1), including the formation of circular dorsal ruffles (CDRs) during macropinocytosis (Gu et al., 2011), and clathrin-dependent and -independent endocytic pathways. Direct recruitment of endocytic regulators to integrins constitutes a general mechanism that can drive integrin endocytosis. Examples of this include the recruitment of HS1 associated protein X-1 (HAX-1) to the cyoplasmic tail of integrin β6 (Ramsay et al., 2007) and the recruitment of Rab21 to the cytoplasmic tail of integrin α subunits (Pellinen et al., 2006). Protein kinase C alpha (PKCα) controls caveolar endocytosis (Smart et al., 1995), and can itself bind to β1 integrins (i.e. integrins that contain a β1 subunit and any α subunit) and regulate integrin internalisation (Ng et al., 1999). In endothelial cells, recruitment of active α5β1 integrin to the neuropilin–GIPC1 (for GAIP C-terminus-interacting protein) complex regulates endocytosis of this integrin directly from fibrillar adhesions (Valdembri et al., 2009). Xenopus GIPC1 (also known as kermit2) regulates α5β1 integrin internalisation into Rab21-positive vesicles (Spicer et al., 2010), which suggests that mechanisms that regulate integrin internalisation are conserved.
Box 1. Endocytosis.
Endocytosis regulates membrane lipid and protein composition, thereby allowing cellular functions to be controlled. Many distinct routes of internalisation have been identified, and these are typically characterised by the mechanism employed, the cargo transported and/or the morphology of the internalising structure (Hansen and Nichols, 2009). Generally, endocytic processes are classified as clathrin-dependent endocytosis (CDE) or clathrin-independent endocytosis (CIE), whereby CIE describes several distinct endocytic routes (Doherty and McMahon, 2009).
Clathrin-dependent endocytosis
CDE is the best-characterised internalisation route and involves the recruitment of clathrin triskelia to the membrane, where they are assembled to form a clathrin-coated pit (CCP). This is preceded by a nucleation step, wherein factors such as phosphatidylinositol 4,5-diphosphate [PtdIns(4,5)P2], epidermal growth factor receptor pathway substrate 15 (EPS15), intersectins and FCH domain only (FCHO) proteins define the site of CCP formation. This ‘nucleation module’ recruits the clathrin-adaptor AP-2, along with cargo-specific adaptor proteins that mediate cargo selection, and, at the same time, clathrin assembles to form the CCP. The GTPase dynamin is subsequently recruited to the neck of the CCP, where it polymerises. GTP hydrolysis by dynamin finally leads to membrane scission (McMahon and Boucrot, 2011).
Clathrin-independent endocytosis
In general, less is known about the mechanisms involved in CIE, and, in particular, how cargoes are recruited. Caveolae are flask-shaped invaginations of the membrane that are 50–80 nm in diameter and enriched in cholesterol, sphingolipids and oligomeric caveolin-1. Endocytosis through caveolae requires the function of dynamin (Mayor and Pagano, 2007). A second type of lipid-raft-associated CIE involves clathrin- and dynamin-independent carriers (CLICs) or glycosylphosphatidylinositol (GPI)-enriched early endosomal compartments (GEECs), which are endomembrane compartments that are formed by clathrin- and dynamin-independent endocytosis. It is thought that BAR domain proteins such as the Rho GTPase activating protein 26 (ARHGAP26, also known as GRAF) confer membrane curvature that might be sufficient for scission. The cargoes of CLIC and GEEC pathways include GPI-anchored proteins, but given that many transmembrane proteins (including flotillins) are found within CLIC and GEEC structures it is likely that these pathways constitute a general internalisation route for a variety of proteins (Howes et al., 2010b; Hansen and Nichols, 2009). Finally, macropinocytosis produces large endocytic vesicles (with a diameter >500 nm), and this process is often associated with fluid uptake as well as internalisation of membrane-associated proteins that are recruited into the macropinocytic cup. Growth-factor stimulation promotes actin polymerisation and the formation of circular dorsal ruffles that is associated with the initiation of macropinocytosis. However, little is known about the precise mechanisms that govern scission and internalisation (Doherty and McMahon, 2009).
Clathrin-dependent endocytosis
The clathrin adaptors disabled homologue 2 (DAB2) and NUMB interact, through their phosphotyrosine-binding (PTB) domains, with the NPxY-motifs in the β-integrin cytoplasmic tails (Calderwood et al., 2003), and several lines of evidence support the notion that clathrin-adaptors regulate integrin endocytosis. Microtubule-induced focal adhesion (FA) disassembly has been shown to require clathrin-dependent endocytosis (CDE) (Chao and Kunz, 2009; Ezratty et al., 2009). Indeed clathrin and DAB2 localise to FAs in the mid-region of moving cells and regulate α5β1 integrin internalisation to facilitate migration. NUMB binds to β1 and β3 integrins, and localises around focal contacts at the leading edge of migrating cells together with clathrin. Phosphorylation of NUMB downstream of the PAR3 (also known as PARD3)–atypical PKC (aPKC) complex regulates integrin internalisation at these sites to promote migration (Nishimura and Kaibuchi, 2007). Inactive integrins are also internalised in a clathrin-dependent manner: DAB2 and AP2 regulate endocytosis of α1β1, α2β1 and α3β1 (but not α5β1) integrins from the dorsal surface of the cell, and the rate of DAP2–AP2-mediated endocytosis correlates positively with migration specifically towards ligands for these integrins (Teckchandani et al., 2009).
Clathrin-independent endocytosis
Accumulating evidence indicates that integrins can follow clathrin-independent endocytic routes. Indeed, β1 integrins are found within clathrin-independent carriers (CLICs, see Box 1) (Howes et al., 2010a). Rab21 overexpression circumvents the requirement for CDE to internalise β1 integrins in dividing cells by driving a form of clathrin-independent endocytosis (CIE) (Pellinen et al., 2008). Cholesterol depletion experiments have shown that integrins can enter the cell in a raft-dependent fashion (Fabbri et al., 2005), and α5β1 and αvβ3 integrins are thought to associate with caveolin-1 (Gálvez et al., 2004; Wickström et al., 2002). More direct evidence supports a role for caveolae in the internalisation of α5β1 in myofibroblasts (Shi and Sottile, 2008), α2β1 in an osteosarcoma cell line (Upla et al., 2004) and active β1 integrins in bone marrow mesenchymal stem cells (BMMSCs; Du et al., 2011).
Regulation of integrin endocytosis by cell–matrix adhesion
Although integrin endocytosis can be constitutive and ligand-independent, cell adhesion to the ECM through other adhesion receptors can promote integrin endocytosis. For instance, myelin-associated glycoprotein (MAG) inhibits axonal growth in neurons and repels growth cones by increasing the local Ca2+ concentration, thereby stimulating clathrin-dependent endocytosis of β1 integrins to promote growth cone turning (Hines et al., 2010). Syndecan-4 can act as a co-receptor for fibronectin (alongside integrins). In addition, syndecan-4 engagement controls the availability of α5β1 at the plasma membrane in fibroblasts through a mechanism that is consistent with caveolar endocytosis of the integrin (Bass et al., 2011).
Post-endocytic trafficking and recycling
Internalised integrins are rapidly trafficked to early endosomes (EEs), where cargoes are sorted for degradation or recycling (Box 2; Caswell and Norman, 2006). In the presence of fibronectin ligand, α5β1 integrin can be routed to late endosomes and degraded (Dozynkiewicz et al., 2012; Lobert et al., 2010; Tiwari et al., 2011), but the majority of internalised integrins are rapidly recycled back to the plasma membrane (Bretscher, 1989; Bretscher, 1992). Integrins can follow the major recycling routes of other cargoes, such as the transferrin receptor (TfnR, also known as TFRC) (Box 2) (Caswell and Norman, 2006; Caswell et al., 2009; Pellinen and Ivaska, 2006). However, although there are shared mechanisms between these two functionally distinct cargoes, there are also integrin-specific elements that provide spatial and temporal control of adhesion receptor availability at the plasma membrane.
Box 2. Rab and Arf GTPases control endocytic recycling.
Small GTPases are thought of as molecular switches that cycle between a GTP-bound ‘on’ state, and a GDP-bound ‘off’ state, in response to the activities of guanine nucleotide exchange factors (GEFs) and GTPases activating-proteins (GAPs), respectively. This switch controls the ability of the small GTPases to interact with their effector molecules. There are more than 60 Rab proteins and six Arf proteins that localise to distinct membrane compartments, and these families of GTPases control most of the known intracellular trafficking events in eukaryotic cells (Grant and Donaldson, 2009; Stenmark, 2009).
The function of early endosomes, where internalised cargoes are sorted, is dependent on Rab5. Early endosomal cargo can be directed to late endosomes through a maturation process that involves the exchange of Rab5 for Rab7 on the endosomal membrane (Rink et al., 2005). Late endosomal proteins are either lysosomally degraded or recycled through alternative pathways [e.g. the Rab9–retromer pathway (Seaman et al., 1998; Lombardi et al., 1993) or the Rab25–CLIC3 (for chloride intracellular channel 3) pathway (Dozynkiewicz et al., 2012)]. Early endosomal cargoes can also be recycled through ‘short-loop’ (fast) or ‘long-loop’ (slow) recycling pathways. The short-loop recycling pathway is regulated by Rab4 and Rab35, and is characterised by cargoes that exit the early endosome to recycle to the plasma membrane. Long-loop recycling involves trafficking of cargo through the perinuclear recycling compartment (PNRC) and is thought to primarily require the activity of Rab11 and Arf6, but roles for Rab8, Rab10 and Rab22a have also been described (Grant and Donaldson, 2009; Stenmark, 2009).
Rab and Arf proteins act together to coordinate the main steps of membrane trafficking. For example, Rab9 mediates vesicle budding by recruiting sorting adaptors to promote cargo selection into budding recycling vesicles on late endosomes (Carroll et al., 2001). Rab GTPases can also regulate tethering, docking and fusion events by recruiting tethering factors, SNAREs and the exocyst complex, and they can promote vesicle transport by recruiting both microtubule- and actin-based motor proteins directly or through additional effectors (Grant and Donaldson, 2009; Stenmark, 2009). By contrast, Arf6 regulates phospholipid signalling, which is important in many trafficking steps, but can also provide links to microtubule motors kinesin and dynein through interactions with scaffold proteins and provide a link to vesicle transport (Donaldson and Jackson, 2011).
Rab4-dependent recycling
αvβ3 integrins recycle through the Rab4-dependent ‘short-loop’ pathway (Box 2), which returns the heterodimers from EEs back to the plasma membrane without transiting through the perinuclear recycling compartment (PNRC) (Roberts et al., 2001; Jones et al., 2009; di Blasio et al., 2010). PKD1 (for protein kinase D1), when autophosphorylated on Ser916 in response to growth factor stimulation, interacts with the extreme C-terminus of integrin β3, and this interaction is required for Rab4-dependent recycling of this integrin (Woods et al., 2004; White et al., 2007; di Blasio et al., 2010). Rab4-dependent trafficking of αvβ3 is key to the control of directionally persistent migration (di Blasio et al., 2010; White et al., 2007; Woods et al., 2004) and branching morphogenesis of endothelial vessels (Jones et al., 2009). Although α5β1 does not follow the Rab4 recycling pathway in platelet-derived growth factor (PDGF)-stimulated fibroblasts (Roberts et al., 2001), recent studies have indicated that β1 integrins can follow a Rab4-dependent route to the plasma membrane: epidermal growth factor (EGF) promotes β1 integrin recycling in HeLa cells in a manner that requires the raft-associated membrane protein supervillin (Fang et al., 2010), and recycling of inactive β1 integrins is Rab4-dependent in breast cancer cell lines (Arjonen et al., 2012).
Rab11- and Arf6-dependent recycling
Integrins can also follow a second, ‘long-loop’ recycling pathway through the PNRC (Box 2), and this is dependent on the GTPases Rab11 and Arf6 and is linked to cell migration (Powelka et al., 2004; Caswell and Norman, 2006; Roberts et al., 2004). For β1 integrins, many of the mechanistic details are shared with other cargoes. EHD1 (for EH-domain containing 1), which is recruited to tubules emanating from the PNRC by MICAL-like 1 (MICALL1) and phosphoinositides, regulates the recycling of both β1 integrins and the TfnR (Jović et al., 2007; Jović et al., 2009; Naslavsky and Caplan, 2011; Sharma et al., 2009). In addition, Myotubularin, a phosphoinositide phosphatase, is required for the exit of integrins from intracellular compartments in Drosophila (Ribeiro et al., 2011). SNARE function is crucial in docking and fusion of vesicles throughout the endosomal system, and several Q-SNAREs (e.g. syntaxin 6, SNAP29, syntaxin 4 and SNAP23) and R-SNAREs (e.g. VAMP2 and VAMP3) have been shown to regulate β1 integrin trafficking (Veale et al., 2010; Tiwari et al., 2011; Skalski and Coppolino, 2005; Rapaport et al., 2010; Hasan and Hu, 2010).
Although integrins share much of the basic machinery for Rab11-dependent recycling with the TfnR, stages of the long-loop recycling pathway that are specifically required for integrin recycling have been identified. Rab21 binds to a conserved motif within the α-subunits of β1 integrin heterodimers and specifically promotes their internalisation and trafficking through early endosomes to the PNRC (Pellinen et al., 2008; Pellinen et al., 2006). Here, p120RasGAP (also known as RASA1) displaces Rab21, thereby promoting the release of the integrin-containing vesicles from the perinuclear region and their recycling to the plasma membrane (Mai et al., 2011). In carcinoma cells, Rab-coupling protein (RCP), a member of the Rab11-family interacting proteins (RAB11FIPs), which act as Rab11 effectors, has a crucial role in regulating integrin recycling but is dispensable for TfnR recycling through Rab11 recycling endosomes (Caswell et al., 2008; Muller et al., 2009). Both α5β1 and αvβ3 integrins follow a Rab11-dependent route in unstimulated fibroblasts. This requires the inactivation of glycogen synthase kinase 3 beta (GSK3β) through phosphorylation by AKT (also known as PKB) (Roberts et al., 2004). Serum stimulation can also promote trafficking of β1 integrins through this route, and AKT activity is required to phosphorylate ACAP1 (for ArfGAP with coiled-coil, ankyrin repeat and PH domains 1), which recruits β1 integrins to a recycling coat complex that contains clathrin (Powelka et al., 2004; Li et al., 2005; Li et al., 2007). PKCε phosphorylates vimentin, a type III intermediate filament protein, and releases β1-integrin-containing vesicles from intermediate filaments in the perinuclear region to allow recycling of β1 integrins (Ivaska et al., 2005; Ivaska et al., 2002). The events regulated by AKT and PKCε are specific to integrin trafficking, and are not required for recycling of TfnR.
Cellular functions of integrin trafficking
Many of the studies described above provide compelling evidence that integrin trafficking contributes directly to cellular processes such as cell migration. Endocytic mechanisms can contribute to the turnover of adhesion complexes, and, in this way, directly regulate interactions with the ECM and proteins involved in adhesion signalling [e.g. focal adhesion kinase (FAK) phosphorylation (Ezratty et al., 2005; Ezratty et al., 2009)]. It is unclear, however, whether trafficking pathways can influence the activation status of integrins or the ability of integrins to mediate adhesive contacts with the substratum, and in some cases this is evidently not the case (Caswell et al., 2007; Caswell et al., 2008). Tracking integrin trafficking using antibody surface-labelling approaches has indicated that internalised integrins can subsequently localise to focal complexes and focal adhesions, but only after extended periods (i.e. hours) following antibody exposure (Gu et al., 2011). Given that the t1/2 of integrin recycling is often <15 minutes, this could indicate that integrins do not recycle directly to focal adhesions (Caswell and Norman, 2006). Accumulating evidence suggests that instead integrin trafficking influences intracellular signalling, either directly or by influencing trafficking of other cargoes.
Integrin trafficking dictates Rho GTPase signalling
Integrin trafficking pathways can directly impact on Rho GTPase signalling. Rab21-mediated recycling of α5β1 is required for the activation of RhoA at the cleavage furrow and, hence, the completion of cytokinesis (Pellinen et al., 2008). Rho GTPases are master regulators of the cytoskeleton, and balancing RhoA and Rac activities is crucial for controlling the directional persistence of migrating cells (Danen et al., 2005). In fibroblasts, Rab4-dependent recycling of αvβ3, and the activity of this integrin, suppresses recycling of α5β1, thereby promoting directionally persistent migration that is characterised by a broad leading lamellipodium (Caswell et al., 2008; White et al., 2007). Inhibition of αvβ3 recycling or ligand binding enhances recycling of α5β1 and signalling through the ROCK (RhoA–Rho-associated, coiled-coil containing protein kinase) pathway, which, in turn, leads to phosphorylation of the downstream effector cofilin and rapid random migration (White et al., 2007).
Integrin trafficking promotes tumour cell invasion and metastasis
Integrin trafficking influences the ability of cells to move in three-dimensional matrices, as well as on two-dimensional substrates. Hypoxia-driven invasion through a laminin-rich matrix requires Rab11, and there is evidence that this involves the Rab11-dependent recycling of α6β4 (Yoon, 2005). αvβ6 promotes invasion in squamous cell carcinoma, and the interaction of this integrin with HAX1 allows its clathrin-dependent endocytosis, which in turn promotes carcinoma cell motility in organotypic culture (Ramsay et al., 2007).
Inhibition of αvβ3, or expression of cancer-associated mutant forms of p53, promotes α5β1 recycling in carcinoma cell lines (Caswell et al., 2008; Muller et al., 2009). This requires the interaction of β1 integrin with RCP (Caswell et al., 2008), which is itself upregulated in breast cancer (Zhang et al., 2009). The actin nucleation promoting factor (NPF) WASH (for WAS protein family homologue) has a role in α5β1 recycling through this pathway, presumably by remodelling actin on endosomes (Zech et al., 2011). RCP-dependent α5β1 trafficking promotes invasive migration in three-dimensional matrices, which is characterised by the extension of invasive pseudopods. The production of phosphatidic acid within the pseudopod tip is required to localise RCP and permit integrin recycling (Rainero et al., 2012). Rather than influence α5β1 activity, α5β1 and RCP recruit EGF receptor 1 (EGFR1) and coordinate its recycling, which, in turn, potentiates EGFR activation and downstream signalling to promote invasion (Caswell et al., 2008; Muller et al., 2009). In this context, integrins and RCP act as key components of a ‘recyclosome’ complex, which recruits receptor tyrosine kinases (RTKs), including EGFR1, ErbB2 and Met, promotes their recycling and potentiates signalling to induce metastasis (Muller et al., 2009; Caswell et al., 2008; Muller et al., 2012; P. T. Caswell, unpublished observations). Interestingly, in neuronal axons, Rab11 and RCP can control trafficking of β1 integrins to promote axonal extension (Eva et al., 2010), indicating that this mechanism is not unique to cancer cells.
Overexpression of Rab25 (also known as Rab11c) is associated with aggressive ovarian cancers (Cheng et al., 2004). Rab25 specifically regulates trafficking of α5β1 by directly interacting with β1 integrin (Caswell et al., 2007). Inactive integrins that become internalised into Rab25 vesicles at the front of invasive cancer cells are recycled back to the plasma membrane within this subcellular region, thus promoting their spatial restriction (Caswell et al., 2007; Caswell and Norman, 2008; Caswell et al., 2009). Those integrins that remain in the active conformation following internalisation, however, are sorted through Rab25-positive late endosomes to lysosomes, moving from the front of the cell towards the rear. CLIC3 regulates the subsequent recycling of active α5β1 from lysosomes to the plasma membrane. This allows Rab25 to control pseudopod extension at the front of the cell and coordinate retraction of the cell body by trafficking active integrins towards the rear of the cell to promote integrin signalling and forward movement (Dozynkiewicz et al., 2012).
Integrin trafficking and signalling regulates endocytic transport of other cargoes
Integrin trafficking pathways can indirectly influence intracellular signalling by controlling endocytic trafficking of other cell surface receptors. In BMMSCs, soft substrates promote endocytosis of β1 integrins through caveolae, and this is required for the internalisation of bone marrow morphogenetic protein (BMP) receptor IA (BMPRIA). BMPRIA colocalises with integrins in an intracellular compartment, which implies that these endocytic cargoes co-internalise, and that this, in turn, inhibits BMP-induced SMAD signalling, which allows cells to differentiate along the neuronal rather than osteogenic or myogenic lineage (Du et al., 2011).
αvβ3 integrin controls receptor trafficking, suppressing the recycling of α5β1 integrin, and thereby leads to changes in Rho GTPase signalling in fibroblasts and promotes receptor-tyrosine kinase trafficking and signalling in carcinoma cells as discussed above. Inhibiting αvβ3 with small molecule inhibitors such as Cilengitide in endothelial cells drives Rab4-dependent vascular endothelial growth factor receptor 2 (VEGFR2) recycling, protecting this receptor from degradation in the presence of VEGF ligand and increasing its levels on the cell surface (Reynolds et al., 2009). This promotes VEGF-driven endothelial cell migration, sprouting of aortic explants and tumour angiogenesis in vivo, which ultimately leads to enhanced tumorigenesis.
Clearly, integrins are cargoes of intracellular trafficking pathways; however, the relationship between integrins and endocytic traffic is complex because integrins themselves govern signals that control endocytic flux. In some instances adhesion negatively regulates endocytosis: engagement of integrin β1 by ligand can slow the dynamics of clathrin-coated structures, and adhesion of cells to fibronectin decreases the rate of TfnR endocytosis (Batchelder and Yarar, 2010). By contrast, β1 integrin engagement promotes endocytosis of the platelet-derived growth factor receptor (PDGFR) during fibroblast chemotaxis. In this scenario, integrins are required to maintain the stability of the actin NPF neural Wiscott–Aldrich syndrome protein (N-WASP), which controls the formation of CDRs and the internalisation of PDGF through macropinocytosis (King et al., 2011). Signalling from β1 integrins is also required for the fusion of vesicle-associated membrane protein 7 (VAMP7) vesicles with the plasma membrane in extending neurites on laminin substrates (Gupton and Gertler, 2010).
Integrin engagement also regulates the trafficking of caveolae and/or lipid rafts. Loss of adhesion triggers internalisation of lipid rafts, which is controlled by the shift of phosphorylated caveolin from adhesion complexes to caveolae (del Pozo et al., 2005; del Pozo et al., 2004). Following re-adhesion, lipid rafts recycle to the plasma membrane from the PNRC in an Arf6-dependent manner (Balasubramanian et al., 2007). RalA regulates exocytosis through the exocyst complex (Balasubramanian et al., 2010), which provides a platform for the activation of Rac. Keratinocytes that lack integrin β1, or the adhesion signalling protein integrin-linked kinase (ILK), also show a defect in the membrane targeting of caveolae. Caveolar endocytosis occurs independently of β1 integrins and ILK, but integrin signalling promotes the recruitment of a complex containing IQGAP (IQ motif containing GTPase activating protein) and mDia1 (also known as DIAPH1) to the plasma membrane, which in turn locally captures and stabilises microtubules to permit re-exocytosis of caveolin-1 (Wickström et al., 2010).
Future perspectives
Integrin trafficking is a dynamic process that is important for the regulation of cellular processes, including migration and cytokinesis. Technical limitations have hindered our understanding of integrin trafficking in vivo, but these are being overcome in some model systems (Yuan et al., 2010; Ribeiro et al., 2011), and furthering these studies to directly visualise trafficking events in vivo is a priority. Trafficking pathways can promote spatial restriction or en masse movement of integrins, and, interestingly, some regulators, such as Rab25, have a role in both (Caswell et al., 2007; Dozynkiewicz et al., 2012). Further studies are necessary to determine the sorting steps that control the decision to recycle integrins to the membrane from which they were initially internalised or to more distal sites. It will also be important to determine how the directional movement of integrins interfaces with downstream signalling pathways.
Unlike most cargoes, integrins are almost uniquely positioned in that they are cargoes for pathways that they themselves regulate. Further investigation is needed to determine whether integrins form central components of multi-cargo trafficking complexes that are controlled by adhesion signalling. This also raises an interesting question: are integrins able to elicit signals when they are transiting the endosomal system? The presence of active integrins on endomembrane compartments has now been noted by several investigators, and the recent identification of the integrin inactivator sharpin (Rantala et al., 2011) suggests that integrins could remain active on intracellular compartments until inactivated. Endomembranes are surfaces that provide a scaffold for signalling pathways such as the AKT (Schenck et al., 2008) and ROCK–myosin light chain 2 (MLC2) pathways (Sturge et al., 2006). This opens up the exciting possibility that integrins could associate with downstream signalling components on endosomes to propagate signals from a lipid and protein environment distinct from the adhesion plaque.
Supplementary Material
Footnotes
Funding
The work of our laboratories is supported by the Wellcome Trust (P.T.C.) and Cancer Research UK (J.C.N). R.E.B is supported by a BBSRC studentship and a Research Impact Scholarship from the ‘Your Manchester’ Fund.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.095810/-/DC1.
References
- Arjonen A., Alanko J., Veltel S., Ivaska J. (2012). Distinct recycling of active and inactive β1 integrins. Traffic 2012, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian N., Scott D. W., Castle J. D., Casanova J. E., Schwartz M. A. (2007). Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat. Cell Biol. 9, 1381–1391 10.1038/ncb1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian N., Meier J. A., Scott D. W., Norambuena A., White M. A., Schwartz M. A. (2010). RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr. Biol. 20, 75–79 10.1016/j.cub.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass M. D., Williamson R. C., Nunan R. D., Humphries J. D., Byron A., Morgan M. R., Martin P., Humphries M. J. (2011). A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis. Dev. Cell 21, 681–693 10.1016/j.devcel.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder E. M., Yarar D. (2010). Differential requirements for clathrin-dependent endocytosis at sites of cell-substrate adhesion. Mol. Biol. Cell 21, 3070–3079 10.1091/mbc.E09-12-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. (1989). Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 8, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. (1992). Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. EMBO J. 11, 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D. A., Fujioka Y., de Pereda J. M., García–Alvarez B., Nakamoto T., Margolis B., McGlade C. J., Liddington R. C., Ginsberg M. H. (2003). Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 100, 2272–2277 10.1073/pnas.262791999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K. S., Hanna J., Simon I., Krise J., Barbero P., Pfeffer S. R. (2001). Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292, 1373–1376 10.1126/science.1056791 [DOI] [PubMed] [Google Scholar]
- Caswell P. T., Norman J. C. (2006). Integrin trafficking and the control of cell migration. Traffic 7, 14–21 10.1111/j.1600-0854.2005.00362.x [DOI] [PubMed] [Google Scholar]
- Caswell P., Norman J. (2008). Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 18, 257–263 10.1016/j.tcb.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Caswell P. T., Spence H. J., Parsons M., White D. P., Clark K., Cheng K. W., Mills G. B., Humphries M. J., Messent A. J., Anderson K. I.et al. (2007). Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510 10.1016/j.devcel.2007.08.012 [DOI] [PubMed] [Google Scholar]
- Caswell P. T., Chan M., Lindsay A. J., McCaffrey M. W., Boettiger D., Norman J. C. (2008). Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 183, 143–155 10.1083/jcb.200804140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell P. T., Vadrevu S., Norman J. C. (2009). Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10, 843–853 10.1038/nrm2799 [DOI] [PubMed] [Google Scholar]
- Chao W-T., Kunz J. (2009). Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 583, 1337–1343 10.1016/j.febslet.2009.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. W., Lahad J. P., Kuo W-L., Lapuk A., Yamada K., Auersperg N., Liu J., Smith–McCune K., Lu K. H., Fishman D.et al. (2004). The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 10, 1251–1256 10.1038/nm1125 [DOI] [PubMed] [Google Scholar]
- Danen E. H. J., van Rheenen J., Franken W., Huveneers S., Sonneveld P., Jalink K., Sonnenberg A. (2005). Integrins control motile strategy through a Rho-cofilin pathway. J. Cell Biol. 169, 515–526 10.1083/jcb.200412081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M. A., Alderson N. B., Kiosses W. B., Chiang H-H., Anderson R. G. W., Schwartz M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839–842 10.1126/science.1092571 [DOI] [PubMed] [Google Scholar]
- del Pozo M. A., Balasubramanian N., Alderson N. B., Kiosses W. B., Grande–García A., Anderson R. G. W., Schwartz M. A. (2005). Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat. Cell Biol. 7, 901–908 10.1038/ncb1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Blasio L., Droetto S., Norman J., Bussolino F., Primo L. (2010). Protein kinase D1 regulates VEGF-A-induced alphavbeta3 integrin trafficking and endothelial cell migration. Traffic 11, 1107–1118 10.1111/j.1600-0854.2010.01077.x [DOI] [PubMed] [Google Scholar]
- Doherty G. J., McMahon H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Jackson C. L. (2011). ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 12, 362–375 10.1038/nrm3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozynkiewicz M. A., Jamieson N. B., Macpherson I., Grindlay J., Berghe P. V. D., Thun A. V., Morton J. P., Gourley C., Timpson P., Nixon C.et al. (2012). Rab25 and CLIC3 Collaborate to Promote Integrin Recycling from Late Endosomes/Lysosomes and Drive Cancer Progression. Dev. Cell 22, 131–145 10.1016/j.devcel.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Chen X., Liang X., Zhang G., Xu J., He L., Zhan Q., Feng X-Q., Chien S., Yang C. (2011). Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. USA 108, 9466–9471 10.1073/pnas.1106467108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva R., Dassie E., Caswell P. T., Dick G., ffrench–Constant C., Norman J. C., Fawcett J. W. (2010). Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J. Neurosci. 30, 11654–11669 10.1523/JNEUROSCI.2425-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty E. J., Partridge M. A., Gundersen G. G. (2005). Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581–590 10.1038/ncb1262 [DOI] [PubMed] [Google Scholar]
- Ezratty E. J., Bertaux C., Marcantonio E. E., Gundersen G. G. (2009). Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 187, 733–747 10.1083/jcb.200904054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M., Di Meglio S., Gagliani M. C., Consonni E., Molteni R., Bender J. R., Tacchetti C., Pardi R. (2005). Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the alphaL/beta2 integrin, LFA-1, during leukocyte chemotaxis. Mol. Biol. Cell 16, 5793–5803 10.1091/mbc.E05-05-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z., Takizawa N., Wilson K. A., Smith T. C., Delprato A., Davidson M. W., Lambright D. G., Luna E. J. (2010). The membrane-associated protein, supervillin, accelerates F-actin-dependent rapid integrin recycling and cell motility. Traffic 11, 782–799 10.1111/j.1600-0854.2010.01062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. D., Donaldson J. G. (2009). Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 10.1038/nrm2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Noss E. H., Hsu V. W., Brenner M. B. (2011). Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J. Cell Biol. 193, 61–70 10.1083/jcb.201007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton S. L., Gertler F. B. (2010). Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev. Cell 18, 725–736 10.1016/j.devcel.2010.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez B. G., Matías–Román S., Yáñez–Mó M., Vicente–Manzanares M., Sánchez–Madrid F., Arroyo A. G. (2004). Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol. Biol. Cell 15, 678–687 10.1091/mbc.E03-07-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C. G., Nichols B. J. (2009). Molecular mechanisms of clathrin-independent endocytosis. J. Cell Sci. 122, 1713–1721 10.1242/jcs.033951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan N., Hu C. (2010). Vesicle-associated membrane protein 2 mediates trafficking of alpha5beta1 integrin to the plasma membrane. Exp. Cell Res. 316, 12–23 10.1016/j.yexcr.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Hines J. H., Abu–Rub M., Henley J. R. (2010). Asymmetric endocytosis and remodeling of beta1-integrin adhesions during growth cone chemorepulsion by MAG. Nat. Neurosci. 13, 829–837 10.1038/nn.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes M. T., Kirkham M., Riches J., Cortese K., Walser P. J., Simpson F., Hill M. M., Jones A., Lundmark R., Lindsay M. R.et al. 2010a). Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 190675–691 10.1083/jcb.201002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes M. T., Mayor S., Parton R. G.2010b). Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 22519–527 10.1016/j.ceb.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Humphries J. D., Byron A., Humphries M. J. (2006). Integrin ligands at a glance. J. Cell Sci. 119, 3901–3903 10.1242/jcs.03098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Ivaska J., Whelan R. D. H., Watson R., Parker P. J. (2002). PKCe controls the traffic of b1 integrins in motile cells. EMBO J. 21, 3608–3619 10.1093/emboj/cdf371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J., Vuoriluoto K., Huovinen T., Izawa I., Inagaki M., Parker P. J. (2005). PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 24, 3834–3845 10.1038/sj.emboj.7600847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. C., Caswell P. T., Moran–Jones K., Roberts M., Barry S. T., Gampel A., Mellor H., Norman J. C. (2009). VEGFR1 (Flt1) regulates Rab4 recycling to control fibronectin polymerization and endothelial vessel branching. Traffic 10, 754–766 10.1111/j.1600-0854.2009.00898.x [DOI] [PubMed] [Google Scholar]
- Jović M., Naslavsky N., Rapaport D., Horowitz M., Caplan S. (2007). EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J. Cell Sci. 120, 802–814 10.1242/jcs.03383 [DOI] [PubMed] [Google Scholar]
- Jović M., Kieken F., Naslavsky N., Sorgen P. L., Caplan S. (2009). Eps15 homology domain 1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling. Mol. Biol. Cell 20, 2731–2743 10.1091/mbc.E08-11-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. J., Worth D. C., Scales T. M. E., Monypenny J., Jones G. E., Parsons M. (2011). β1 integrins regulate fibroblast chemotaxis through control of N-WASP stability. EMBO J. 30, 1705–1718 10.1038/emboj.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate K. R., Wickström S. A., Fässler R. (2009). Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23, 397–418 10.1101/gad.1758709 [DOI] [PubMed] [Google Scholar]
- Li J., Ballif B. A., Powelka A. M., Dai J., Gygi S. P., Hsu V. W. (2005). Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev. Cell 9, 663–673 10.1016/j.devcel.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Li J., Peters P. J., Bai M., Dai J., Bos E., Kirchhausen T., Kandror K. V., Hsu V. W. (2007). An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 178, 453–464 10.1083/jcb.200608033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobert V. H., Brech A., Pedersen N. M., Wesche J., Oppelt A., Malerød L., Stenmark H. (2010). Ubiquitination of α5β1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev. Cell 19, 148–159 10.1016/j.devcel.2010.06.010 [DOI] [PubMed] [Google Scholar]
- Lombardi D., Soldati T., Riederer M. A., Goda Y., Zerial M., Pfeffer S. R. (1993). Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12, 677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai A., Veltel S., Pellinen T., Padzik A., Coffey E., Marjomäki V., Ivaska J. (2011). Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J. Cell Biol. 194, 291–306 10.1083/jcb.201012126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S., Pagano R. E. (2007). Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8, 603–612 10.1038/nrm2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- Muller P. A. J., Caswell P. T., Doyle B., Iwanicki M. P., Tan E. H., Karim S., Lukashchuk N., Gillespie D. A., Ludwig R. L., Gosselin P.et al. (2009). Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- Muller P A., Trinidad A G., Timpson P., Morton J P., Zanivan S., van den Berghe P V., Nixon C., Karim S A., Caswell P T., Noll J E., et al. (2012). Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene [Epub ahead of print] 10.1038/onc.2012.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N., Caplan S. (2011). EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 21, 122–131 10.1016/j.tcb.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T., Shima D., Squire A., Bastiaens P. I. H., Gschmeissner S., Humphries M. J., Parker P. J. (1999). PKCalpha regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 18, 3909–3923 10.1093/emboj/18.14.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Kaibuchi K. (2007). Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell 13, 15–28 10.1016/j.devcel.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Pellinen T., Ivaska J. (2006). Integrin traffic. J. Cell Sci. 119, 3723–3731 10.1242/jcs.03216 [DOI] [PubMed] [Google Scholar]
- Pellinen T., Arjonen A., Vuoriluoto K., Kallio K., Fransen J. A. M., Ivaska J. (2006). Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J. Cell Biol. 173, 767–780 10.1083/jcb.200509019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H., Grosse R., Kitzing T., Rantala J. K., Kallioniemi O.et al. (2008). Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371–385 10.1016/j.devcel.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Powelka A. M., Sun J., Li J., Gao M., Shaw L. M., Sonnenberg A., Hsu V. W. (2004). Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5, 20–36 10.1111/j.1600-0854.2004.00150.x [DOI] [PubMed] [Google Scholar]
- Rainero E., Caswell P. T., Muller P. A. J., Grindlay J., McCaffrey M. W., Zhang Q., Wakelam M. J. O., Vousden K. H., Graziani A., Norman J. C. (2012). Diacylglycerol kinase α controls RCP-dependent integrin trafficking to promote invasive migration. J. Cell Biol. 196, 277–295 10.1083/jcb.201109112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay A. G., Keppler M. D., Jazayeri M., Thomas G. J., Parsons M., Violette S., Weinreb P., Hart I. R., Marshall J. F. (2007). HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res. 67, 5275–5284 10.1158/0008-5472.CAN-07-0318 [DOI] [PubMed] [Google Scholar]
- Rantala J. K., Pouwels J., Pellinen T., Veltel S., Laasola P., Mattila E., Potter C. S., Duffy T., Sundberg J. P., Kallioniemi O.et al. (2011). SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat. Cell Biol. 13, 1315–1324 10.1038/ncb2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D., Lugassy Y., Sprecher E., Horowitz M. (2010). Loss of SNAP29 impairs endocytic recycling and cell motility. PLoS ONE 5, e9759 10.1371/journal.pone.0009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. R., Hart I. R., Watson A. R., Welti J. C., Silva R. G., Robinson S. D., Da Violante G., Gourlaouen M., Salih M., Jones M. C.et al. (2009). Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 15, 392–400 10.1038/nm.1941 [DOI] [PubMed] [Google Scholar]
- Ribeiro I., Yuan L., Tanentzapf G., Dowling J. J., Kiger A. (2011). Phosphoinositide regulation of integrin trafficking required for muscle attachment and maintenance. PLoS Genet. 7, e1001295 10.1371/journal.pgen.1001295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J., Ghigo E., Kalaidzidis Y., Zerial M. (2005). Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 10.1016/j.cell.2005.06.043 [DOI] [PubMed] [Google Scholar]
- Roberts M., Barry S., Woods A., van der Sluijs P., Norman J. (2001). PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11, 1392–1402 10.1016/S0960-9822(01)00442-0 [DOI] [PubMed] [Google Scholar]
- Roberts M. S., Woods A. J., Dale T. C., Van Der Sluijs P., Norman J. C. (2004). Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol. Cell. Biol. 24, 1505–1515 10.1128/MCB.24.4.1505-1515.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A., Goto–Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., Zerial M. (2008). The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133, 486–497 10.1016/j.cell.2008.02.044 [DOI] [PubMed] [Google Scholar]
- Seaman M. N., McCaffery J. M., Emr S. D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142, 665–681 10.1083/jcb.142.3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Giridharan S. S., Rahajeng J., Naslavsky N., Caplan S. (2009). MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol. Biol. Cell 20, 5181–5194 10.1091/mbc.E09-06-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Sottile J. (2008). Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J. Cell Sci. 121, 2360–2371 10.1242/jcs.014977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalski M., Coppolino M. G. (2005). SNARE-mediated trafficking of alpha5beta1 integrin is required for spreading in CHO cells. Biochem. Biophys. Res. Commun. 335, 1199–1210 10.1016/j.bbrc.2005.07.195 [DOI] [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Anderson R. G. (1995). Hormonal regulation of caveolae internalization. J. Cell Biol. 131, 929–938 10.1083/jcb.131.4.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E., Suckert C., Al–Attar H., Marsden M. (2010). Integrin alpha5beta1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation. PLoS ONE 5, e10665 10.1371/journal.pone.0010665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. (2009). Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- Sturge J., Wienke D., Isacke C. M. (2006). Endosomes generate localized Rho-ROCK-MLC2-based contractile signals via Endo180 to promote adhesion disassembly. J. Cell Biol. 175, 337–347 10.1083/jcb.200602125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teckchandani A., Toida N., Goodchild J., Henderson C., Watts J., Wollscheid B., Cooper J. A. (2009). Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J. Cell Biol. 186, 99–111 10.1083/jcb.200812160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Jung J-J., Inamdar S. M., Brown C. O., Goel A., Choudhury A. (2011). Endothelial cell migration on fibronectin is regulated by syntaxin 6-mediated alpha5beta1 integrin recycling. J. Biol. Chem. 286, 36749–36761 10.1074/jbc.M111.260828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upla P., Marjomäki V., Kankaanpää P., Ivaska J., Hyypiä T., Van Der Goot F. G., Heino J. (2004). Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol. Biol. Cell 15, 625–636 10.1091/mbc.E03-08-0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdembri D., Caswell P. T., Anderson K. I., Schwarz J. P., König I., Astanina E., Caccavari F., Norman J. C., Humphries M. J., Bussolino F.et al. (2009). Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 7, e25 10.1371/journal.pbio.1000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale K. J., Offenhäuser C., Whittaker S. P., Estrella R. P., Murray R. Z. (2010). Recycling endosome membrane incorporation into the leading edge regulates lamellipodia formation and macrophage migration. Traffic 11, 1370–1379 10.1111/j.1600-0854.2010.01094.x [DOI] [PubMed] [Google Scholar]
- White D. P., Caswell P. T., Norman J. C. (2007). alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 177, 515–525 10.1083/jcb.200609004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström S. A., Alitalo K., Keski–Oja J. (2002). Endostatin associates with integrin α5β1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 62, 5580–5589 [PubMed] [Google Scholar]
- Wickström S. A., Lange A., Hess M. W., Polleux J., Spatz J. P., Krüger M., Pfaller K., Lambacher A., Bloch W., Mann M.et al. (2010). Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell 19, 574–588 10.1016/j.devcel.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A. J., White D. P., Caswell P. T., Norman J. C. (2004). PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 23, 2531–2543 10.1038/sj.emboj.7600267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S O., Shin S., Mercurio A M. (2005). Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the α6β4 integrin. Cancer Res. 65, 2761–2769 10.1158/0008-5472.CAN-04-4122 [DOI] [PubMed] [Google Scholar]
- Yuan L., Fairchild M. J., Perkins A. D., Tanentzapf G. (2010). Analysis of integrin turnover in fly myotendinous junctions. J. Cell Sci. 123, 939–946 10.1242/jcs.063040 [DOI] [PubMed] [Google Scholar]
- Zech T., Calaminus S. D. J., Caswell P., Spence H. J., Carnell M., Insall R. H., Norman J., Machesky L. M. (2011). The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J. Cell Sci. 124, 3753–3759 10.1242/jcs.080986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu X., Datta A., Govindarajan K., Tam W. L., Han J., George J., Wong C., Ramnarayanan K., Phua T. Y.et al. (2009). RCP is a human breast cancer-promoting gene with Ras-activating function. J. Clin. Invest. 119, 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.