Summary
The extracellular matrix (ECM) is an intricate network of proteins that surrounds cells and has a central role in establishing an environment that is conducive to tissue-specific cell functions. In the case of stem cells, this environment is the stem cell niche, where ECM signals participate in cell fate decisions. In this Commentary, we describe how changes in ECM composition and mechanical properties can affect cell shape and stem cell differentiation. Using chondrogenic differentiation as a model, we examine the changes in the ECM that occur before and during mesenchymal stem cell differentiation. In particular, we focus on the main ECM protein fibronectin, its temporal expression pattern during chondrogenic differentiation, its potential effects on functions of differentiating chondrocytes, and how its interactions with other ECM components might affect cartilage development. Finally, we discuss data that support the possibility that the fibronectin matrix has an instructive role in directing cells through the condensation, proliferation and/or differentiation stages of cartilage formation.
Key words: Fibronectin; Chondrogenesis; Stem cell differentiation; Extracellular matrix, SOX9
Introduction
The extracellular matrix (ECM) is a fibrillar network of proteins that has an essential role in determining tissue architecture by providing a framework for cell adhesion (Hynes and Yamada, 2012; Mecham, 2011). Cell–ECM interactions largely depend on integrin cell-surface receptors. Binding of these receptors to sites on proteins within the ECM transduces environmental information to the cell interior, which directs tissue-specific cell functions. ECM signals are communicated through these receptors to regulate cellular functions such as proliferation, migration, survival and differentiation (Geiger et al., 2001; Geiger and Yamada, 2011). All cells that reside within tissues, including stem cells, interact with the ECM. A fundamental property of stem cells is the ability to maintain a balance between self-renewal and differentiation, and these cell fate decisions are affected by signals from the ECM within the stem cell niche.
The protein composition of the ECM varies from one tissue to the next. The main fibrillar components of an ECM can be divided into two groups: collagens and cell adhesive glycoproteins (e.g. laminins and fibronectin) (Mecham, 2011). Collagens are triple-helix proteins that assemble into multimeric fibrils through end-to-end and lateral interactions. The principal integrins for collagen binding are integrin α1β1 and integrin α2β1 (Hynes and Naba, 2012; Hynes and Yamada, 2012). The ECM glycoproteins fibronectin and laminin are multidomain proteins that contain binding sites for integrins, collagen and other ECM proteins, glycosaminoglycans, as well as self-association sites (Singh et al., 2010; Yurchenco and Patton, 2009). The multidomain structure of these proteins, thus, provides a mechanism for connecting cells to the ECM network by allowing simultaneous interaction with integrins, collagens and other ECM components. Integrin receptors that bind laminins include integrins α3β1, α6β1 and α7β1, whereas fibronectin binds to – among others – integrins α5β1, α4β1 and αvβ3 (Hynes and Naba, 2012; Hynes and Yamada, 2012). The connections that are formed between the cytoplasmic domains of integrin receptors and the cytoskeleton subsequently organize intracellular actin filaments to induce specific cell morphologies (Geiger and Yamada, 2011; Schwartz, 2010). The composition and organization of the ECM determine the physical properties of a tissue (such as compliance, topology and insolubility), and these properties influence cell adhesion, cell shape and the activation of intracellular signaling pathways.
Because the ECM has a crucial role in tissue structure and function, interactions between stem cells and the surrounding ECM are likely to participate in decisions regarding the stem cell fate. Although embryonic and adult stem cells are known to express integrin receptors that bind various ECM proteins (Kapur et al., 2001; Klees et al., 2007; Wagner et al., 2005), the contributions that specific ECM proteins make to stem cell function have not been thoroughly investigated. So far, the main limitation to studies on the interaction between stem cells and the ECM is the lack of information about the composition of the stem cell niche. In this Commentary, we discuss the current knowledge of the role of the ECM in the stem cell niche and the regulation of stem cell functions. In addition, we focus on fibronectin, an ubiquitous ECM component that has been the focus of many studies of ECM assembly, ECM mechanical properties and cell–ECM interactions, and on chondrogenesis, a stem cell differentiation process that depends on changes in ECM protein expression and deposition.
The ECM in stem cell differentiation
The ECM in the stem cell niche
Within tissues, stem cells reside in the stem cell niche, which is composed of various support cells, the ECM and soluble factors (Lo Celso and Scadden, 2011). Interactions within the niche are important for maintaining stem cells in an undifferentiated state, but also contribute to the decision to differentiate. The severe defects in early development that are observed in β1-integrin knockout mice suggest that these receptors and their ECM ligands are important for stem cell fate decisions. The lack of β1 integrin affects the morphogenesis and survival of embryonic inner cell mass cells (Stephens et al., 1995) and, in culture, β1-integrin-null embryonic stem (ES) cells fail to adhere to the ECM (Fassler et al., 1995). Likewise, laminins and fibronectin have essential roles in early differentiation events (Aszodi et al., 2006; George et al., 1993; Li et al., 2002). The developmental requirements for the ECM and for receptors that recognize specific extracellular proteins raise important questions about how these ECM proteins control stem cell differentiation.
The hematopoietic stem cell niche has been particularly well studied and consists of multiple cell types, including osteoblastic cells, endothelial cells and adipocytes (Lo Celso and Scadden, 2011), as well as the ECM proteins fibronectin, collagens and vitronectin (Whetton and Graham, 1999). Mesenchymal support cells in the niche produce and assemble an ECM, and the composition of this ECM can have a role in maintaining stem cell properties – as has been shown recently with human mesenchymal stem cells grown on an ECM derived from bone marrow stromal cells (Lai et al., 2010). The repertoire of stem cell integrin receptors determines the extent of cell–ECM binding. Hematopoietic stem cells, for example, express α4β1 integrin, which binds to fibronectin and to receptors on neighboring cells (Whetton and Graham, 1999), so this receptor can tether the stem cells to the ECM and to support cells within the bone marrow. The multimeric ECM protein tenascin-C is a stem cell niche component of the hair follicle bulge region (Tumbar et al., 2004) and is also present in the bone marrow (Klein et al., 1993). Tenascin-C binds to fibronectin and modulates cell adhesion (Hsia and Schwarzbauer, 2005). This raises the possibility that this protein influences hematopoietic stem cell interactions with the ECM in their niche.
The interplay between stem cells and mesenchymal support cells might affect the composition of the niche ECM. Tumor metastasis studies suggest that cancer stem cells can induce resident fibroblasts to express certain ECM proteins in order to make the tissue environment permissive for colonization. In particular, both fibronectin (Kaplan et al., 2005) and periostin (Malanchi et al., 2012) have been linked to the development of a metastatic niche. Although these studies were carried out with cancer stem cells, it would not be surprising to find that normal stem cells also have an impact on the composition of the niche ECM. In fact, both embryonic and adult stem cells express ECM proteins (Hunt et al., 2012; Lai et al., 2010), a fact that raises the possibility that incorporation of stem cell proteins into the matrix network contribute to cell fate decisions. The role of ECM composition and its impact on stem cell behavior in vivo is a complex problem that requires further investigation.
ECM compliance and cell shape in stem cell differentiation
The composition of the ECM substrate in vitro can have a direct effect on stem cell differentiation. It has been shown that plating ES cells on laminin or fibronectin induces differentiation, whereas self-renewal is maintained when the cells are plated on type I or type IV collagen substrates (Hayashi et al., 2007). Different types of laminin have differential effects on stem cell differentiation. For example, laminin-511, but not several other laminin types, can support self-renewal (Domogatskaya et al., 2008). Laminin-322 stimulates osteogenic differentiation of mesenchymal stem cells, whereas laminin-111 promotes neural differentiation (Klees et al., 2005; Klees et al., 2007; Mruthyunjaya et al., 2010). Fibronectin can mediate ES cell differentiation towards a meso-endodermal lineage (Hayashi et al., 2007) by upregulating the expression of integrin α5β1 (Pimton et al., 2011). Under different culture conditions, however, fibronectin promotes ES cell self-renewal (Hunt et al., 2012).
In adult stem cells, fibronectin can promote differentiation along skeletal lineages while suppressing adipogenic differentiation (Martino et al., 2009; Ogura et al., 2004; Wang et al., 2010). Recent results have shown that chondrogenesis is enhanced when mesenchymal stem cells are expanded on a three-dimensional decellularized ECM compared with cells that are grown on a planar ECM-coated surface (Pei et al., 2011). By contrast, planar fibronectin substrates have been shown to enhance mesenchymal stem cell migration (Veevers-Lowe et al., 2011). The effects of fibronectin on cell fate also extend to differentiated cells: 3T3 adipocytes show reduced lipogenic gene expression when grown on fibronectin (Spiegelman and Ginty, 1983). Differential effects of specific ECM components (collagen, laminin, fibronectin) and ECM architecture (planar versus decellularized) on self-renewal and differentiation of ES and adult stem cells indicate that cell fate decisions depend on the context. Thus, it is important to determine the identity and organization of niche components in order to understand the interactions of stem cells with their niche.
Cell shape is determined by receptor-mediated interactions with proteins in the ECM and is linked to the regulation of cell growth (see, for example, Folkman and Moscona, 1978). In addition, cell shape has a role in determining lineage-specific differentiation of stem cells. For example, mesenchymal stem cell spreading, which involves Rho GTPase activity and tension generated by cell contractility, supports osteogenic differentiation (McBeath et al., 2004; Wang et al., 2011). By contrast, restricting cell spreading by exposing cells to a specific substrate composition or plating them at increasing densities antagonizes osteogenesis and promotes adipogenic (Wang et al., 2011). These results suggest that differentiation can be influenced by stem cell shape, which is determined by multiple factors including the composition and properties of the ECM.
The composition of the ECM also determines its mechanical properties, but the inherent complexity of the ECM network makes it difficult to manipulate these properties. One way to modulate substrate stiffness is through the use of hydrogels (e.g. polyacrylamide gels) of varying elasticity (Pelham and Wang, 1997). In-vitro studies have provided convincing evidence that stem cells respond to signals that are generated as a result of varying substrate stiffness and use this information to determine their differentiation fate. ES cells grown on a soft gel maintain a state of self-renewal (Winer et al., 2009) and express high levels of self-renewal markers such as NANOG and OCT4 (also known as POU5F1), even in the absence of the self-renewal factor leukemia inhibitory factor (LIF) (Chowdhury et al., 2010). Varying the compliance of collagen-coated gels has been shown to have different effects on mesenchymal stem cells. A substrate that mimics the stiffness of brain tissue promotes neurogenic differentiation, whereas stiffer substrates induce myogenic or osteogenic properties (Engler et al., 2006). Similarly, neural stem cells that are grown on a substrate with the approximate stiffness of the ECM in brain tissue upregulate neuronal markers (Saha et al., 2008); on stiffer substrates, however, they suppress neurogenesis through Rho activation (Keung et al., 2011). Together, these and other studies indicate that the composition and stiffness of the ECM contribute to the determination of stem cell fate. However, the details of when, where and how the ECM influences stem cell fate remain to be uncovered.
As described above, some ECM components of stem cell niches have been identified, and experiments using cells in culture provided additional information on how stem cells respond when they adhere to ECM proteins such as fibronectin, collagens or laminins. However, mechanistic information about stem cells and the ECM is limited. Stem cell microenvironments are likely to differ from one tissue to the next, and between embryonic and adult tissues. Below we describe insights into stem cell differentiation and the ECM that have been gleaned from analyses of cartilage development. Studies of this process have benefited from the availability of complementary in vivo and in vitro systems. Developmental information has been gained from analyses of limb buds in vivo and from the existence of many non-lethal skeletal mutations that affect ECM molecules, whereas chondrogenic culture systems have allowed the dissection of molecular events and cellular changes throughout the early stages of the process.
Differentiation of mesenchymal cells
Steps involved in chondrogenesis
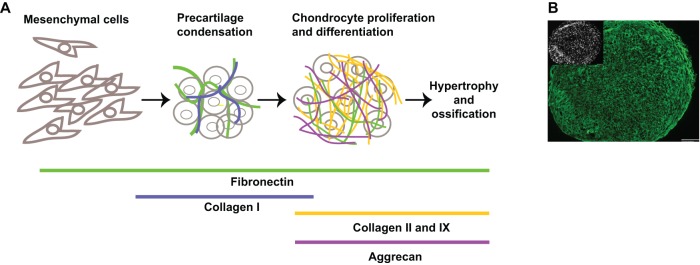
One process that has provided new insights into the functions of the ECM during cell differentiation is chondrogenesis, because the composition of the matrix changes dramatically as mesenchymal cells differentiate (Sundelacruz and Kaplan, 2009). The initiation of skeletal development in the limb bud has provided substantial information about the very early steps of chondrogenesis in vivo. During embryogenesis, prechondrogenic mesenchymal cells that reside within the ECM of the limb bud rearrange into condensed cell aggregates. This formation of a compact cell mass is accompanied by changes in cell shape (from spread to rounded) and the deposition of pre-cartilaginous ECM (DeLise et al., 2000) (Fig. 1A). Condensed cells are readily detected by staining with labeled peanut agglutinin – which binds to a galactose moiety that is upregulated in cell aggregates during chondrogenic differentiation in vivo and in vitro (Aulthouse and Solursh, 1987) – or, at later stages, with Alcian Blue, which detects glycosaminoglycan moieties on ECM proteoglycans. Condensation is a prerequisite for chondrogenic differentiation (Bobick et al., 2009; DeLise et al., 2000) and depends on the expression of the cell–cell adhesion proteins N-cadherin and neural cell adhesion molecule (NCAM). Antibody-blocking experiments and the modulation of gene expression in the limb bud confirmed the importance of these two molecules in mediating cell–cell interactions during condensation (Bobick et al., 2009). Changes in protein expression also occur during condensation. In particular, the expression of fibronectin is rapidly upregulated when mesenchymal cells condense (Kulyk et al., 1989).
Fig. 1.
The stages of chondrogenesis. (A) The diagram illustrates the principal steps of chondrogenesis and indicates some of the main ECM proteins that are expressed at different stages throughout the process. The ECM proteins fibronectin (green), collagen I (blue), collagen II and IX (orange) and aggrecan (violet) are shown. (B) Mesenchymal stem cells were induced to undergo chondrogenesis in pellet culture for 12 days in chondrogenic medium containing TGF-β3. At day 12, pellets were harvested, embedded and cut into 5 µm-thick sections, which were fixed and stained. Anti-human fibronectin (HFN7.1) monoclonal antibody staining was followed by Alexa-Fluor-488-conjugated goat anti-mouse immunoglobulin G. The image shows that the fibronectin matrix is present throughout the section (green). Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) and the inset shows the cell density within the section. Scale bar: 100 µm.
Following the aggregation of mesenchymal cells, the levels of cell–cell adhesion molecules drop. However, cells remain closely associated, possibly as a result of the assembly of pre-cartilage ECM between differentiating cells (Fig. 1B). The importance of this ECM for cell condensation and subsequent differentiation is evident from knockdown or blocking experiments in which targeting fibronectin, versican or collagen causes defects in chondrogenesis (Kamiya et al., 2006; White et al., 2003; Wilson et al., 2011). As differentiation proceeds, a matrix that is rich in fibronectin and type I collagen is replaced with one that contains type II collagen and aggrecan as the main components (Bobick et al., 2009; Sundelacruz and Kaplan, 2009). Interactions between proteins within the matrix and their receptors on the surface of differentiating chondrocytes might provide environmental signals that contribute to the progression of differentiation. Ultimately, in vivo, the ECM is crucial for defining cartilage structure and flexibility as well as chondrocyte fate when the cells progress through the proliferating, hypertrophic and terminal stages (Lefebvre and Bhattaram, 2010) (Box 1).
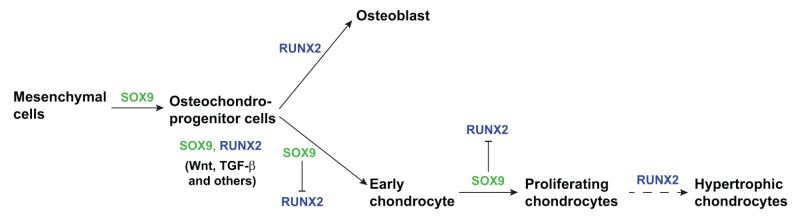
Box 1: Transcriptional regulation of a mesenchymal cell differentiation.
The vertebrate skeleton forms through a process called endochondral ossification. Multipotent mesenchymal cells in the limb buds condense and differentiate into osteochondroprogenitor cells that are the precursors for cartilage and bone tissue (Hartmann, 2009; Kronenberg, 2003). Chondrocytes develop in the center of the condensate, while the surrounding cells form the perichondrium – which later gives rise to osteoblasts. The differentiated chondrocytes proliferate and mature into hypertrophic chondrocytes, which are growth-arrested cells that deposit mineralized matrix as a template for bone formation by osteoblasts (Kronenberg, 2003) (see Figure). The decision for progenitor cells to differentiate depends on the integration of signals arising from soluble extracellular factors – such as TGF-β family members, Wnt and others (Lefebvre and Bhattaram, 2010) – and on two main transcription factors: SOX9 for chondrogenesis and Runt-domain transcription factor (RUNX2) for osteogenesis (Komori et al., 1997; Otto et al., 1997). These two transcription factors are expressed by osteochondroprogenitor cells (Akiyama et al., 2005) and both are regulated by the effects of other transcription factors (Hartmann, 2009). SOX9 has a dominant role in promoting chondrogenic differentiation by positively regulating progenitor differentiation and negatively regulating RUNX2 (Zhou et al., 2006). Two other SOX family transcription factors, SOX5 and SOX6, cooperate with SOX9 to promote chondrogenesis. In mouse embryos that lack these factors, differentiation is impaired but not blocked (Lefebvre et al., 1998). RUNX2 expression stimulates differentiation of the osteoblast lineage and also contributes to chondrocyte development by regulating maturation into hypertrophic chondrocytes (Hinoi et al., 2006; Karsenty, 2008).
Deciphering the molecular mechanisms of chondrogenesis has been facilitated by the ability to recapitulate the steps in high-density micromass- or pellet-culture systems in vitro. Using this approach, various mesenchymal cell types, ES cells and mesenchymal stem cells can be induced to undergo chondrogenesis and follow the in vivo differentiation steps in the same order (Chen et al., 2009; Diekman et al., 2010; Richter, 2009; Toh et al., 2010). Aggregating cells by pelleting or plating them at a high density, followed by growth in chondrogenic culture medium that contains members of the transforming growth factor beta (TGF-β) family and other growth factors, results in the completion of the condensation step. In addition, this process activates those changes in gene expression that are required for differentiation (Chimal-Monroy and Diaz de Leon, 1999; Kawakami et al., 2006). In particular, the expression of type II collagen and aggrecan is upregulated at this point, which promotes the deposition of a cartilage-specific ECM.
Cell shape and chondrogenic differentiation
Mesenchymal cell shape appears to have a substantial role in the initiation of the chondrogenic program. Prior to condensation, cells have a typical mesenchymal morphology, but they become more rounded as cell–cell interactions increase during condensation (Fig. 1A). Early studies have shown that treating limb mesenchymal cells with cytochalasin D, which depolymerizes actin filaments, stimulates chondrogenic differentiation and blocks the anti-chondrogenic effects of ectoderm-conditioned medium (Zanetti et al., 1990; Zanetti and Solursh, 1984). More recently, synthetic substrates that were designed to control cell shape have provided evidence in support of the idea that restricting cell spreading promotes differentiation of mesenchymal stem cells towards chondrocytes (Gao et al., 2010).
Cell shape can be affected by changing cell density: as cell density increases, cell spreading decreases. Furthermore, the comparison of multipotent mouse cells grown at different densities has shown a correlation between a round nuclear shape and an increase in the expression of chondrogenic differentiation markers (McBride and Knothe Tate, 2008). Differentiated chondrocytes also respond to changes in cell shape and density. Dispersed chondrocytes have a stellate morphology. Moreover, in these cells, the synthesis of sulfated glycosaminoglycans is substantially lower than in chondrocytes that are cultured at high density or in pellet cultures – where cells maintain a more rounded morphology (Abbott and Holtzer, 1966). These results suggest a correlation between a rounded chondrocyte shape and the maintenance of a differentiated phenotype.
Transcriptional regulation during chondrogenesis
Differentiation and expression of chondrogenic ECM proteins depends on the transcription factor sex-determining region Y protein (SRY)-box 9 (SOX9), which is a master regulator of chondrogenic differentiation both in vivo and in vitro (Box 1). SOX9 is expressed during condensation in the developing chick limb, and its misexpression by retroviral transduction in early fore- and hindlimb regions causes the formation of ectopic cartilage, which indicates that SOX9 is sufficient to promote chondrogenesis (Healy et al., 1999). Defects in SOX9 binding to DNA cause campomelic dysplasia, a congenital deformity that is characterized by bowing of the long bones as well as other skeletal and reproductive defects (Foster et al., 1994), which highlights an essential role for SOX9 in regulating chondrogenic gene expression. Analyses of heterozygous SOX9+/− mice have confirmed a role for this transcription factor in chondrogenesis (Bi et al., 1999). Furthermore, using SOX9−/− ES cells to generate chimeric mice has shown that these cells are excluded from the mesenchymal condensation of wild type cells (Bi et al., 1999), and live imaging of SOX9 chimeras has shown that this exclusion corresponds to the formation of a less rounded, more spread-out cell phenotype (Barna and Niswander, 2007). Whereas overexpression of SOX9 in chick micromass cultures increases the extent of condensation, the complete absence of Sox9 in mouse ES cells does not prevent pre-cartilage condensation or upregulation of certain prechondrogenic markers (Hargus et al., 2008). Taken together, these findings indicate that SOX9 is not required during condensation. However, it is expressed in skeletal precursors and has been shown to be essential for differentiation in a variety of systems (Akiyama, 2008; Bi et al., 1999) (Box 1). SOX9 has an important role in regulating changes in the expression of ECM proteins during chondrogenesis. It directly regulates expression of the main cartilage collagen gene COL2A1 in chondroprogenitor cells and in chondrocytes (Akiyama, 2008; Bell et al., 1997; Ng et al., 1997; Zhao et al., 1997).
ECM deposition during chondrogenesis
Chondrogenesis is an excellent system for studying changes in ECM production and deposition, because the components of mesenchymal, pre-cartilaginous and cartilaginous matrices are altered as chondrocytes differentiate (Table 1). Undifferentiated mesenchymal cells and the condensing mesenchyme deposit a fibronectin-rich matrix (Dessau et al., 1980; Kulyk et al., 1989) that also contains versican (Kamiya et al., 2006; Kimata et al., 1986), type I collagen (Dessau et al., 1980) and hyaluronan (Knudson and Knudson, 2001). Tenascin-C is expressed during condensation and, as condensed cells undergo differentiation, levels of tenascin-C and collagen I decrease (Dessau et al., 1980; Kosher et al., 1986; Mackie et al., 1987). By contrast, a cartilaginous matrix consisting of collagen types II and IX, and the proteoglycans aggrecan and versican develops during this stage (Choocheep et al., 2010; Knudson and Knudson, 2001; Kravis and Upholt, 1985; Kulyk et al., 1991). Concomitant with changes in the molecular composition of the ECM, receptors for specific ECM proteins are expressed by differentiating chondrocytes (Djouad et al., 2007).
Table 1.
Changes of main ECM components during chondrogenesis
| ECM protein | Mesenchyme | Pre-cartilage matrix | Cartilage matrix |
| Collagen I | Yes | Yes | No |
| Collagen II | No | No | Yes |
| Collagen IX | No | No | Yes |
| Fibronectin | Yes | Yes | Yes |
| Tenascin-C | No | Yes | No |
| Aggrecan | No | No | Yes |
| Versican | Yes | Yes | Yes |
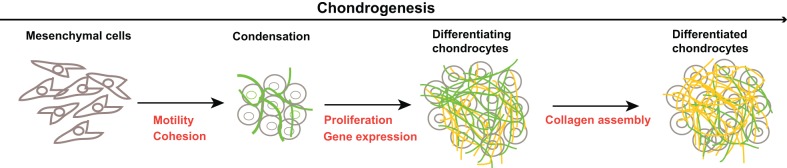
Although fibronectin is not usually considered a principal cartilage protein, it is present throughout differentiation and persists in mature cartilaginous tissue. Fibronectin is abundant in the developing mesenchyme, and its expression is upregulated during cell condensation in vivo and in vitro (Dessau et al., 1980; Kulyk et al., 1989). Its level peaks early in the differentiation phase, declines somewhat, and then remains at detectable levels as type II collagen and proteoglycans accumulate. In addition, fibronectin is abundant throughout the matrix of the growth plate and other types of cartilaginous tissue (Melnick et al., 1981; DeLise et al., 2000; J.E.S., unpublished observations), which suggests a continuing role for fibronectin in chondrocyte differentiation after birth. Furthermore, fibronectin directly interacts with many of the proteins that are expressed at various stages of differentiation (Table 1), including tenascin-C, versican, glycosaminoglycans and a range of collagens (Hynes, 1990; Parsons et al., 2011; Singh et al., 2010). One potential role for fibronectin during chondrogenesis is to promote proliferation during the differentiation phase (Fig. 1A; Fig. 2). Along these lines, fibronectin–integrin signaling has been shown to regulate cell-cycle progression (Schwartz and Assoian, 2001). Increased expression of fibronectin during condensation would result in upregulation of the fibronectin matrix (Fig. 1B), thus generating additional contact sites between the cells and fibronectin that could positively influence cell proliferation.
Fig. 2.
Potential roles for fibronectin in facilitating progression through the chondrogenesis stages. The principal chondrogenesis steps are illustrated, with fibronectin (green) and collagen matrices (orange) highlighted. Specific cell activities that might involve fibronectin are indicated in red at each step. Please refer to the text for further details.
In addition to changes in fibronectin expression, the fibronectin isoforms that are expressed vary during chondrogenesis. Fibronectin is alternatively spliced at three sites, namely the extra type III domains EIIIA and EIIIB and the variable (V)/IIICS region (Schwarzbauer and DeSimone, 2011). Mice that lack either the EIIIA or EIIIB exon do not develop overt skeletal defects (Fukuda et al., 2002; George et al., 1993; Muro et al., 2003). However, the simultaneous deletion of both exons causes cardiovascular defects and, in certain genetic backgrounds, embryonic lethality (Astrof et al., 2007). As cartilage develops, the proportions of fibronectin that contain EIIIA or EIIIB change dramatically. Fibronectin containing both EIIIA and EIIIB is prevalent in the developing mesenchyme (Bennett et al., 1991), and antibody-blocking experiments have indicated that fibronectin containing the EIIIA exon participates in cell condensation (Gehris et al., 1997). During differentiation, inclusion of the EIIIA exon is reduced both in vivo and in vitro (Gehris et al., 1996), such that mRNA of cartilage fibronectin includes exon EIIIB but not exon EIIIA (White et al., 2003). This specific pattern of fibronectin transcript splicing suggests that the balance of isoforms contributes to chondrogenesis, but their exact functions remain to be determined.
Both fibronectin and its main cell surface receptor integrin α5β1 are expressed during condensation and throughout differentiation (Djouad et al., 2007; Goessler et al., 2009). In order to assemble cartilage matrix that is rich in type II collagen, integrins α1β1 and α2β1 – which act as collagen receptors – are also highly expressed during differentiation (Goessler et al., 2009). Numerous signaling pathways that lie downstream of integrins or are activated by chondrogenic factors have been linked to various stages of this process. Activation of focal adhesion kinase (FAK) and paxillin occurs during pre-cartilage condensation (Bang et al., 2000). Extracellular-signal-regulated kinases 1 and 2 (ERKs1/2, also known as MAPK3 and MAPK1, respectively) are activated by binding of preadipocyte factor 1 (Pref-1, also known as DLK1, a transmembrane protein that contains EGF-like repeats) to fibronectin, which leads to upregulation of SOX9 and inhibition of adipogenesis (Wang et al., 2010). Perhaps ERK signaling is one way by which fibronectin can modulate a differentiation switch between lineages. The Rho family of GTPases also responds to adhesion of the cells to fibronectin-based matrices and has been implicated in chondrogenesis. Rho has been linked to the regulation of SOX9 (Kumar and Lassar, 2009; Woods and Beier, 2006; Woods et al., 2005; Woods et al., 2007a), and the activation of the GTPase Rac is required for N-cadherin expression during condensation (Wang et al., 2007; Woods et al., 2007b). The number of other signaling pathways that control chondrogenic and osteogenic differentiation shows the complexity of initiating and regulating these processes (Augello and De Bari, 2010; Beier and Loeser, 2010; Bobick et al., 2009).
Fibronectin matrix assembly, chondrogenesis and disease
The ECM, its composition and organization, are central to the correct development of the skeleton. Numerous genetic diseases that cause overt defects in the formation and function of skeletal tissues have been linked to mutations in collagens, proteoglycans, and other ECM components in humans. Some of these mutations directly affect ECM assembly, whereas others affect the production or secretion of ECM components and, thus, decrease cell viability – which has a more general effect on skeletogenesis. Mutations in proteins that indirectly contribute to ECM assembly also cause skeletal defects. Prime examples of this category include mutations in SOX9 that are responsible for campomelic dysplasia (Foster et al., 1994), and mutations in components of the glycosaminoglycan synthesis and processing pathways that control post-translational modification of key proteoglycans in developing cartilage and bone (Zak et al., 2002).
Mutations in fibronectin that affect the skeleton have not been identified in humans, perhaps because severe mutations cause lethality (George et al., 1993). However, a connection between fibronectin matrix assembly and early steps in chondrogenesis has been suggested by the effect of mutations in the glycosaminoglycan synthesis pathways (Galante and Schwarzbauer, 2007). Mutations in human diastrophic dysplasia sulfate transporter (DTDST, also known as SLC26A2) cause a variety of skeletal dysplasias, including achondrogenesis and chondrodysplasias (Rossi and Superti-Furga, 2001). These mutations reduce sulfate import and thus affect the level of sulfation on proteins and glycosaminoglycans that are produced by the affected cells. Mice carrying a knock-in mutation from one of the human alleles show a partial loss of function of the sulfate transporter, and this causes defects in chondrocyte size, proliferation and terminal differentiation (Forlino et al., 2005; Gualeni et al., 2010). We have linked this gene to fibronectin matrix assembly in a microarray screen and have shown that reduced sulfation of proteoglycans and downregulation of the cell-surface proteoglycan syndecan-2 substantially reduces fibronectin matrix assembly (Galante and Schwarzbauer, 2007). This role for DTDST in the production of the fibronectin matrix raises the possibility that the fibronectin matrix has a role in the early stages of chondrogenesis and that defects in these early processes can lead to later skeletal defects.
Fibronectin matrix organization is also affected in mouse limbs with the conditional knockout of another glycosaminoglycan-related molecule, exostosin 1 (EXT1), which is involved in heparan sulfate synthesis (Zak et al., 2002). Differentiation of cells isolated from EXT1-knockout mouse limbs is impaired in a micromass culture and, in vivo, fibronectin matrix organization in the perichondrium is less compact than in wild-type animals, which correlates with a more diffuse distribution of bone morphogenetic proteins (BMPs) and glycosaminoglycans in the matrix (Matsumoto et al., 2010). Mice lacking a related enzyme, EXT2, also have defects in chondrocyte differentiation (Stickens et al., 2005). Together these findings indicate that glycosaminoglycan synthesis and modification pathways are crucial for establishing the appropriate matrix required for differentiation. Perhaps defects in these pathways and downstream perturbations of the matrix initiate a cascade of changes that ultimately lead to chondrodysplasias or other skeletal defects.
Does fibronectin have a structural or instructive role during chondrogenesis?
Given that fibronectin is present before and throughout chondrogenesis and is also found in the matrices of various types of cartilage, it seems likely that the fibronectin matrix has a role in these tissues. One possibility is that fibronectin has a structural role by providing a scaffold for cell adhesion and differentiation. Another, not mutually exclusive, possibility is that fibronectin signals through integrins to activate intracellular pathways that regulate changes in gene expression during chondrogenesis. The ERK and Rho GTPase signaling pathways are downstream of fibronectin and have been linked to chondrogenesis (Augello and De Bari, 2010; Beier and Loeser, 2010). In addition, fibronectin signals can support cell survival and growth (Schwartz and Assoian, 2001) and thus might contribute to cell viability during differentiation and in mature cartilage. Whereas some information is available about the signals that regulate chondrogenic differentiation, the contributions of fibronectin to chondrogenic signaling require further examination.
Cell condensation involves two processes: the movement of cells to the aggregation sites and cell cohesion to form and maintain the aggregate (Fig. 2). A primary function of fibronectin during embryonic development is to support mesenchymal cell migration (George et al., 1993). Its presence in the limb bud mesenchyme is likely to provide a substrate that allows cells to move and form a pre-cartilage cell aggregate. Cell–cell connections that are mediated by a fibronectin matrix are required for tumor cells to adhere to each other in vitro (Robinson et al., 2004). The assembly of the fibronectin matrix might have a similar role in limb buds by facilitating aggregate formation and providing a framework for the organization of mesenchymal cells in cell aggregates.
As cells shift into the differentiation phase, N-cadherin and NCAM expression is reduced, which would result in cells being released from strong interactions with each other. The fibronectin matrix surrounding the condensed cells (Fig. 1B; Fig. 2) might serve to maintain cells in close proximity, thus facilitating a cell organization that is conducive to differentiation. How these cells maintain a rounded shape while being surrounded by an adhesive fibronectin matrix might be crucial to the success of this phase of differentiation.
A final potential role for fibronectin is in the organization of the cartilage matrix. The fibronectin matrix has been shown to support deposition of collagens, latent TGF-β-binding protein (LTBP) and other ECM proteins (Dallas et al., 2005; Hynes, 2009; Kadler et al., 2008; Kutsuna et al., 2011; Parsons et al., 2011; Singh et al., 2010). Inhibition of fibronectin matrix assembly or interference with protein binding to fibronectin severely reduces matrix incorporation of these proteins. This dependency on fibronectin suggests that the organization of fibronectin fibrils direct the oriented deposition of cartilage ECM proteins and, in that way, provide spatial cues in developing cartilage. Such an instructive role might be particularly important in the later stages of chondrogenesis and, perhaps, also in mature cartilage, where fibronectin matrix fibrils might provide a framework for oriented collagen fibrillogenesis (Kadler et al., 2008). Whether fibronectin acts as a scaffold for cell organization or also directs the orientation of collagen and other cartilage ECM proteins requires further investigation. However, it has become clear that fibronectin is implicated in events throughout chondrogenesis and future studies might uncover its specific functions in this process.
Conclusions and perspectives
As described in this Commentary, there is substantial evidence to implicate protein components of the ECM in the regulation of stem cell behaviors. Certain ECM ligands and their receptors have been linked to lineage-specific differentiation and to the control of self-renewal. As the composition of the ECM can vary from tissue to tissue, stem cell decisions could be differentially affected depending on the location of the niche. The questions of when, where and how the ECM mediates its effects on stem cell fate decisions, therefore, require further investigation.
Using chondrogenesis as an example, we have illustrated how a single transcription factor (in this case, SOX9) can have a key regulatory role in stem cell differentiation. SOX9 is expressed in skeletal precursors and is required to promote the chondrogenic differentiation pathway while preventing osteogenesis. Substantial changes in the synthesis of ECM proteins occur during differentiation and – in chondrocytes – SOX9 regulates the expression of the main cartilage ECM proteins type II collagen and aggrecan. However, the possibility that changes in the interaction of matrix proteins and receptors trigger pathways that directly control the synthesis or activity of SOX9 needs to be evaluated.
We know that type I collagen is present early in the chondrogenic microenvironment and that it is eventually replaced by collagen II. Fibronectin is upregulated as condensation occurs and remains at detectable levels throughout differentiation and beyond. The levels of other proteins and of proteoglycans also change as cartilage matures. Some information is available about the ECM signals that regulate this differentiation process. Specific ECM components could be responsible for key regulatory steps or the organization of the ECM that results from coordinated assembly of fibronectin with collagens and proteoglycans could provide a permissive environment for differentiation. For example, a fibronectin–collagen matrix might act as a scaffold for cell organization, thus providing an architectural context that supports efficient differentiation. Understanding the organization and assembly of the niche microenvironment will not only provide mechanistic information about stem cell fate decisions but will also be important for the development of biomaterials for use in cartilage regeneration in the future (Box 2).
Box 2: Stem-cell-based therapy for cartilage regeneration.
Damage to cartilage as a result of injury or disease is difficult to treat because of the limited regenerative ability of cartilage tissue. Mesenchymal stem cells have the potential to be used in cell-based therapies because they can be induced to differentiate into cartilage or bone. They also can be propagated in culture, which contrasts with differentiated chondrocytes, as the latter undergo de-differentiation in vitro (Schulze-Tanzil, 2009). There was some success in using mesenchymal stem cells to repair injured articular cartilage in animal models (see, for example, Wakitani et al., 1994). However, optimizing the microenvironment with appropriate ECM proteins and soluble factors to promote chondrogenic differentiation of implanted stem cells has been a challenge (Chung et al., 2011). One approach is to use polymer-based, biodegradable, three-dimensional scaffolds that mimic the mesenchymal ECM in composition and stiffness. A variety of polymers including synthetic hydrogels and polysaccharide-based biological molecules are being used for transplantation (Sundelacruz and Kaplan, 2009; Vinatier et al., 2009). Gels of polymeric ECM proteins such as collagen or fibrin and hydrogels with attached ECM protein fragments have also shown promise as supports for mesenchymal stem cell differentiation and the induction of cartilage-specific gene expression (Bosnakovski et al., 2006; Connelly et al., 2011; Vinatier et al., 2009). Synthetic materials are also being used to address the problem of culturing stem cells for the extended periods needed for full differentiation. For example, incorporation of microspheres that contain TGF-β or other growth factors into hydrogel scaffolds has shown some improvement in terms of the quality of the cartilage matrix (Bian et al., 2011; Park et al., 2011). A deeper understanding of the functions of the key components of the chondrogenic microenvironment will be important for future development of biomaterials for cartilage regeneration.
Acknowledgments
The authors thank Charles Miller for helpful comments on this Commentary.
Footnotes
Funding
The authors were supported by the National Institutes of Health [grant numbers CA044627, GM059383, and CA160611 to J.E.S.]. Deposited in PMC for release after 12 months.
References
- Abbott J., Holtzer H. (1966). The loss of phenotypic traits by differentiated 3. The reversible behavior of chondrocytes in primary cultures. J. Cell Biol. 28, 473–487 10.1083/jcb.28.3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H. (2008). Control of chondrogenesis by the transcription factor Sox9. Mod. Rheumatol. 18, 213–219 10.1007/s10165-008-0048-x [DOI] [PubMed] [Google Scholar]
- Akiyama H., Kim J. E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T.et al. (2005). Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665–14670 10.1073/pnas.0504750102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S., Crowley D., Hynes R. O. (2007). Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 311, 11–24 10.1016/j.ydbio.2007.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A., Legate K. R., Nakchbandi I., Fässler R. (2006). What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 22, 591–621 10.1146/annurev.cellbio.22.010305.104258 [DOI] [PubMed] [Google Scholar]
- Augello A., De Bari C. (2010). The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 21, 1226–1238 10.1089/hum.2010.173 [DOI] [PubMed] [Google Scholar]
- Aulthouse A. L., Solursh M. (1987). The detection of a precartilage, blastema-specific marker. Dev. Biol. 120, 377–384 10.1016/0012-1606(87)90240-5 [DOI] [PubMed] [Google Scholar]
- Bang O. S., Kim E. J., Chung J. G., Lee S. R., Park T. K., Kang S. S. (2000). Association of focal adhesion kinase with fibronectin and paxillin is required for precartilage condensation of chick mesenchymal cells. Biochem. Biophys. Res. Commun. 278, 522–529 10.1006/bbrc.2000.3831 [DOI] [PubMed] [Google Scholar]
- Barna M., Niswander L. (2007). Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev. Cell 12, 931–941 10.1016/j.devcel.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Beier F., Loeser R. F. (2010). Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J. Cell. Biochem. 110, 573–580 10.1002/jcb.22604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D. M., Leung K. K., Wheatley S. C., Ng L. J., Zhou S., Ling K. W., Sham M. H., Koopman P., Tam P. P., Cheah K. S. (1997). SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174–178 10.1038/ng0697-174 [DOI] [PubMed] [Google Scholar]
- Bennett V. D., Pallante K. M., Adams S. L. (1991). The splicing pattern of fibronectin mRNA changes during chondrogenesis resulting in an unusual form of the mRNA in cartilage. J. Biol. Chem. 266, 5918–5924 [PubMed] [Google Scholar]
- Bi W., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22, 85–89 10.1038/8792 [DOI] [PubMed] [Google Scholar]
- Bian L., Zhai D. Y., Tous E., Rai R., Mauck R. L., Burdick J. A. (2011). Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425–6434 10.1016/j.biomaterials.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobick B. E., Chen F. H., Le A. M., Tuan R. S. (2009). Regulation of the chondrogenic phenotype in culture. Birth Defects Res. C Embryo Today 87, 351–371 10.1002/bdrc.20167 [DOI] [PubMed] [Google Scholar]
- Bosnakovski D., Mizuno M., Kim G., Takagi S., Okumura M., Fujinaga T. (2006). Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol. Bioeng. 93, 1152–1163 10.1002/bit.20828 [DOI] [PubMed] [Google Scholar]
- Chen W. H., Lai M. T., Wu A. T., Wu C. C., Gelovani J. G., Lin C. T., Hung S. C., Chiu W. T., Deng W. P. (2009). In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 60, 450–459 10.1002/art.24265 [DOI] [PubMed] [Google Scholar]
- Chimal–Monroy J., Díaz de León L. (1999). Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta1, beta2, beta3 and beta5 during the formation of precartilage condensations. Int. J. Dev. Biol. 43, 59–67 [PubMed] [Google Scholar]
- Choocheep K., Hatano S., Takagi H., Watanabe H., Kimata K., Kongtawelert P., Watanabe H. (2010). Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J. Biol. Chem. 285, 21114–21125 10.1074/jbc.M109.096479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F., Li Y., Poh Y. C., Yokohama–Tamaki T., Wang N., Tanaka T. S. (2010). Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 5, e15655 10.1371/journal.pone.0015655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung R., Foster B. K., Xian C. J. (2011). Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cells Int. 2011, 570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly J. T., Petrie T. A., García A. J., Levenston M. E. (2011). Fibronectin- and collagen-mimetic ligands regulate bone marrow stromal cell chondrogenesis in three-dimensional hydrogels. Eur. Cell. Mater. 22, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas S. L., Sivakumar P., Jones C. J., Chen Q., Peters D. M., Mosher D. F., Humphries M. J., Kielty C. M. (2005). Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 280, 18871–18880 10.1074/jbc.M410762200 [DOI] [PubMed] [Google Scholar]
- DeLise A. M., Fischer L., Tuan R. S. (2000). Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309–334 10.1053/joca.1999.0306 [DOI] [PubMed] [Google Scholar]
- Dessau W., von der Mark H., von der Mark K., Fischer S. (1980). Changes in the patterns of collagens and fibronectin during limb-bud chondrogenesis. J. Embryol. Exp. Morphol. 57, 51–60 [PubMed] [Google Scholar]
- Diekman B. O., Rowland C. R., Lennon D. P., Caplan A. I., Guilak F. (2010). Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng. Part A 16, 523–533 10.1089/ten.tea.2009.0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouad F., Delorme B., Maurice M., Bony C., Apparailly F., Louis–Plence P., Canovas F., Charbord P., Noël D., Jorgensen C. (2007). Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res. Ther. 9, R33 10.1186/ar2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domogatskaya A., Rodin S., Boutaud A., Tryggvason K. (2008). Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells 26, 2800–2809 10.1634/stemcells.2007-0389 [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Fässler R., Pfaff M., Murphy J., Noegel A. A., Johansson S., Timpl R., Albrecht R. (1995). Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 128, 979–988 10.1083/jcb.128.5.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. (1978). Role of cell shape in growth control. Nature 273, 345–349 10.1038/273345a0 [DOI] [PubMed] [Google Scholar]
- Forlino A., Piazza R., Tiveron C., Della Torre S., Tatangelo L., Bonafè L., Gualeni B., Romano A., Pecora F., Superti–Furga A.et al. (2005). A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum. Mol. Genet. 14, 859–871 10.1093/hmg/ddi079 [DOI] [PubMed] [Google Scholar]
- Foster J. W., Dominguez–Steglich M. A., Guioli S., Kwok C., Weller P. A., Stevanović M., Weissenbach J., Mansour S., Young I. D., Goodfellow P. N.et al. (1994). Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530 10.1038/372525a0 [DOI] [PubMed] [Google Scholar]
- Fukuda T., Yoshida N., Kataoka Y., Manabe R., Mizuno–Horikawa Y., Sato M., Kuriyama K., Yasui N., Sekiguchi K. (2002). Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 62, 5603–5610 [PubMed] [Google Scholar]
- Galante L. L., Schwarzbauer J. E. (2007). Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. J. Cell Biol. 179, 999–1009 10.1083/jcb.200707150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., McBeath R., Chen C. S. (2010). Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells 28, 564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehris A. L., Oberlender S. A., Shepley K. J., Tuan R. S., Bennett V. D. (1996). Fibronectin mRNA alternative splicing is temporally and spatially regulated during chondrogenesis in vivo and in vitro. Dev. Dyn. 206, 219–230 [DOI] [PubMed] [Google Scholar]
- Gehris A. L., Stringa E., Spina J., Desmond M. E., Tuan R. S., Bennett V. D. (1997). The region encoded by the alternatively spliced exon IIIA in mesenchymal fibronectin appears essential for chondrogenesis at the level of cellular condensation. Dev. Biol. 190, 191–205 10.1006/dbio.1997.8693 [DOI] [PubMed] [Google Scholar]
- Geiger B., Yamada K. M. (2011). Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3, 3 10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Bershadsky A., Pankov R., Yamada K. M. (2001). Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, 793–805 10.1038/35099066 [DOI] [PubMed] [Google Scholar]
- George E. L., Georges–Labouesse E. N., Patel–King R. S., Rayburn H., Hynes R. O. (1993). Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119, 1079–1091 [DOI] [PubMed] [Google Scholar]
- Goessler U. R., Bugert P., Bieback K., Stern–Straeter J., Bran G., Sadick H., Hbrmann K., Riedel F. (2009). In vitro analysis of integrin expression in stem cells from bone marrow and cord blood during chondrogenic differentiation. J. Cell. Mol. Med. 13, 1175–1184 10.1111/j.1582-4934.2008.00451.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gualeni B., Facchini M., De Leonardis F., Tenni R., Cetta G., Viola M., Passi A., Superti–Furga A., Forlino A., Rossi A. (2010). Defective proteoglycan sulfation of the growth plate zones causes reduced chondrocyte proliferation via an altered Indian hedgehog signalling. Matrix Biol. 29, 453–460 10.1016/j.matbio.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Hargus G., Kist R., Kramer J., Gerstel D., Neitz A., Scherer G., Rohwedel J. (2008). Loss of Sox9 function results in defective chondrocyte differentiation of mouse embryonic stem cells in vitro. Int. J. Dev. Biol. 52, 323–332 10.1387/ijdb.072490gh [DOI] [PubMed] [Google Scholar]
- Hartmann C. (2009). Transcriptional networks controlling skeletal development. Curr. Opin. Genet. Dev. 19, 437–443 10.1016/j.gde.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Furue M. K., Okamoto T., Ohnuma K., Myoishi Y., Fukuhara Y., Abe T., Sato J. D., Hata R., Asashima M. (2007). Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 25, 3005–3015 10.1634/stemcells.2007-0103 [DOI] [PubMed] [Google Scholar]
- Healy C., Uwanogho D., Sharpe P. T. (1999). Regulation and role of Sox9 in cartilage formation. Dev. Dyn. 215, 69–78 [DOI] [PubMed] [Google Scholar]
- Hinoi E., Bialek P., Chen Y. T., Rached M. T., Groner Y., Behringer R. R., Ornitz D. M., Karsenty G. (2006). Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 20, 2937–2942 10.1101/gad.1482906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia H. C., Schwarzbauer J. E. (2005). Meet the tenascins: multifunctional and mysterious. J. Biol. Chem. 280, 26641–26644 10.1074/jbc.R500005200 [DOI] [PubMed] [Google Scholar]
- Hunt G. C., Singh P., Schwarzbauer J. E. (2012). Endogenous production of fibronectin is required for self-renewal of cultured mouse embryonic stem cells. Exp. Cell Res. 16,318, 1820–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Fibronectins. New York, NY: Springer-Verlag; 1990. p. 546. [Google Scholar]
- Hynes R. O. (2009). The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Naba A. (2012). Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 4, a004903 10.1101/cshperspect.a004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Extracellular Matrix Biology. Cold Spring Harbor, NY: CSHL Press; 2012. p. 397. [Google Scholar]
- Kadler K. E., Hill A., Canty–Laird E. G. (2008). Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 20, 495–501 10.1016/j.ceb.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N., Watanabe H., Habuchi H., Takagi H., Shinomura T., Shimizu K., Kimata K. (2006). Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J. Biol. Chem. 281, 2390–2400 10.1074/jbc.M509341200 [DOI] [PubMed] [Google Scholar]
- Kaplan R. N., Riba R. D., Zacharoulis S., Bramley A. H., Vincent L., Costa C., MacDonald D. D., Jin D. K., Shido K., Kerns S. A.et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur R., Cooper R., Zhang L., Williams D. A. (2001). Cross-talk between alpha(4)beta(1)/alpha(5)beta(1) and c-Kit results in opposing effect on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood 97, 1975–1981 10.1182/blood.V97.7.1975 [DOI] [PubMed] [Google Scholar]
- Karsenty G. (2008). Transcriptional control of skeletogenesis. Annu. Rev. Genomics Hum. Genet. 9, 183–196 10.1146/annurev.genom.9.081307.164437 [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Rodriguez–León J., Izpisúa Belmonte J. C. (2006). The role of TGFbetas and Sox9 during limb chondrogenesis. Curr. Opin. Cell Biol. 18, 723–729 10.1016/j.ceb.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Keung A. J., de Juan–Pardo E. M., Schaffer D. V., Kumar S. (2011). Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29, 1886–1897 10.1002/stem.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K., Oike Y., Tani K., Shinomura T., Yamagata M., Uritani M., Suzuki S. (1986). A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J. Biol. Chem. 261, 13517–13525 [PubMed] [Google Scholar]
- Klees R. F., Salasznyk R. M., Kingsley K., Williams W. A., Boskey A., Plopper G. E. (2005). Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Mol. Biol. Cell 16, 881–890 10.1091/mbc.E04-08-0695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klees R. F., Salasznyk R. M., Vandenberg S., Bennett K., Plopper G. E. (2007). Laminin-5 activates extracellular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biol. 26, 106–114 10.1016/j.matbio.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Beck S., Müller C. A. (1993). Tenascin is a cytoadhesive extracellular matrix component of the human hematopoietic microenvironment. J. Cell Biol. 123, 1027–1035 10.1083/jcb.123.4.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. B., Knudson W. (2001). Cartilage proteoglycans. Semin. Cell Dev. Biol. 12, 69–78 10.1006/scdb.2000.0243 [DOI] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M.et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 10.1016/S0092-8674(00)80258-5 [DOI] [PubMed] [Google Scholar]
- Kosher R. A., Kulyk W. M., Gay S. W. (1986). Collagen gene expression during limb cartilage differentiation. J. Cell Biol. 102, 1151–1156 10.1083/jcb.102.4.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravis D., Upholt W. B. (1985). Quantitation of type II procollagen mRNA levels during chick limb cartilage differentiation. Dev. Biol. 108, 164–172 10.1016/0012-1606(85)90018-1 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332–336 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- Kulyk W. M., Upholt W. B., Kosher R. A. (1989). Fibronectin gene expression during limb cartilage differentiation. Development 106, 449–455 [DOI] [PubMed] [Google Scholar]
- Kulyk W. M., Coelho C. N., Kosher R. A. (1991). Type IX collagen gene expression during limb cartilage differentiation. Matrix 11, 282–288 10.1016/S0934-8832(11)80236-2 [DOI] [PubMed] [Google Scholar]
- Kumar D., Lassar A. B. (2009). The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol. Cell. Biol. 29, 4262–4273 10.1128/MCB.01779-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna T., Inoue H., Takeda H., Takahashi T., Yamamoto H., Miura H., Higashiyama S. (2011). Fibronectin regulates proteoglycan production balance in transforming growth factor-β1-induced chondrogenesis. Int. J. Mol. Med. 28, 829–834 [DOI] [PubMed] [Google Scholar]
- Lai Y., Sun Y., Skinner C. M., Son E. L., Lu Z., Tuan R. S., Jilka R. L., Ling J., Chen X. D. (2010). Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 19, 1095–1107 10.1089/scd.2009.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Bhattaram P. (2010). Vertebrate skeletogenesis. Curr. Top. Dev. Biol. 90, 291–317 10.1016/S0070-2153(10)90008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V., Li P., de Crombrugghe B. (1998). A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718–5733 10.1093/emboj/17.19.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Harrison D., Carbonetto S., Fassler R., Smyth N., Edgar D., Yurchenco P. D. (2002). Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157, 1279–1290 10.1083/jcb.200203073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C., Scadden D. T. (2011). The haematopoietic stem cell niche at a glance. J. Cell Sci. 124, 3529–3535 10.1242/jcs.074112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Thesleff I., Chiquet–Ehrismann R. (1987). Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J. Cell Biol. 105, 2569–2579 10.1083/jcb.105.6.2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I., Santamaria–Martínez A., Susanto E., Peng H., Lehr H. A., Delaloye J. F., Huelsken J. (2012). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 10.1038/nature10694 [DOI] [PubMed] [Google Scholar]
- Martino M. M., Mochizuki M., Rothenfluh D. A., Rempel S. A., Hubbell J. A., Barker T. H. (2009). Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 30, 1089–1097 10.1016/j.biomaterials.2008.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Irie F., Mackem S., Yamaguchi Y. (2010). A mouse model of chondrocyte-specific somatic mutation reveals a role for Ext1 loss of heterozygosity in multiple hereditary exostoses. Proc. Natl. Acad. Sci. USA 107, 10932–10937 10.1073/pnas.0914642107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- McBride S. H., Knothe Tate M. L. (2008). Modulation of stem cell shape and fate A: the role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng. Part A 14, 1561–1572 10.1089/ten.tea.2008.0112 [DOI] [PubMed] [Google Scholar]
- Mecham R. P. (2011). The Extracellular Matrix: an Overview. Biology of Extracellular Matrix (ed. Mecham R P.), pp. 425 Berlin, Germany: Springer-Verlag [Google Scholar]
- Melnick M., Jaskoll T., Brownell A. G., MacDougall M., Bessem C., Slavkin H. C. (1981). Spatiotemporal patterns of fibronectin distribution during embryonic development. I. Chick limbs. J. Embryol. Exp. Morphol. 63, 193–206 [PubMed] [Google Scholar]
- Mruthyunjaya S., Manchanda R., Godbole R., Pujari R., Shiras A., Shastry P. (2010). Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK-MEK/ERK signaling pathways. Biochem. Biophys. Res. Commun. 391, 43–48 10.1016/j.bbrc.2009.10.158 [DOI] [PubMed] [Google Scholar]
- Muro A. F., Chauhan A. K., Gajovic S., Iaconcig A., Porro F., Stanta G., Baralle F. E. (2003). Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 162, 149–160 10.1083/jcb.200212079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L. J., Wheatley S., Muscat G. E., Conway–Campbell J., Bowles J., Wright E., Bell D. M., Tam P. P., Cheah K. S., Koopman P. (1997). SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 183, 108–121 10.1006/dbio.1996.8487 [DOI] [PubMed] [Google Scholar]
- Ogura N., Kawada M., Chang W. J., Zhang Q., Lee S. Y., Kondoh T., Abiko Y. (2004). Differentiation of the human mesenchymal stem cells derived from bone marrow and enhancement of cell attachment by fibronectin. J. Oral Sci. 46, 207–213 10.2334/josnusd.46.207 [DOI] [PubMed] [Google Scholar]
- Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W., Beddington R. S., Mundlos S., Olsen B. R.et al. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 10.1016/S0092-8674(00)80259-7 [DOI] [PubMed] [Google Scholar]
- Park J. S., Yang H. N., Woo D. G., Jeon S. Y., Park K. H. (2011). The promotion of chondrogenesis, osteogenesis, and adipogenesis of human mesenchymal stem cells by multiple growth factors incorporated into nanosphere-coated microspheres. Biomaterials 32, 28–38 10.1016/j.biomaterials.2010.08.088 [DOI] [PubMed] [Google Scholar]
- Parsons P., Gilbert S. J., Vaughan–Thomas A., Sorrell D. A., Notman R., Bishop M., Hayes A. J., Mason D. J., Duance V. C. (2011). Type IX collagen interacts with fibronectin providing an important molecular bridge in articular cartilage. J. Biol. Chem. 286, 34986–34997 10.1074/jbc.M111.238188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M., He F., Kish V. L. (2011). Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng. Part A 17, 3067–3076 10.1089/ten.tea.2011.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham R. J., Jr and Wang Y. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94, 13661–13665 10.1073/pnas.94.25.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimton P., Sarkar S., Sheth N., Perets A., Marcinkiewicz C., Lazarovici P., Lelkes P. I. (2011). Fibronectin-mediated upregulation of α5β1 integrin and cell adhesion during differentiation of mouse embryonic stem cells. Cell Adhes. Migr. 5, 73–82 10.4161/cam.5.1.13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W. (2009). Mesenchymal stem cells and cartilage in situ regeneration. J. Intern. Med. 266, 390–405 10.1111/j.1365-2796.2009.02153.x [DOI] [PubMed] [Google Scholar]
- Robinson E. E., Foty R. A., Corbett S. A. (2004). Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol. Biol. Cell 15, 973–981 10.1091/mbc.E03-07-0528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., Superti–Furga A. (2001). Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2): 22 novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum. Mutat. 17, 159–171 10.1002/humu.1 [DOI] [PubMed] [Google Scholar]
- Saha K., Keung A. J., Irwin E. F., Li Y., Little L., Schaffer D. V., Healy K. E. (2008). Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438 10.1529/biophysj.108.132217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze–Tanzil G. (2009). Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann. Anat. 191, 325–338 10.1016/j.aanat.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Schwartz M. A. (2010). Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2, a005066 10.1101/cshperspect.a005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Assoian R. K. (2001). Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553–2560 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., DeSimone D. W. (2011). Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 3, a005041 10.1101/cshperspect.a005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Carraher C., Schwarzbauer J. E. (2010). Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 26, 397–419 10.1146/annurev-cellbio-100109-104020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. (1983). Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell 35, 657–666 10.1016/0092-8674(83)90098-3 [DOI] [PubMed] [Google Scholar]
- Stephens L. E., Sutherland A. E., Klimanskaya I. V., Andrieux A., Meneses J., Pedersen R. A., Damsky C. H. (1995). Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9, 1883–1895 10.1101/gad.9.15.1883 [DOI] [PubMed] [Google Scholar]
- Stickens D., Zak B. M., Rougier N., Esko J. D., Werb Z. (2005). Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132, 5055–5068 10.1242/dev.02088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S., Kaplan D. L. (2009). Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 20, 646–655 10.1016/j.semcdb.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh W. S., Lee E. H., Richards M., Cao T. (2010). In vitro derivation of chondrogenic cells from human embryonic stem cells. Methods Mol. Biol. 584, 317–331 10.1007/978-1-60761-369-5_17 [DOI] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W. E., Rendl M., Fuchs E. (2004). Defining the epithelial stem cell niche in skin. Science 303, 359–363 10.1126/science.1092436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veevers–Lowe J., Ball S. G., Shuttleworth A., Kielty C. M. (2011). Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J. Cell Sci. 124, 1288–1300 10.1242/jcs.076935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinatier C., Bouffi C., Merceron C., Gordeladze J., Brondello J. M., Jorgensen C., Weiss P., Guicheux J., Noël D. (2009). Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr. Stem Cell Res. Ther. 4, 318–329 10.2174/157488809789649205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Wein F., Seckinger A., Frankhauser M., Wirkner U., Krause U., Blake J., Schwager C., Eckstein V., Ansorge W.et al. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 33, 1402–1416 10.1016/j.exphem.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Wakitani S., Goto T., Pineda S. J., Young R. G., Mansour J. M., Caplan A. I., Goldberg V. M. (1994). Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 76, 579–592 [DOI] [PubMed] [Google Scholar]
- Wang G., Woods A., Agoston H., Ulici V., Glogauer M., Beier F. (2007). Genetic ablation of Rac1 in cartilage results in chondrodysplasia. Dev. Biol. 306, 612–623 10.1016/j.ydbio.2007.03.520 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao L., Smas C., Sul H. S. (2010). Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol. Cell. Biol. 30, 3480–3492 10.1128/MCB.00057-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. K., Yu X., Cohen D. M., Wozniak M. A., Yang M. T., Gao L., Eyckmans J., Chen C. S. (2011). Bone Morphogenetic Protein-2-Induced Signaling and Osteogenesis Is Regulated by Cell Shape, RhoA/ROCK, and Cytoskeletal Tension. Stem Cells Dev. 21, 1176–1186 10.1089/scd.2011.0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Graham G. J. (1999). Homing and mobilization in the stem cell niche. Trends Cell Biol. 9, 233–238 10.1016/S0962-8924(99)01559-7 [DOI] [PubMed] [Google Scholar]
- White D. G., Hershey H. P., Moss J. J., Daniels H., Tuan R. S., Bennett V. D. (2003). Functional analysis of fibronectin isoforms in chondrogenesis: Full-length recombinant mesenchymal fibronectin reduces spreading and promotes condensation and chondrogenesis of limb mesenchymal cells. Differentiation 71, 251–261 10.1046/j.1432-0436.2003.7104502.x [DOI] [PubMed] [Google Scholar]
- Wilson D. G., Phamluong K., Li L., Sun M., Cao T. C., Liu P. S., Modrusan Z., Sandoval W. N., Rangell L., Carano R. A.et al. (2011). Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J. Cell Biol. 193, 935–951 10.1083/jcb.201007162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer J. P., Janmey P. A., McCormick M. E., Funaki M. (2009). Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng. Part A 15, 147–154 10.1089/ten.tea.2007.0388 [DOI] [PubMed] [Google Scholar]
- Woods A., Beier F. (2006). RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J. Biol. Chem. 281, 13134–13140 10.1074/jbc.M509433200 [DOI] [PubMed] [Google Scholar]
- Woods A., Wang G., Beier F. (2005). RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 280, 11626–11634 10.1074/jbc.M409158200 [DOI] [PubMed] [Google Scholar]
- Woods A., Wang G., Beier F. (2007a). Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J. Cell. Physiol. 213, 1–8 10.1002/jcp.21110 [DOI] [PubMed] [Google Scholar]
- Woods A., Wang G., Dupuis H., Shao Z., Beier F. (2007b). Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J. Biol. Chem. 282, 23500–23508 10.1074/jbc.M700680200 [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Patton B. L. (2009). Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 15, 1277–1294 10.2174/138161209787846766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak B. M., Crawford B. E., Esko J. D. (2002). Hereditary multiple exostoses and heparan sulfate polymerization. Biochim. Biophys. Acta 1573, 346–355 10.1016/S0304-4165(02)00402-6 [DOI] [PubMed] [Google Scholar]
- Zanetti N. C., Solursh M. (1984). Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J. Cell Biol. 99, 115–123 10.1083/jcb.99.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti N. C., Dress V. M., Solursh M. (1990). Comparison between ectoderm-conditioned medium and fibronectin in their effects on chondrogenesis by limb bud mesenchymal cells. Dev. Biol. 139, 383–395 10.1016/0012-1606(90)90307-5 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Eberspaecher H., Lefebvre V., de Crombrugghe B. (1997). Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. 209, 377–386 [DOI] [PubMed] [Google Scholar]
- Zhou G., Zheng Q., Engin F., Munivez E., Chen Y., Sebald E., Krakow D., Lee B. (2006). Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 103, 19004–19009 10.1073/pnas.0605170103 [DOI] [PMC free article] [PubMed] [Google Scholar]