Summary
Integrins are the primary receptors of cells adhering to the extracellular matrix, and play key roles in various cellular processes including migration, proliferation and survival. The expression and distribution of integrins at the cell surface is controlled by endocytosis and recycling. The present study examines the function of syntaxin 6 (STX6), a t-SNARE located in the trans-Golgi network, in integrin trafficking. STX6 is overexpressed in many types of human cancer. We show that depletion of STX6 inhibits chemotactic cell migration and the delivery of the laminin receptor α3β1 integrin to the cell surface, whereas STX6 overexpression stimulates chemotactic cell migration, integrin delivery, and integrin-initiated activation of focal adhesion kinase. These data indicate that STX6 plays a rate-limiting role in cell migration and integrin trafficking. In STX6-depleted cells, α3β1 integrin is accumulated in recycling endosomes that contain the v-SNARE VAMP3. Importantly, we show that STX6 and VAMP3 form a v-/t-SNARE complex, VAMP3 is required in α3β1 integrin delivery to the cell surface, and endocytosed α3β1 integrin traffics to both VAMP3 and STX6 compartments. Collectively, our data suggest a new integrin trafficking pathway in which endocytosed integrins are transported from VAMP3-containing recycling endosomes to STX6-containing trans-Golgi network before being recycled to the plasma membrane.
Key words: Integrin, Endocytic recycling, Cell migration, SNARE
Introduction
Integrins are a family of heterodimeric receptors that mediate cell adhesion to extracellular matrix (ECM) proteins such as fibronectin, laminin, collagen and vitronectin (Hynes, 2002). Integrins play essential roles in various cellular processes including migration, differentiation, proliferation and survival, and in many pathological conditions including cancer progression and metastasis (Guo and Giancotti, 2004; Hood and Cheresh, 2002). The α- and β-integrins are paired in the endoplasmic reticulum and delivered to the cell surface by vesicular trafficking (Tiwari et al., 2011b). Cell surface integrins are constantly endocytosed, transported to endosomes and then recycled back to the plasma membrane (Bretscher, 1989; Caswell and Norman, 2006). Endocytic recycling of integrins is particularly important in cell migration. In migrating cells, integrins serve as traction points to stabilize protrusion formation and move the cell body forward (Ridley et al., 2003; Webb et al., 2002). To provide fresh traction points, integrins are endocytosed and recycled to the cell front (Kiosses et al., 2001; Laukaitis et al., 2001; Lawson and Maxfield, 1995; Palecek et al., 1996; Pierini et al., 2000; Regen and Horwitz, 1992; Woods et al., 2004).
Endocytosis of integrins takes place through clathrin-coated pits, caveolin or lipid rafts at the plasma membrane (Caswell and Norman, 2006; De Deyne et al., 1998; Wary et al., 1998) and involves the adaptor protein Dab2 (Teckchandani et al., 2009). Recycling of integrins to the cell surface occurs by at least two pathways. In the long-loop pathway, α5β1 integrin travels to early endosomes, then to the perinuclear recycling endosomes before recycling to the plasma membrane (Powelka et al., 2004; Roberts et al., 2004; Yoon et al., 2005). In the short-loop pathway, αvβ3 integrin recycles directly from early endosomes to the plasma membrane (Roberts et al., 2001). Although the majority of endocytosed α5β1 molecules are recycled to the cell surface (Bretscher, 1989), a fraction of endocytosed α5β1 molecules bound with the fibronectin ligands are sorted into multivesicular endosomes and degraded in lysosomes (Lobert et al., 2010). In the current study, we present evidence that endocytosed integrins traffic from recycling endosomes to the trans-Golgi network (TGN).
The final step in vesicular delivery, the fusion of transport vesicles with target membranes, is catalyzed by the interactions of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins on vesicles (v-SNAREs) and on target membranes (t-SNAREs) (Bonifacino and Glick, 2004; Jahn et al., 2003; Rothman, 1994). A few SNAREs have been implicated in integrin trafficking. Cleavage of the v-SNARE VAMP3 by tetanus toxin impairs cell migration and reduces cell surface α5β1 integrin expression in CHO-K1 cells (Proux-Gillardeaux et al., 2005; Skalski and Coppolino, 2005). In macrophages, VAMP3 interacts with plasma membrane t-SNAREs syntaxin 4 and SNAP-23 to mediate endocytic recycling of α5β1 integrin (Veale et al., 2010). We showed that silencing of VAMP3 inhibits β1 integrin trafficking in PANC-1 pancreatic cancer cells (Luftman et al., 2009). In addition, we reported that VAMP2 mediates α5β1 integrin trafficking (Hasan and Hu, 2010), and that syntaxin 3 and syntaxin 4, but not syntaxin 2, mediate the trafficking of α5β1 and α3β1 integrins in HeLa cells (Day et al., 2011). In the current study, we show that syntaxin 6, a t-SNARE in the TGN, plays a rate-limiting role in integrin trafficking.
Syntaxin 6 (STX6) is broadly expressed in tissues and cell lines (Bock et al., 1996). Immunogold labeling analyses revealed that approximately 70% of STX6 proteins are present in the TGN, with the remaining STX6 proteins in endosomes and transport vesicles (Bock et al., 1997). A SNARE motif retains STX6 proteins in the TGN, while a tyrosine-based sorting signal YGRL retrieves STX6 proteins that have escaped to the plasma membrane (Watson and Pessin, 2000). STX6 has been shown to be involved in the trafficking from the TGN to endosomes (Bock et al., 1997), and from endosomes to the TGN (Mallard et al., 2002) and lysosomes (Kuliawat et al., 2004). STX6 has also been shown to be involved in homotypic fusion of immature secretory granules (Wendler et al., 2001) and early endosomes (Brandhorst et al., 2006). Interestingly, the tumor suppressor protein p53 binds to the promoter of STX6 and activates STX6 transcription (Zhang et al., 2008).
Here we report that STX6 is overexpressed in human cancers. By manipulating STX6 expression levels, we determined the roles of STX6 in integrin trafficking and chemotactic cell migration. Our data suggest that endocytosed integrins traffic from VAMP3-containing recycling endosomes to STX6-containing TGN before returning to the plasma membrane.
Results
STX6 is overexpressed in cancers
Using the ONCOMINE cancer gene expression microarray database (Rhodes et al., 2004), with P = 0.05 and fold change = 1.5 as cut-off, we found that among the 36 known SNAREs in humans, STX6 is the most frequently overexpressed in cancers (Fig. 1, and data not shown). Compared with normal tissues, STX6 expression is elevated in breast, colon, liver, pancreatic, prostate, bladder, skin, testicular, tongue, cervical, lung and gastric cancers (Biewenga et al., 2008; D'Errico et al., 2009; Hou et al., 2010; Kaiser et al., 2007; Korkola et al., 2006; LaTulippe et al., 2002; Richardson et al., 2006; Riker et al., 2008; Sanchez-Carbayo et al., 2006; Segara et al., 2005; Wurmbach et al., 2007; Ye et al., 2008) (Fig. 1). Elevated STX6 expression in almost all major types of cancers suggests that STX6 overexpression is important in cancer. Using HeLa human cervical cancer cells as a model, we determined the roles of STX6 in cell migration, proliferation, integrin trafficking and signaling.
Fig. 1.
STX6 overexpression in human cancers. STX6 mRNA expression in 7 normal breast, 40 ductal breast carcinoma (Richardson et al., 2006); 5 normal colon, 17 cecum adenocarcinoma (Kaiser et al., 2007); 10 normal liver, 35 hepatocellular carcinoma (Wurmbach et al., 2007); 6 normal pancreas, 11 pancreatic carcinoma (Segara et al., 2005); 3 normal prostate gland, 32 prostate carcinoma (LaTulippe et al., 2002); 48 normal bladder, 81 bladder urothelial carcinoma (Sanchez-Carbayo et al., 2006); 4 normal skin, 11 skin squamous cell carcinoma (Riker et al., 2008); 6 normal testis, 101 adult male germ cell tumor (Korkola et al., 2006); 12 normal tongue, 26 tongue squamous cell carcinoma (Ye et al., 2008); 5 normal cervix, 40 cervical squamous cell carcinoma (Biewenga et al., 2008); 65 normal lung, 27 squamous cell lung carcinoma (Hou et al., 2010); and 31 normal gastric mucosa, 4 gastric adenocarcinoma (D'Errico et al., 2009) samples was analyzed using microarrays. Dark gray boxes represent tumor tissues and light gray boxes represent normal tissues. Box plots were generated at www.oncomine.org. P, P-value; FC, fold change.
STX6 silencing inhibits chemotactic cell migration and integrin trafficking
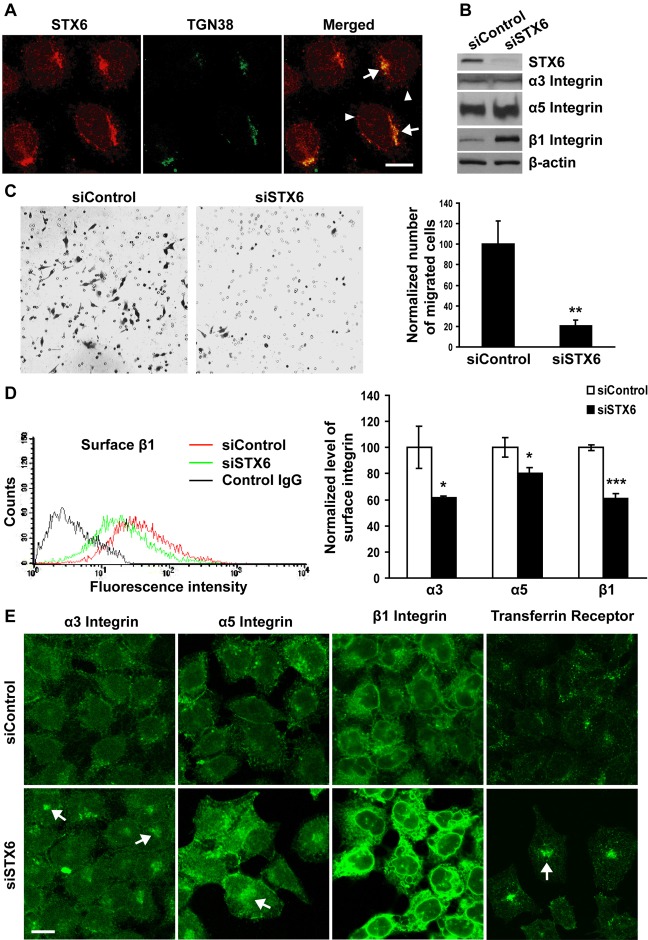
Immunocytochemistry revealed that in HeLa cells, the majority of STX6 proteins were localized in the TGN that was labeled by an antibody to TGN38 (arrows, Fig. 2A). Additional vesicular STX6 staining was detected in the cytoplasm (arrowheads, Fig. 2A). Such subcellular distribution is consistent with previously reported STX6 distribution (Bock et al., 1997). We then silenced STX6 by siRNAs (Fig. 2B), and performed transwell migration assay (Hasan and Hu, 2010) to determine the effects of STX6 knockdown on cell migration, using Matrigel as a chemoattractant. STX6 knockdown inhibited chemotactic cell migration by 80% (Fig. 2C), suggesting that STX6 is required in chemotactic migration.
Fig. 2.
Silencing of STX6 inhibits chemotactic cell migration and integrin delivery to the cell surface. (A) Permeabilized HeLa cells were dual labeled with a polyclonal antibody to STX6 and a monoclonal antibody to TGN38. Representative single-slice confocal images are shown. Superimposed images (merged) demonstrate the overlapping distribution of STX6 with TGN38. Arrows, TGN; arrowheads, vesicles. (B–E) HeLa cells were transfected with non-targeting control or STX6 siRNAs. (B) 60 h after transfection, cell lysates were immunoblotted with an antibody to STX6, α3 integrin, α5 integrin or β1 integrin. The same membranes were blotted with an antibody to β-actin as a loading control. (C) 48 h after transfection, cells were harvested and loaded to the top chambers of transwells. Matrigel (20 µg/ml) was included in the bottom chambers as a chemoattractant. After 20 h at 37°C, unmigrated cells were removed, and migrated cells were Giemsa stained. Five random images were taken at 10× magnification using a light microscope, for each transwell. The number of migrated cells in each image was quantified using ImageJ software and averaged for each experimental group. Representative images of migrated cells are shown. In the bar graph, the number of migrated cells transfected with the STX6 siRNA was normalized to the number of migrated cells transfected with the control siRNA. Error bars represent standard deviation of three independent experiments. **P<0.01 vs cells transfected with the control siRNA. (D) FACS analysis of cell surface integrin expression. 60 h after transfection, unpermeabilized cells were stained with non-specific control mouse IgG (blank control) or a monoclonal antibody to α3, α5 or β1 integrins. The levels of cell surface integrin staining were quantified by flow cytometry. (Left panel) the FACS profile of STX6 knockdown cells stained with the control IgG (black curve) overlapped completely with the profile of control cells stained with the control IgG (not shown). (Right panel) the mean fluorescence intensity of cell surface α3, α5 and β1 integrins in cells transfected with the STX6 siRNA was normalized to the intensity in cells transfected with the control siRNA. Error bars represent standard deviation of four independent experiments. *P<0.05; ***P<0.001 vs cells transfected with the control siRNA. (E) The transfected cells were permeabilized and stained with an antibody to α3 integrin, α5 integrin, β1 integrin or transferrin receptor. Arrows, perinuclear areas. Representative single-slice confocal images of four independent experiments are shown. Scale bars: 20 µm.
To determine if STX6 is involved in integrin trafficking, HeLa cells transfected with the control or STX6 siRNAs were immunostained for cell surface α3, α5 and β1 integrins. The levels of cell surface integrin staining were measured by flow cytometry as we previously reported (Hasan and Hu, 2010). Compared with control cells, STX6 knockdown cells had a 39%, 20% and 39% reduction in cell surface expression of α3, α5 and β1 integrins, respectively (Fig. 2D). To investigate if reduced cell surface integrin expression was caused by inhibition of integrin trafficking, the transfected cells were permeabilized and stained with integrin antibodies. Different from control cells, in which α3 and α5 integrins were detected in dispersed vesicles in the cytoplasm (Fig. 2E), in STX6 knockdown cells α3 and α5 integrins were present in vesicles concentrated in perinuclear areas (arrows, Fig. 2E). Since α3 and α5 integrins only assemble with the β1 subunit to form heterodimeric receptors (Hynes, 2002), the subcellular distribution of α3 and α5 represents the distribution of α3β1 and α5β1 integrins, respectively. Further, STX6 knockdown did not alter total cellular expression of α3 and α5 integrins (Fig. 2B). Therefore, the reduced cell surface expression and increased vesicular accumulation was a result of impaired trafficking of α3β1 and α5β1 integrins in the absence of STX6.
In addition to perinuclear vesicles, large amount of β1 integrin proteins were detected in other membrane-bound compartments, likely the endoplasmic reticulum, in STX6 knockdown cells (Fig. 2E). Since β1 integrin can form heterodimers with 12 different α subunits (Hynes, 2002), the more severe trafficking defects of β1 relative to α3 and α5 integrins indicated that in addition to α3β1 and α5β1 integrins, trafficking of other integrins was disrupted by STX6 silencing. Consistent with brighter intracellular β1 integrin staining, total cellular level of β1 integrin protein was increased in STX6 knockdown cells (Fig. 2B). In addition, we determined the effects of STX6 knockdown on the trafficking of transferrin receptor, which undergoes repeated endocytic recycling (Presley et al., 1997). Like α3 and α5 integrins, transferrin receptor was concentrated in perinuclear areas in STX6 knockdown cells (Fig. 2E).
We next sought to identify the subcellular compartment(s) where integrins are accumulated in STX6 knockdown cells. We focused on α3 integrin, whose distinct accumulation patterns (Fig. 2E) suggest that it is trapped in specific compartment(s). HeLa cells transfected with the control or STX6 siRNAs were dual labeled with an antibody to α3 integrin and an antibody to the early endosome marker protein EEA1 or the lysosome probe LysoTracker. In control cells, colocalization of α3 integrin with EEA1 or LysoTracker was not detectable (Fig. 3A,B). In STX6 knockdown cells, small fractions of α3 integrin molecules were clearly detected in both EEA1 and LysoTracker compartments, as shown by yellow vesicles in the merged channel (arrowheads, Fig. 3A,B), suggesting that in the absence of STX6, α3 integrin molecules are retained in early endosomes and some α3 integrin molecules are delivered to lysosomes for degradation. In a later part of this paper, we show that in STX6 knockdown cells α3 integrin was trapped mostly in VAMP3-containing recycling endosomes (Fig. 8).
Fig. 3.
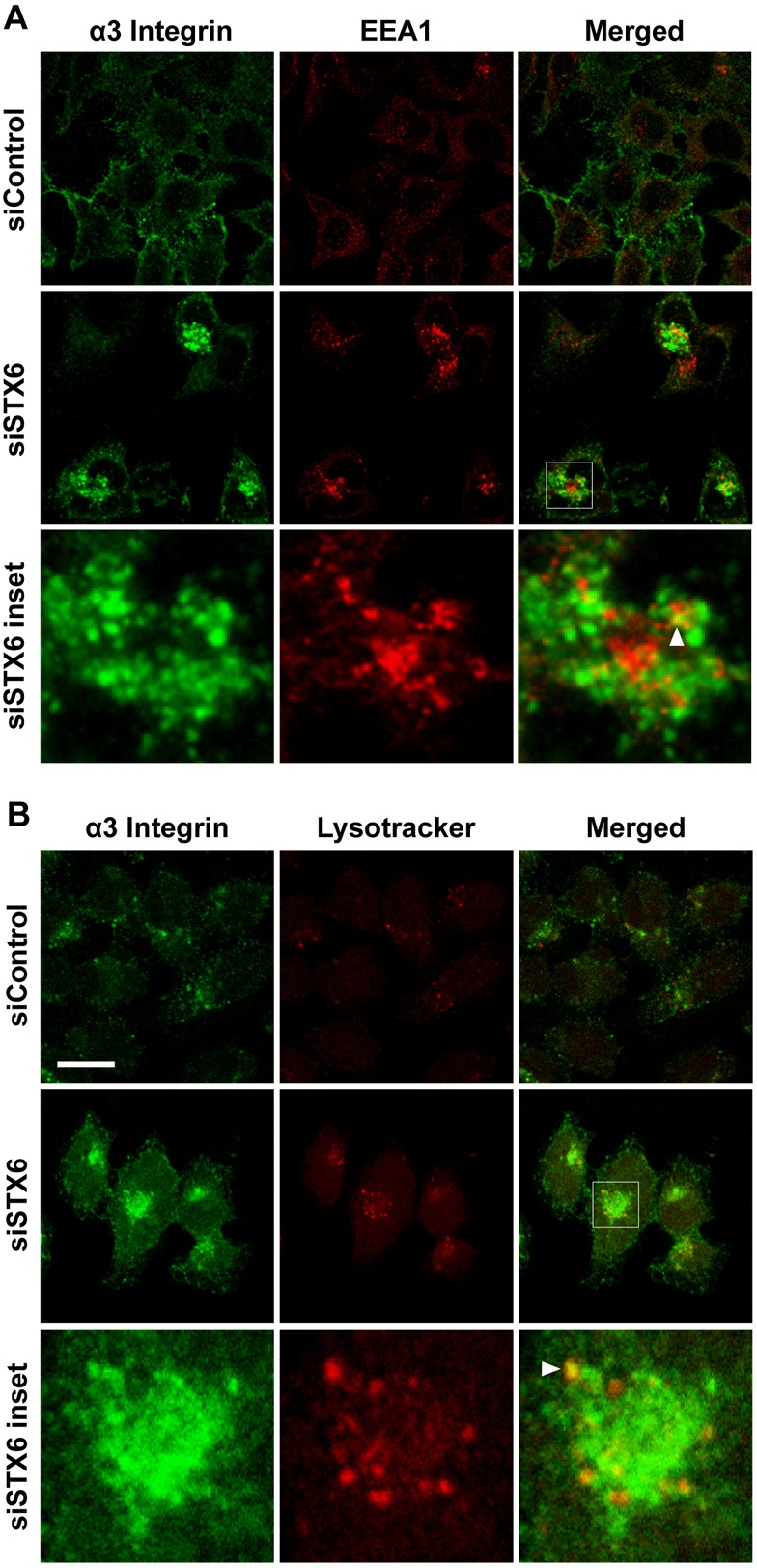
α3 Integrin is detected in early endosomes and lysosomes in STX6 knockdown cells. (A,B) 60 h after transfection with the control or STX6 siRNAs, HeLa cells were permeabilized and dual stained with an antibody to α3 integrin and an antibody to the early endosome marker EEA1 (A) or the lysosome probe LysoTracker Red (B). siSTX6 inset panels: enlarged view of the boxed regions in the merged images showing the colocalization (arrowheads) of α3 integrin and EEA1 or Lysotracker. Representative single-slice confocal images of three independent experiments are shown. Scale bar: 50 µm.
Fig. 8.
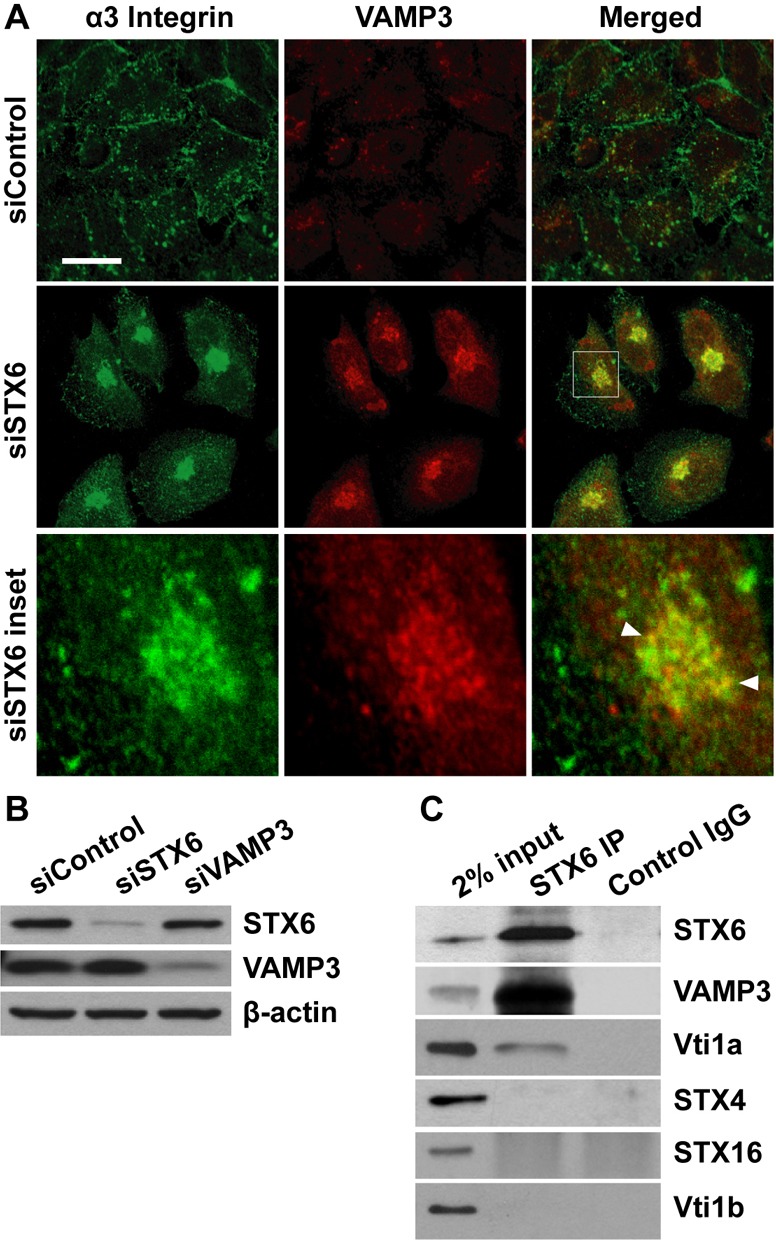
VAMP3 and STX6 form a v-/t-SNARE complex. (A) α3 integrin is accumulated in VAMP3 compartments in STX6 knockdown cells. 60 h after transfection with the control or STX6 siRNAs, HeLa cells were permeabilized and dual stained with an antibody to α3 integrin and an antibody to VAMP3. siSTX6 inset panels: enlarged view of the boxed region in the merged image showing the colocalization (arrowheads) of α3 integrin and VAMP3. Representative single-slice confocal images of three independent experiments are shown. Scale bar: 50 µm. (B) Lysates of HeLa cells transfected with the control, STX6 or VAMP3 siRNAs were immunoblotted with an antibody to STX6, VAMP3 or β-actin. (C) Lysates of HeLa (2 mg) cells were immunoprecipitated with a STX6 antibody or control rabbit IgG, followed by immunoblotting analysis for STX6, VAMP3, Vti1a, STX4, STX16 and Vti1b. 2% (40 µg) input lysates serve as a loading control.
Since in addition to cell adhesion and migration, integrins exert control on cell proliferation and survival (Guo and Giancotti, 2004), we assessed the effects of STX6 knockdown on cell proliferation. Compared with the control siRNA, STX6 siRNA reduced BrdU incorporation by 34% at 48 h and by 85% at 72 h after transfection (Fig. 4A), indicating that STX6 knockdown inhibits cell proliferation. To determine if STX6 knockdown triggers apoptosis, cells were incubated with a FITC-conjugated caspase inhibitor VAD-FMK to label apoptotic cells (arrows, Fig. 4B). In control cells, apoptosis was not detected until 72 h after transfection of the control siRNA, when 2% of the cells were labeled by FITC–VAD-FMK (Fig. 4B). At 60 and 72 h after STX6 siRNA transfection, 7% and 33% of the cells were apoptotic, respectively (Fig. 4B), indicating that STX6 knockdown led to apoptosis.
Fig. 4.

STX6 silencing inhibits cell proliferation and triggers apoptosis. (A) 0, 24, 48, 72 and 96 h after transfection with the control or STX6 siRNAs, proliferation of HeLa cells was determined using the BrdU cell proliferation assay by absorbance at 450 nm. Error bars represent standard deviation of four independent replicates. Representative results of one of three independent experiments are shown. (B) 24, 48, 60 and 72 h after siRNA transfection, the CaspACETM FITC–VAD-FMK in situ marker was added to label apoptotic cells. Shown are representative confocal and phase-contrast images of cells at 72 h after transfection. Scale bar: 20 µm. At each time point, the number of apoptotic cells that were labeled by FITC–VAD-FMK, and the total number of cells in the phase-contrast channel were counted and quantified (graph). Error bars represent standard deviation of three independent experiments.
Most analyses of STX6 knockdown (Figs 2, 3) were conducted at 60 h after siRNA transfection, when 7% of the cells underwent apoptosis. As apoptotic cells shrank and condensed (Fig. 4B), our analyses were focused on spreading cells that showed distinct morphology from apoptotic cells. To further validate that the STX6 knockdown effects on cell migration and integrin trafficking were not the results of apoptosis, we performed analyses at 24 h after STX6 siRNA transfection, when apoptosis was not detected (Fig. 4B; supplementary material Fig. S1B). Consistent with the observations at 60 h, 24 h treatment of STX6 siRNA reduced cell surface expression of α3 integrin by 25% (supplementary material Fig. S1C) and accumulated α3 integrin in vesicles in the cytoplasm (arrows, supplementary material Fig. S1D). Furthermore, 24 h treatment of STX6 siRNA impaired cell adhesion to laminin (a 28% decrease at 30 min) (supplementary material Fig. S2A), and inhibited chemotactic migration to Matrigel (a 66% decrease) or 10% fetal bovine serum (an 85% decrease) (supplementary material Fig. S2B). Taken together, the STX6 knockdown studies demonstrated that STX6 is required in chemotactic cell migration and vesicular trafficking of α3 integrin to the cell surface.
STX6 overexpression stimulates cell migration, spreading and adhesion
The inhibitory effects of STX6 silencing prompted us to investigate the effects of STX6 overexpression on cell migration and integrin trafficking. cDNA of human STX6 was amplified by PCR from an I.M.A.G.E. clone and cloned into the pCMV-3Tag-1a vector to generate a construct that encodes an N-terminal FLAG-tagged STX6. When the plasmid was transfected into DU145 prostate cancer cells, STX6 overexpression was demonstrated by immunoblotting analysis (Fig. 5A). Strikingly, STX6 overexpression led to a 4.8-fold increase in DU145 chemotactic cell migration (Fig. 5B). To further analyze the effects of STX6 overexpression, we developed HeLa cell lines that stably express FLAG–STX6. In a representative cell line (STX6OE21), endogenous STX6 protein level was not altered, while total cellular STX6 protein level was increased by 74% (supplementary material Fig. S3A). In addition, microarray analysis showed an 87% increase of STX6 mRNA level in STX6OE21 cells compared with HeLa cells (supplementary material Table S1). Consistent with the results in DU145 cells, STX6OE21 cells migrated 80% faster than HeLa cells (supplementary material Fig. S3B), despite proliferating at the same rate (supplementary material Fig. S3C). When FLAG–STX6 and endogenous STX6 proteins in STX6OE21 cells were depleted by STX6 siRNA transfection, the stimulatory effects of STX6 overexpression on cell migration were reversed (Fig. 5C,D), further demonstrating that STX6 overexpression stimulates chemotactic cell migration. Accordingly, we found that overexpression of STX6 in PANC-1 pancreatic cancer cells increased cell migration in the transwell migration assay (data not shown). In cancers that have elevated STX6 expression (Fig. 1), motility of cancer cells may be increased by STX6 overexpression.
Fig. 5.

STX6 overexpression stimulates chemotactic cell migration. (A,B) DU145 cells were transfected with the empty vector pCMV-3Tag-1 or a plasmid that overexpresses FLAG–STX6 (STX6OE). 24 h after transfection (A) cell lysates were immunoblotted with an antibody to STX6. The same membrane was blotted with an antibody to β-actin as a loading control. (B) Chemotactic cell migration was analyzed using the transwell migration assay as described in Fig. 2C. The number of migrated cells transfected with FLAG–STX6 (STX6OE) was normalized to the number of migrated cells transfected with the empty vector. Error bars represent standard deviation of three independent experiments. ***P<0.001 vs the control cells. (C,D) STX6OE21 cells that stably express FLAG–STX6 were transfected with the control or STX6 siRNAs for 24 h. (C) Lysates of HeLa cells and the transfected STX6OE21 cells were immunoblotted with an antibody to STX6. β-actin was used as a loading control. (D) Motility of HeLa and STX6OE21 cells transfected with the control or STX6 siRNAs was compared using the transwell migration assay. Error bars represent standard deviation of three independent experiments. ***P<0.001.
To determine the effects of STX6 overexpression on cell spreading, we seeded HeLa and STX6OE21 cells to cell culture plates, and analyzed cell morphology at different time points. 20 minutes after seeding, 42% of STX6OE21 cells have spread, whereas only 20% of the control cells have spread (Fig. 6A), indicating that STX6 overexpression promotes cell spreading. In addition, STX6OE21 cells adhered to cell culture plates faster than HeLa cells (a 71% and 30% increase at 30 min and 60 min, respectively; Fig. 6B). To delineate the involvement of STX6 in integrin-mediated cell adhesion to ECM proteins, we seeded HeLa and STX6OE21 cells to plates coated with laminin or Matrigel. STX6OE21 cells adhered much faster to laminin (a 4.6 and 3.9-fold increase at 15 min and 30 min, respectively) and Matrigel (a 2.3 and 2.4-fold increase at 15 min and 30 min, respectively) than HeLa cells (Fig. 6B). Increased cell adhesion to ECM proteins suggested that STX6 overexpression might enhance cell surface expression and/or delivery of integrin receptors.
Fig. 6.
STX6 overexpression promotes cell spreading and adhesion. (A) HeLa and STX6OE21 cells were harvested and added to cell culture plates. After 30 min, unattached cells were washed away, and adherent cells were imaged using a light microscope. The number and percentage of spreading cells (arrows) in 10 random images were counted and quantified (bar graph). Error bars represent standard deviation of three independent experiments. (B) HeLa and STX6OE21 cells were harvested and added to cell culture plates, or plates coated with laminin or Matrigel. After 15–60 min, unattached cells were washed away, and the number of adherent cells was measured using a colorimetric assay by absorbance at 490 nm. Error bars represent standard deviation of three independent experiments. *P<0.05; **P<0.01; ***P<0.001 vs HeLa cells.
STX6 promotes integrin delivery to the cell surface and activation of focal adhesion kinase
FACS analysis showed that STX6 overexpression had no effects on cell surface expression of α3, α5 and β1 integrins (Fig. 7A). Nor were total cellular protein (Fig. 7B) and mRNA (supplementary material Table S1) levels of the integrins altered in STX6OE21 cells compared with HeLa cells. Interestingly, when seeded to laminin-coated plates, the STX6-overexpressing cells exhibited much higher cell surface levels of α3 and β1 integrins after 30 min of cell adhesion (Fig. 7C). These data suggested that although STX6 overexpression had no effects on total cellular and static cell surface expression of integrins, it accelerated the delivery of α3β1 integrin to the cell surface during cell adhesion to laminin.
Fig. 7.
STX6 promotes integrin delivery and activation of FAK. (A) FACS analysis of cell surface integrin expression. Unpermeabilized HeLa and STX6OE21 cells were stained with an antibody to α3, α5 or β1 integrins. The levels of cell surface integrin staining were measured by flow cytometry. Error bars represent standard deviation of four independent experiments. (B) Lysates of HeLa and STX6OE21 cells were immunoblotted with an antibody to α3, α5 or β1 integrins. β-actin was used as a loading control. (C) STX6 overexpression promotes integrin delivery during cell adhesion. HeLa and STX6OE21 cells were harvested and added to laminin-coated glass coverslips. After 30 min, unattached cells were washed away, and adherent cells were fixed and stained with an antibody to α3 or β1 integrins. Representative confocal images of three independent experiments are shown. Scale bar: 50 µm. In the box plots, the staining intensity of α3 integrin in HeLa (n = 97) and STX6OE21 cells (n = 152), and the staining intensity of β1 integrin in HeLa (n = 81) and STX6OE21 cells (n = 110) were quantified using ImageJ software and analyzed. (D) Left panel: HeLa and STX6OE21 cells were seeded to laminin-coated plates. After 4 h, unattached cells were washed away and adherent cells were lysed. 30 µg of the cell lysates were immunoblotted with an antibody to phospho-FAK (pY397), total FAK, phospho-paxillin (pY118), total paxillin or β-actin. Right panel: before seeding to laminin-coated plates HeLa and STX6OE21 cells (in suspension) were lysed, and cell lysates were analyzed by immunoblotting. Representative images of three independent immunoblotting experiments are shown.
In addition to anchoring the cell to ECM, integrins are important mediators of cell signaling (ffrench-Constant and Colognato, 2004). The binding of ECM proteins to the extracellular domains of integrins leads to integrin activation, which recruits and activates signaling molecules such as focal adhesion kinase (FAK) (Wozniak et al., 2004; Zhao and Guan, 2009). FAK further activates downstream signaling molecules including the adaptor protein paxillin (Wozniak et al., 2004). Activated FAK and paxillin are phosphorylated at tyrosine 397 (Y397) and tyrosine 118 (Y118), respectively. To investigate whether STX6 overexpression modulates integrin-initiated signaling, HeLa and STX6OE21 cells were seeded to cell culture plates coated with laminin. After 4 h, the levels of total FAK, phospho-FAK (Y397), total paxillin and phospho-paxillin (Y118) were analyzed by immunoblotting. Compared with control cells, STX6 overexpression cells showed similar levels of total FAK and paxillin, but increased levels of phospho-FAK and phospho-paxillin when adhered to laminin (Fig. 7D). However, HeLa and STX6OE21 cells in suspension exhibited similar levels of phospho-FAK and phospho-paxillin (Fig. 7D), indicating that the increased phosphorylation of FAK and paxillin in STX6OE21 cells was cell adhesion dependent.
STX6 and VAMP3 form a v-/t-SNARE complex
The perinuclear accumulation of integrins in STX6 knockdown cells (Fig. 2E) suggests that integrins may be concentrated in recycling endosomes. The v-SNARE VAMP3 is known to reside in recycling endosomes (Galli et al., 1994; McMahon et al., 1993), and has been shown to mediate integrin endocytic recycling (Tayeb et al., 2005; Veale et al., 2010). Indeed, dual labeling experiments showed extensive colocalization of accumulated α3 integrin with VAMP3 in STX6 knockdown cells (arrowheads, Fig. 8A). These data indicated that in the absence of STX6 integrin molecules were trapped in VAMP3 compartments, and suggested that STX6 and VAMP3 may be functionally linked in integrin trafficking. In control cells, VAMP3 was detected in dispersed vesicles in the cytoplasm (Fig. 8A). In STX6 knockdown cells, VAMP3 was present in aggregated vesicles in perinuclear areas (Fig. 8A), although total cellular VAMP3 protein level was not altered (Fig. 8B). These results indicated that the distribution of VAMP3-containing recycling endosomes is modulated in the absence of STX6.
Assembly of complexes between v- and t-SNAREs drives vesicle fusion (Bonifacino and Glick, 2004; Jahn et al., 2003; Rothman, 1994). We next examined whether VAMP3 and STX6 interact in HeLa cells. Immunoprecipitation with a STX6 antibody clearly detected a VAMP3/STX6 interaction (Fig. 8C). In addition, STX6 co-precipitated with Vti1a, a t-SNARE localized predominantly in the TGN (Kreykenbohm et al., 2002). In contrast, STX6 did not co-precipitate with the plasma membrane t-SNARE STX4 or the TGN and endosome-localized t-SNAREs STX16 and Vti1b (Kreykenbohm et al., 2002; Mallard et al., 2002), nor was VAMP3 precipitated by control rabbit IgG (Fig. 8C). These data demonstrated the specificity of the VAMP3/STX6 interaction. The outcome of a vesicle fusion event driven by a v- and t-SNARE interaction is the transfer of cargo molecules (e.g., integrins) from the v-SNARE compartments into the t-SNARE compartments. The accumulation of α3 integrin in VAMP3 compartments in STX6 knockdown cells (Fig. 8A), and the formation of the v-/t-SNARE complex between VAMP3 and STX6 suggested that integrins are delivered from VAMP3-containing recycling endosomes to STX6-containing TGN.
We next investigated the role of VAMP3 in the trafficking of α3β1 integrin. siRNA silencing of VAMP3 had no effect on total cellular protein levels of α3 and β1 integrins (Fig. 9A), but reduced cell surface expression of α3 and β1 integrins by 27% and 19%, respectively (Fig. 9B). Like in STX6 knockdown cells (Fig. 2E), α3 integrin was accumulated in perinuclear vesicles and colocalized with EEA1 and LysoTracker in VAMP3 knockdown cells (Fig. 9C), suggesting that VAMP3 mediates α3β1 trafficking. The matching accumulative distributions of α3 integrin in STX6 and VAMP3 knockdown cells provide further evidence that STX6 and VAMP3 interact to mediate integrin trafficking.
Fig. 9.
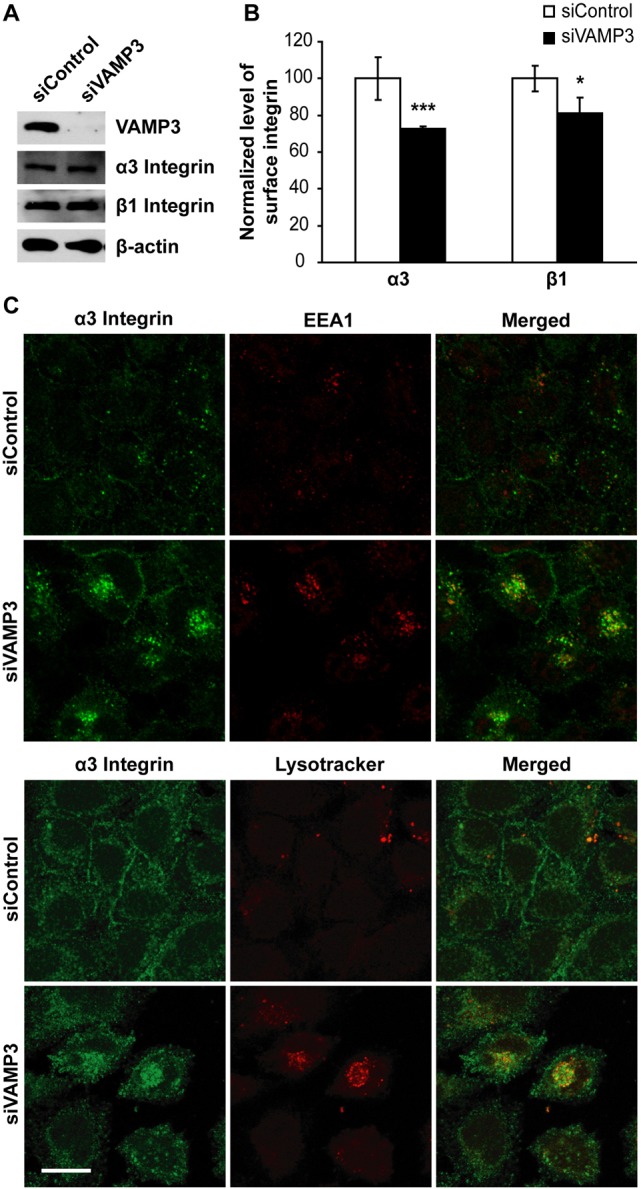
VAMP3 is required in α3β1 integrin trafficking to the cell surface. HeLa cells were transfected with control or VAMP3 siRNAs. (A) 72 h after transfection cell lysates were immunoblotted with an antibody to VAMP3, α3 integrin, β1 integrin or β-actin. (B) The transfected cells were stained with an antibody to α3 or β1 integrins, and the levels of cell surface integrin staining were measured by flow cytometry. The mean fluorescence intensity of cell surface α3 and β1 integrins in VAMP3 knockdown cells was normalized to the intensity in control cells. Error bars represent standard deviation of four independent experiments. *P<0.05; ***P<0.001 vs control cells. (C) The transfected cells were permeabilized and dual labeled with an antibody to α3 integrin and an antibody to EEA1 or LysoTracker Red. Shown are representative single-slice confocal images of four independent experiments. Scale bar: 50 µm.
If STX6 and VAMP3 function together in α3β1 trafficking, knockdown of both STX6 and VAMP3 would not have additive effects on α3β1 integrin trafficking compared with STX6 or VAMP3 knockdown. To test this, we depleted STX6 and VAMP3 simultaneously (supplementary material Fig. S4A) and measured the effects of double knockdown on cell surface α3 integrin expression. Indeed, compared with single knockdown, double knockdown of STX6 and VAMP3 had no additive effect on reducing α3 integrin cell surface expression (supplementary material Fig. S4B).
Endocytosed α3 integrin traffics to VAMP3 and STX6 compartments
To determine whether endocytosed integrins traffic to VAMP3 and STX6 compartments, we tagged cell surface α3 integrin with a monoclonal antibody at 4°C. As expected, when the cells were fixed immediately and dual stained with antibodies, tagged α3 integrin was restricted to the plasma membrane and did not colocalize with VAMP3 or STX6 (Fig. 10A). To characterize endocytosis, after tagging cells with the α3 integrin antibody, we incubated the cells at 37°C to resume vesicular trafficking. After 30 min, the cells were fixed and endocytosed α3 integrin and VAMP3 or STX6 were dual stained. Within 30 min, endocytosed α3 integrin had entered VAMP3 and STX6 compartments (Fig. 10B). 29% of the vesicles that contained endocytosed α3 integrin were labeled by a VAMP3 antibody (n = 70 cells), and 38% of the vesicles that contained endocytosed α3 integrin were labeled by a STX6 antibody (n = 70 cells; arrowheads, Fig. 10B). Together, the results in Fig. 10 indicated that endocytosed α3 integrin traffics to VAMP3 and STX6 compartments.
Fig. 10.

Endocytosed α3 integrin traffics to VAMP3 and STX6 compartments. (A) Cell surface α3 integrin in HeLa cells was tagged by incubation with a monoclonal antibody at 4°C. (B) Vesicular trafficking was resumed by incubating the cells at 37°C for 30 min. The cells were fixed and permeabilized. Cell surface (A) and endocytosed (B) α3 integrin molecules were visualized using FITC-conjugated secondary antibody. VAMP3 and STX6 were stained with polyclonal antibodies and Rhodamine-conjugated secondary antibody. Representative single-slice confocal images of three independent experiments are shown. Scale bar: 20 µm.
Discussion
STX6 is overexpressed in many types of cancers, including lung, breast, colon, liver, prostate, cervical and pancreatic cancers. Using HeLa cervical cancer cells as a model, we show that knockdown of STX6, a t-SNARE in the TGN, inhibits chemotactic migration, whereas STX6 overexpression stimulates chemotactic cell migration. STX6 knockdown inhibits the delivery of α3β1 and α5β1 integrins to the cell surface, and accumulates α3β1 integrin in recycling endosomes that contain VAMP3, whereas STX6 overexpression enhances α3β1 integrin delivery during cell adhesion, and promotes integrin-initiated activation of FAK. Furthermore, we show that STX6 and VAMP3 form a v-/t-SNARE complex, and VAMP3 knockdown inhibits α3β1 integrin trafficking to the cell surface. Importantly, we show that endocytosed α3β1 integrin traffics to VAMP3 and STX6 compartments. These data suggest a new integrin trafficking pathway in which endocytosed integrins are transported from VAMP3-containing recycling endosomes to STX6-containing TGN before being recycled to the cell surface (Fig. 11).
Fig. 11.
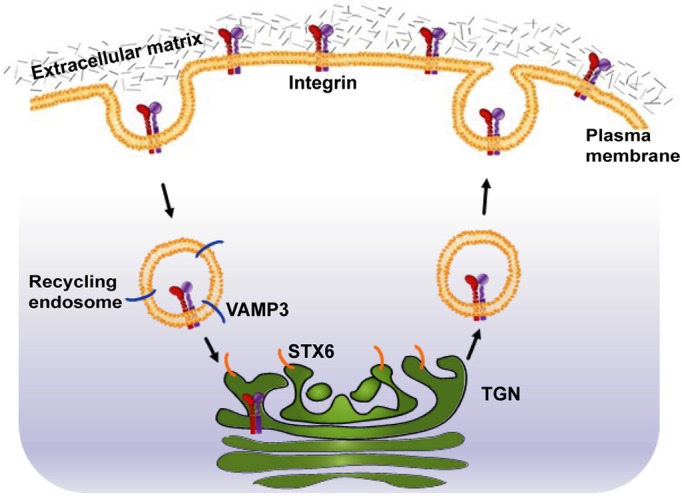
An integrin endocytic recycling pathway mediated by STX6 and VAMP3.
In macrophages, STX6 mediates the trafficking and secretion of tumor necrosis factor α (TNFα) (Murray et al., 2005b). In muscle cells and adipocytes, STX6 is involved in the recycling of the insulin-responsive aminopeptidase (IRAP) from the cell surface to the insulin-responsive compartment (Watson et al., 2008), and in the trafficking of the glucose transporter GLUT4 to a storage compartment (Perera et al., 2003). In endothelial cells, STX6 regulates the maintenance of a Golgi-associated pool of vascular endothelial growth factor receptor 2 (VEGFR2) (Manickam et al., 2011), and endocytic recycling of the fibronectin receptor α5β1 integrin (Tiwari et al., 2011a). Here, we report that in HeLa cells STX6 plays a rate-limiting role in vesicular trafficking of the laminin receptor α3β1 integrin. Our finding is consistent with the view that within a specialized cell type, STX6 fulfills selective functions (Wendler and Tooze, 2001).
The majority of STX6 proteins are localized in the TGN (Bock et al., 1997) (Fig. 2A). TGN takes part in the sorting of lysosomal enzymes, the secretory pathway and the endocytic recycling pathway. The established role of VAMP3 in integrin endocytic recycling (Skalski and Coppolino, 2005; Tayeb et al., 2005; Veale et al., 2010), the accumulation of α3 integrin in VAMP3 compartments in STX6 knockdown cells, the formation of VAMP3/STX6 complex, and the trafficking of endocytosed α3 integrin into VAMP3 and STX6 compartments, suggest that after integrin endocytosis VAMP3 and STX6 catalyze the vesicle fusion reaction that results in the transport of integrins from recycling endosomes to the TGN. The reduction of cell surface integrin expression by VAMP3 and STX6 knockdown provides further evidence that this VAMP3 to STX6 pathway is important for integrin delivery to the cell surface.
The VAMP3/STX6 complex has been reported in macrophages to mediate the transport of TNFα from the Golgi to recycling endosomes (Murray et al., 2005a). Interactions of STX6 with VAMP3 and the TGN-localized t-SNAREs STX16 and Vti1a have been implicated in early/recycling endosomes-to-TGN transport (Mallard et al., 2002). In adipocytes STX6 interacts with STX16 to regulate GLUT4 trafficking (Perera et al., 2003). Our immunoprecipitation analysis detects STX6 interactions with VAMP3 and Vti1a, but not with STX16, suggesting that Vti1a may be the third SNARE protein that mediates integrin transport from recycling endosomes to the TGN. In macrophages STX6 interacts with Vti1b, a t-SNARE in late endosomes and the TGN (Kreykenbohm et al., 2002), to facilitate the exocytosis of TNFα (Murray et al., 2005b). Our immunoprecipitation analysis does not detect an STX6/Vti1b interaction in HeLa cells, suggesting that in different cell types, STX6 may have distinct t-SNARE partners. In macrophages VAMP3 forms a complex with the plasma membrane t-SNAREs syntaxin 4 and SNAP-23 to drive endocytic recycling of α5β1 integrin (Veale et al., 2010). Therefore, VAMP3 can take part in two integrin trafficking events: (1) from recycling endosomes to the TGN; (2) from recycling endosomes to the plasma membrane. Future studies are needed to investigate how VAMP3-mediated integrin trafficking to the TGN and to the plasma membrane is coordinated. A tempting alternative possibility is that the two events are two legs of the same endocytic recycling pathway: after interacting with STX6 to transport integrins to the TGN, VAMP3 functions at the next events, i.e. interacting with syntaxin 4 and SNAP-23 to deliver integrins back to the plasma membrane.
STX6 knockdown in HeLa cells reduces cell surface expression of α3 integrin and accumulates α3 integrin in the VAMP3-containing recycling endosomes (Fig. 8A), with smaller fractions in early endosomes (Fig. 3A) and lysosomes (Fig. 3B). STX6 knockdown does not alter the total cellular level of α3 integrin, suggesting that in STX6 knockdown cells the lysosomal degradation of α3 molecules and the synthesis of new α3 molecules equilibrate. On the other hand, STX6 knockdown reduces cell surface expression of β1 integrin and increases total cellular level of β1 integrin. Since the β1 subunit can form heterodimers with 12 different α subunits, the increased β1 cellular level suggests that the expression of certain heterodimeric integrins may be upregulated in STX6 knockdown cells. A recent study showed that blocking STX6 function in HUVEC endothelial cells reduces total cellular levels of α5 and β1 integrins by more than 50%, through targeting α5β1 integrin to lysosomal degradation (Tiwari et al., 2011a). The disparity in total cellular levels of β1 integrin in STX6-deficient HeLa and HUVEC cells suggests that the synthesis of new integrin molecules and the lysosomal degradation of integrins in HeLa cancer cells and HUVEC endothelial cells may have different kinetics.
In STX6 knockdown cells, α3 and α5 integrins are enriched in perinuclear vesicles, whereas in addition to perinuclear vesicles β1 integrin is accumulated in other membrane-bound compartments, likely the endoplasmic reticulum (Fig. 2E). The more severe trafficking defects of β1 relative to α3 and α5 integrins indicate that along with α3β1 and α5β1 integrins, STX6 may mediate the trafficking of additional heterodimeric integrins. STX6 overexpression promotes cell adhesion to laminin-1 that is used in the current study (Fig. 6B). Since α3β1 integrin binds to laminin-5 and laminin-10/11, but not to laminin-1 (Delwel et al., 1994; Kreidberg, 2000; Nishiuchi et al., 2003), these data suggest that STX6 may be involved in the trafficking of other laminin receptors such as α6β1 integrin, which binds to laminin-1 (Delwel et al., 1994).
Metastasis, the spreading of cancer from a primary site to distant organs, is responsible for 90% of cancer deaths. Metastasis consists of a series of sequential steps, including local tumor growth, migration of cancer cells through basement membrane and invasion into surrounding tissues, intravasation into lymphatic and blood vessels, spread and survival in the circulation, and extravasation and establishing colonies at distant sites (Huber et al., 2005). Integrins are involved in each step of the metastatic cascade, especially in migration and invasion of cancer cells (Goel et al., 2008; Guo and Giancotti, 2004; Hood and Cheresh, 2002). In cell migration, the endocytic recycling pathway is responsible for delivering fresh integrins to the leading edge to stabilize protrusion formation and provide fresh traction points to move the cell body forward (Caswell and Norman, 2006; Caswell et al., 2009). By overexpressing STX6, cancer cells can potentiate their capacities to transport integrins, to migrate, and to metastasize. STX6 may be a valuable target for cancer therapy.
How is STX6 expression elevated in cancers? In breast and colon cancer cells, the tumor suppressor protein p53 and the other two members of the p53 family, i.e. p63 and p73, bind to a p53-responsive element in the STX6 promoter and activate STX6 transcription (Zhang et al., 2008). p53 is mutated in 50% of human cancers. Most of p53 mutations are missense point mutations (Levine, 1997), and 70% of p53 mutants retain transcriptional activity (Kato et al., 2003). Future studies are warranted to investigate if p53 mutants upregulate STX6 expression in cancer cells.
Materials and Methods
Reagents and antibodies
Matrigel and laminin were obtained from BD Biosciences. Giemsa stain solution and mouse monoclonal antibody (mAb) to β-actin were purchased from Sigma. Rabbit polyclonal antibodies (pAbs) to STX6, VAMP3 and STX16 were obtained from Synaptic Systems GmbH. Mouse mAb to α3 integrin (MAB2056), rabbit pAb to α3 integrin (AB1920), mouse mAb to α5 integrin (MAB1999), mouse mAb to total FAK (clone 4.47), and rabbit pAb to phospho-paxillin (Y118) were from Millipore. Goat pAb to β1 integrin (N-20), rabbit pAb to α5 integrin (H-104), mouse mAb to TGN38 (A-5), rabbit pAb to EEA1 (H-300), and rabbit pAb to paxillin (H-114) were from Santa Cruz Biotechnology. Rabbit pAb to phospho-FAK (Y397) was from Invitrogen. Mouse mAb to Vti1a (611220) and mouse mAb to Vti1b (611404) were from BD Biosciences. HRP-, FITC- or Rhodamine-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories. The anti-β1 integrin mouse mAb P5D2, developed by Elizabeth A. Wayner at Fred Hutchinson Cancer Research Center, was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa. The AllStars Negative Control siRNA and the predesigned STX6 siRNA oligo Hs_STX6_10 (targeting sequence CACCAACGAGCTGAGAAATAA) were purchased from QIAGEN. The siRNA oligo that targets VAMP3 has been reported (Luftman et al., 2009).
Construct of FLAG–STX6
The I.M.A.G.E. Clone 4122224 that contains cDNA of human STX6 was obtained from the American Type Culture Collection (ATCC). The coding sequence of STX6 (amino acids 2–255) was amplified by PCR from clone 4122224 with primers CHL28F (5′-GCGGGATCCTCCATGGAGGACCCCTTCTTTG-3′) and CHL28R (5′-TACCGGGCCCTCACAGCACTAAGAAGAGGATGAGC-3′). The PCR product was digested with BamH1 and ApaI and cloned into the BamHI and ApaI sites of the pCMV-3Tag-1a vector (Stratagene), resulting in plasmid pCHL28. Pfu DNA polymerase (Stratagene) was used for PCR cloning, and the coding sequence was confirmed by DNA sequencing.
Cell culture, transfection and development of stable cell lines
HeLa and DU145 cells were obtained from ATCC, and cultured in minimum essential medium α (MEMα) supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. The day before siRNA transfection, HeLa cells were seeded into six-well plates (2.5×105 cells per well) or 24-well plates (5×104 cells per well). siRNAs were transfected at 10 nM using Lipofectamine RNAiMAX according to the manufacturer's instructions (Invitrogen). The AllStars Negative Control siRNA was used as control.
The day before plasmid transfection, 2.5×105 DU145 cells were seeded in each well of six-well plates. 1 µg of empty vector pCMV-3Tag-1a or pCHL28 was transfected in each well. Transfection was done with Lipofectamine LTX according to the manufacturer's instructions (Invitrogen). To develop stable cell lines that express FLAG–STX6, HeLa cells were transfected with pCHL28 using Lipofectamine (Invitrogen). 48 h after transfection, the cells were selected in culture medium containing 0.8 mg/ml of G418. After 2 weeks, two stable clones (STX6OE5 and STX6OE21) were expanded in G418-containing medium. Expression of FLAG–STX6 in the stable cell lines was determined by immunoblotting using a STX6 antibody.
Immunoblotting
Whole cell lysates were prepared by incubating cells for 30 min in lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40) containing complete protease inhibitor cocktail (Roche). Protein concentrations were determined by the Bio-Rad DC Protein Assay. 30–50 µg of cell lysates were loaded into each lane of SDS-PAGE. After electrophoresis, proteins were transferred to Trans-Blot Nitrocellulose Transfer Membrane (Bio-Rad). The membranes were blotted with antibodies to STX6, α3 integrin, α5 integrin, β1 integrin, p-FAK, total FAK or p-paxillin, followed by HRP-conjugated secondary antibodies. Bound antibodies were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific Pierce). The same membranes were then blotted with a mouse mAb to β-actin as a loading control.
Immunocytochemistry
The day before siRNA transfection, HeLa cells were seeded on sterile 12-mm glass coverslips contained in 24-well plates. 24 or 60 h after transfection, the cells were fixed with 4% paraformaldehyde in PBS++ (PBS supplemented with 0.1 g/l CaCl2 and 0.1 g/l MgCl2), permeabilized with 0.2% Triton X-100, and blocked in 10% FBS. Primary antibodies were incubated with the cells at the following dilutions: anti-STX6 pAb, 1:100; anti-TGN38 mAb, 1:50; anti-β1 integrin mAb P5D2, neat conditioned culture medium; anti-α3 integrin mAb, 1:100; and anti-α5 integrin mAb, 1:100. Fluorophore-conjugated secondary antibodies were used at a dilution of 1:500. For double staining, the cells were incubated first with a mixture of the primary antibodies, and then with a mixture of the secondary antibodies. To label lysosomes, cells were incubated first with 100 nM LysoTracker Red DND-99 (Invitrogen) at 37°C for 45 min before fixation. Single-slice confocal images were collected on an Olympus laser scanning confocal microscope. The images were processed with Adobe Photoshop software.
Transwell migration assay
The transwell migration assay was performed as described (Hasan and Hu, 2010). 20 µg/ml of growth-factor-reduced Matrigel or MEMα medium containing 10% fetal bovine serum was added to the lower chambers of the 12-well format transwells (8 µm pore; BD Biosciences) as chemoattractants. 24 or 48 h after siRNA transfection or 24 h after plasmid transfection, HeLa and DU145 cells were harvested with trypsin/EDTA and added to the upper chambers at 8×104 cells per transwell. After 20 h at 37°C, the transwells were fixed in methanol, and stained with Giemsa Stain solution. Unmigrated cells were removed from the top of the membranes using cotton swabs. The membranes were detached from the transwells then affixed to glass slides using Permount mounting medium (Fisher Scientific). To quantify the number of migrated cells, five random images were taken at 10× magnification using a light microscope, for each transwell. The number of migrated cells per image was counted using ImageJ software.
Flow cytometry
To measure the levels of cell surface integrins by flow cytometry (Hasan and Hu, 2010), cells were seeded in 24-well plates. 24 h after seeding of HeLa and STX6OE21 cells, and 24 or 60 h after HeLa cells were transfected with siRNAs, cells were fixed with 1% paraformaldehyde in PBS++ for 15 min, and then blocked in 10% FBS in PBS++ for 15 min. The cells were incubated with 20 µg/ml of control mouse IgG (Sigma), mAbs to α3, α5 or β1 integrins for 60 min at room temperature. After washing, the cells were labeled with FITC-conjugated secondary antibodies (1:200 dilution) for 45 min. After further washing, the cells were scraped off the plates with a cell scraper. 10,000 cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). The mean fluorescence intensity of each sample was obtained using the CellQuest Pro software.
Cell proliferation assay
Two assays were used to measure cell proliferation and showed consistent results. To determine the effect of STX6 knockdown on cell proliferation, the day before siRNA transfection HeLa cells were seeded in 96-well plates at 3×103 cells per well. 0, 24, 48, 72 and 96 h after transfection, proliferation was measured using the BrdU Cell Proliferation Assay according to the manufacturer's instructions (Millipore). Cells were incubated with BrdU for 18 h, and BrdU incorporation was detected immunochemically by absorbance at 450 nm using a 96-well ELISA plate reader.
Proliferation of HeLa and STX6OE21 cells were compared using the CellTiter 96 AQueous One Solution Reagent (Promega) as reported (Hasan and Hu, 2010). 0, 24, 48 and 72 h after HeLa and STX6OE21 cells were seeded in 96-well plates at 1.5×103 cells per well, cell culture medium was replaced with MEMα medium containing no phenol red, and the CellTiter 96 AQueous One Solution Reagent was added to measure the number of living cells. After incubation at 37°C for 90 min, absorbance of the medium at 490 nm was measured in a 96-well ELISA plate reader. Absorbance from wells containing only the MEMα medium but no cells was taken as blank reading.
Apoptosis assay
Apoptotic cells were labeled with the CaspACETM FITC–VAD-FMK In Situ Marker (Promega).
The day before siRNA transfection, HeLa cells were seeded in 24-well plates. 24, 48, 60 and 72 h after siRNA transfection, cells were incubated with 10 µM CaspACETM FITC–VAD-FMK at 37°C for 1 h before fixation. Fluorescence and phase contrast images were collected on an Olympus laser scanning confocal microscope.
Cell adhesion assay
The cell adhesion assay was performed as described previously (Luftman et al., 2009). Each well of 24-well plates was coated with 20 µg of laminin or Matrigel for 1 h at 37°C. The plates were washed once with PBS and blocked with 2% heat-inactivated BSA in PBS. HeLa and STX6OE21 cells were harvested with trypsin/EDTA, counted with a hemacytometer and resuspended in MEMα medium supplemented with 0.5% BSA (MEMα-BSA). 1×105 cells were added to each well and allowed to adhere to the extracellular matrix proteins for various time at 37°C. After incubation, non-attached cells were removed by three washes with MEMα–BSA, and the medium was replaced with MEMα medium containing no Phenol Red. The CellTiter 96 AQueous One Solution Reagent was added to measure the number of attached cells. After incubation at 37°C for 90 min, absorbance of the medium at 490 nm was measured in a 96-well ELISA plate reader.
Microarray analysis
Total RNA was isolated from cells using Trizol (Invitrogen). Two independent culture preparations of wild-type HeLa and STX6OE21 cells were harvested for RNA isolation. The concentration and quality of the resulting RNAs were assessed both through NanoDrop (Thermo Scientific) and Agilent 2100 bioanalyzer. Affymetrix Human Gene 1.0 ST arrays were used to analyze gene expression. Probe labeling, hybridization, and scanning for the arrays were done using standard protocols in the Microarray Core Facility at the University of Louisville. Raw data (Cel files) were analyzed using Partek Genomic Suite (version 6.5). Expression values were normalized on the exon level using RMA normalization. Exon level expression values were summarized by their means to determine gene level expression values. One-way ANOVA was performed and as a multiple test correction, FDR (false discovery rate) was applied to identify differentially expressed genes.
Co-immunoprecipitation
HeLa cell lysates were prepared by incubating cells in the lysis buffer containing complete protease inhibitor cocktail. 2.0 mg of HeLa lysates were incubated with 4 µl of STX6 pAb or control rabbit IgG overnight at 4°C on an orbital rocker. 50 µl of protein A–agarose (Santa Cruz Biotechnology) were added and incubated for 2 h. The agarose beads were collected by centrifugation and washed twice with 600 µl of Tris-buffered saline (TBS). Immune complexes were eluted in 35 µl of 2× SDS-PAGE sample buffer by incubation at 95°C for 5 min. Eluted proteins were separated by 15% SDS-PAGE and analyzed by immunoblotting.
Integrin endocytosis assay
HeLa cells were seeded on glass coverslips placed in 24-well plates. Cell surface α3 integrin molecules were tagged by incubating HeLa cells with a mouse mAb at 4°C. At 4°C, endocytosis is inhibited. Immediately after labeling, some of the cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and labeled with rabbit pAbs to STX6 or VAMP3. In parallel experiments, the tagged cells were incubated at 37°C to resume endocytosis and vesicular trafficking. After 30 min at 37°C, the cells were fixed, permeabilized, and labeled with pAbs to STX6 or VAMP3. The cells were then dual labeled with a FITC-conjugated anti-mouse antibody (to stain endocytosed α3 integrin) and a Rhodamine-conjugated anti-rabbit antibody (to stain STX6 and VAMP3). Colocalization of α3 integrin with STX6 or VAMP3 was examined by confocal fluorescence microscopy.
Supplementary Material
Acknowledgments
We thank Yinlu Chen and Sabine J. Waigel in the Microarray Core Facility at the University of Louisville for assistance in microarray analysis, Ronald Gregg for critically reading the manuscript and Krishna Patel for technical assistance.
Footnotes
Funding
This work was supported by startup funds from the University of Louisville School of Medicine to C.H.; and the National Institutes of Health [grant number CA135123 to C.H.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.102566/-/DC1
References
- Biewenga P., Buist M. R., Moerland P. D., Ver Loren van Themaat E., van Kampen A. H., ten Kate F. J., Baas F. (2008). Gene expression in early stage cervical cancer. Gynecol. Oncol. 108, 520–526 10.1016/j.ygyno.2007.11.024 [DOI] [PubMed] [Google Scholar]
- Bock J. B., Lin R. C., Scheller R. H. (1996). A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 271, 17961–17965 10.1074/jbc.271.30.17961 [DOI] [PubMed] [Google Scholar]
- Bock J. B., Klumperman J., Davanger S., Scheller R. H. (1997). Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol. Biol. Cell 8, 1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. (2004). The mechanisms of vesicle budding and fusion. Cell 116, 153–166 10.1016/S0092-8674(03)01079-1 [DOI] [PubMed] [Google Scholar]
- Brandhorst D., Zwilling D., Rizzoli S. O., Lippert U., Lang T., Jahn R. (2006). Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc. Natl. Acad. Sci. USA 103, 2701–2706 10.1073/pnas.0511138103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. (1989). Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 8, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell P. T., Norman J. C. (2006). Integrin trafficking and the control of cell migration. Traffic 7, 14–21 10.1111/j.1600-0854.2005.00362.x [DOI] [PubMed] [Google Scholar]
- Caswell P. T., Vadrevu S., Norman J. C. (2009). Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10, 843–853 10.1038/nrm2799 [DOI] [PubMed] [Google Scholar]
- D'Errico M., de Rinaldis E., Blasi M. F., Viti V., Falchetti M., Calcagnile A., Sera F., Saieva C., Ottini L., Palli D.et al. (2009). Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur. J. Cancer 45, 461–469 10.1016/j.ejca.2008.10.032 [DOI] [PubMed] [Google Scholar]
- Day P., Riggs K. A., Hasan N., Corbin D., Humphrey D., Hu C. (2011). Syntaxins 3 and 4 mediate vesicular trafficking of α5β1 and α3β1 integrins and cancer cell migration. Int. J. Oncol. 39, 863–871 [DOI] [PubMed] [Google Scholar]
- De Deyne P. G., O'Neill A., Resneck W. G., Dmytrenko G. M., Pumplin D. W., Bloch R. J. (1998). The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J. Cell Sci. 111, 2729–2740 [DOI] [PubMed] [Google Scholar]
- Delwel G. O., de Melker A. A., Hogervorst F., Jaspars L. H., Fles D. L., Kuikman I., Lindblom A., Paulsson M., Timpl R., Sonnenberg A. (1994). Distinct and overlapping ligand specificities of the alpha 3A beta 1 and alpha 6A beta 1 integrins: recognition of laminin isoforms. Mol. Biol. Cell 5, 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench–Constant C., Colognato H. (2004). Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 14, 678–686 [DOI] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. (1994). Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J. Cell Biol. 125, 1015–1024 10.1083/jcb.125.5.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel H. L., Li J., Kogan S., Languino L. R. (2008). Integrins in prostate cancer progression. Endocr. Relat. Cancer 15, 657–664 10.1677/ERC-08-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Giancotti F. G. (2004). Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5, 816–826 10.1038/nrm1490 [DOI] [PubMed] [Google Scholar]
- Hasan N., Hu C. (2010). Vesicle-associated membrane protein 2 mediates trafficking of alpha5beta1 integrin to the plasma membrane. Exp. Cell Res. 316, 12–23 10.1016/j.yexcr.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Hood J. D., Cheresh D. A. (2002). Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2, 91–100 10.1038/nrc727 [DOI] [PubMed] [Google Scholar]
- Hou J., Aerts J., den Hamer B., van Ijcken W., den Bakker M., Riegman P., van der Leest C., van der Spek P., Foekens J. A., Hoogsteden H. C.et al. (2010). Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS ONE 5, e10312 10.1371/journal.pone.0010312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M. A., Kraut N., Beug H. (2005). Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 10.1016/j.ceb.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Jahn R., Lang T., Südhof T. C. (2003). Membrane fusion. Cell 112, 519–533 10.1016/S0092-8674(03)00112-0 [DOI] [PubMed] [Google Scholar]
- Kaiser S., Park Y. K., Franklin J. L., Halberg R. B., Yu M., Jessen W. J., Freudenberg J., Chen X., Haigis K., Jegga A. G.et al. (2007). Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 8, R131 10.1186/gb-2007-8-7-r131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Han S. Y., Liu W., Otsuka K., Shibata H., Kanamaru R., Ishioka C. (2003). Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc. Natl. Acad. Sci. USA 100, 8424–8429 10.1073/pnas.1431692100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses W. B., Shattil S. J., Pampori N., Schwartz M. A. (2001). Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3, 316–320 10.1038/35060120 [DOI] [PubMed] [Google Scholar]
- Korkola J. E., Houldsworth J., Chadalavada R. S., Olshen A. B., Dobrzynski D., Reuter V. E., Bosl G. J., Chaganti R. S. (2006). Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 66, 820–827 10.1158/0008-5472.CAN-05-2445 [DOI] [PubMed] [Google Scholar]
- Kreidberg J. A. (2000). Functions of alpha3beta1 integrin. Curr. Opin. Cell Biol. 12, 548–553 10.1016/S0955-0674(00)00130-7 [DOI] [PubMed] [Google Scholar]
- Kreykenbohm V., Wenzel D., Antonin W., Atlachkine V., von Mollard G. F. (2002). The SNAREs vti1a and vti1b have distinct localization and SNARE complex partners. Eur. J. Cell Biol. 81, 273–280 10.1078/0171-9335-00247 [DOI] [PubMed] [Google Scholar]
- Kuliawat R., Kalinina E., Bock J., Fricker L., McGraw T. E., Kim S. R., Zhong J., Scheller R., Arvan P. (2004). Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells. Mol. Biol. Cell 15, 1690–1701 10.1091/mbc.E03-08-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaTulippe E., Satagopan J., Smith A., Scher H., Scardino P., Reuter V., Gerald W. L. (2002). Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 62, 4499–4506 [PubMed] [Google Scholar]
- Laukaitis C. M., Webb D. J., Donais K., Horwitz A. F. (2001). Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 153, 1427–1440 10.1083/jcb.153.7.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M. A., Maxfield F. R. (1995). Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75–79 10.1038/377075a0 [DOI] [PubMed] [Google Scholar]
- Levine A. J. (1997). p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 10.1016/S0092-8674(00)81871-1 [DOI] [PubMed] [Google Scholar]
- Lobert V. H., Brech A., Pedersen N. M., Wesche J., Oppelt A., Malerød L., Stenmark H. (2010). Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev. Cell 19, 148–159 10.1016/j.devcel.2010.06.010 [DOI] [PubMed] [Google Scholar]
- Luftman K., Hasan N., Day P., Hardee D., Hu C. (2009). Silencing of VAMP3 inhibits cell migration and integrin-mediated adhesion. Biochem. Biophys. Res. Commun. 380, 65–70 10.1016/j.bbrc.2009.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F., Tang B. L., Galli T., Tenza D., Saint–Pol A., Yue X., Antony C., Hong W., Goud B., Johannes L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653–664 10.1083/jcb.200110081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickam V., Tiwari A., Jung J. J., Bhattacharya R., Goel A., Mukhopadhyay D., Choudhury A. (2011). Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6. Blood 117, 1425–1435 10.1182/blood-2010-06-291690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Südhof T. C. (1993). Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364, 346–349 10.1038/364346a0 [DOI] [PubMed] [Google Scholar]
- Murray R. Z., Kay J. G., Sangermani D. G., Stow J. L. (2005a). A role for the phagosome in cytokine secretion. Science 310, 1492–1495 10.1126/science.1120225 [DOI] [PubMed] [Google Scholar]
- Murray R. Z., Wylie F. G., Khromykh T., Hume D. A., Stow J. L. (2005b). Syntaxin 6 and Vti1b form a novel SNARE complex, which is up-regulated in activated macrophages to facilitate exocytosis of tumor necrosis Factor-alpha. J. Biol. Chem. 280, 10478–10483 10.1074/jbc.M414420200 [DOI] [PubMed] [Google Scholar]
- Nishiuchi R., Murayama O., Fujiwara H., Gu J., Kawakami T., Aimoto S., Wada Y., Sekiguchi K. (2003). Characterization of the ligand-binding specificities of integrin alpha3beta1 and alpha6beta1 using a panel of purified laminin isoforms containing distinct alpha chains. J. Biochem. 134, 497–504 10.1093/jb/mvg185 [DOI] [PubMed] [Google Scholar]
- Palecek S. P., Schmidt C. E., Lauffenburger D. A., Horwitz A. F. (1996). Integrin dynamics on the tail region of migrating fibroblasts. J. Cell Sci. 109, 941–952 [DOI] [PubMed] [Google Scholar]
- Perera H. K., Clarke M., Morris N. J., Hong W., Chamberlain L. H., Gould G. W. (2003). Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol. Biol. Cell 14, 2946–2958 10.1091/mbc.E02-11-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini L. M., Lawson M. A., Eddy R. J., Hendey B., Maxfield F. R. (2000). Oriented endocytic recycling of alpha5beta1 in motile neutrophils. Blood 95, 2471–2480 [PubMed] [Google Scholar]
- Powelka A. M., Sun J., Li J., Gao M., Shaw L. M., Sonnenberg A., Hsu V. W. (2004). Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5, 20–36 10.1111/j.1600-0854.2004.00150.x [DOI] [PubMed] [Google Scholar]
- Presley J. F., Mayor S., McGraw T. E., Dunn K. W., Maxfield F. R. (1997). Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J. Biol. Chem. 272, 13929–13936 10.1074/jbc.272.21.13929 [DOI] [PubMed] [Google Scholar]
- Proux–Gillardeaux V., Gavard J., Irinopoulou T., Mège R. M., Galli T. (2005). Tetanus neurotoxin-mediated cleavage of cellubrevin impairs epithelial cell migration and integrin-dependent cell adhesion. Proc. Natl. Acad. Sci. USA 102, 6362–6367 10.1073/pnas.0409613102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen C. M., Horwitz A. F. (1992). Dynamics of beta 1 integrin-mediated adhesive contacts in motile fibroblasts. J. Cell Biol. 119, 1347–1359 10.1083/jcb.119.5.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. R., Yu J., Shanker K., Deshpande N., Varambally R., Ghosh D., Barrette T., Pandey A., Chinnaiyan A. M. (2004). ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A. L., Wang Z. C., De Nicolo A., Lu X., Brown M., Miron A., Liao X., Iglehart J. D., Livingston D. M., Ganesan S. (2006). X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 9, 121–132 10.1016/j.ccr.2006.01.013 [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704–1709 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- Riker A. I., Enkemann S. A., Fodstad O., Liu S., Ren S., Morris C., Xi Y., Howell P., Metge B., Samant R. S.et al. (2008). The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med. Genomics 1, 13 10.1186/1755-8794-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Barry S., Woods A., van der Sluijs P., Norman J. (2001). PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11, 1392–1402 10.1016/S0960-9822(01)00442-0 [DOI] [PubMed] [Google Scholar]
- Roberts M. S., Woods A. J., Dale T. C., Van Der Sluijs P., Norman J. C. (2004). Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol. Cell. Biol. 24, 1505–1515 10.1128/MCB.24.4.1505-1515.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. (1994). Mechanisms of intracellular protein transport. Nature 372, 55–63 10.1038/372055a0 [DOI] [PubMed] [Google Scholar]
- Sanchez–Carbayo M., Socci N. D., Lozano J., Saint F., Cordon–Cardo C. (2006). Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 24, 778–789 10.1200/JCO.2005.03.2375 [DOI] [PubMed] [Google Scholar]
- Segara D., Biankin A. V., Kench J. G., Langusch C. C., Dawson A. C., Skalicky D. A., Gotley D. C., Coleman M. J., Sutherland R. L., Henshall S. M. (2005). Expression of HOXB2, a retinoic acid signaling target in pancreatic cancer and pancreatic intraepithelial neoplasia. Clin. Cancer Res. 11, 3587–3596 10.1158/1078-0432.CCR-04-1813 [DOI] [PubMed] [Google Scholar]
- Skalski M., Coppolino M. G. (2005). SNARE-mediated trafficking of alpha5beta1 integrin is required for spreading in CHO cells. Biochem. Biophys. Res. Commun. 335, 1199–1210 10.1016/j.bbrc.2005.07.195 [DOI] [PubMed] [Google Scholar]
- Tayeb M. A., Skalski M., Cha M. C., Kean M. J., Scaife M., Coppolino M. G. (2005). Inhibition of SNARE-mediated membrane traffic impairs cell migration. Exp. Cell Res. 305, 63–73 10.1016/j.yexcr.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Teckchandani A., Toida N., Goodchild J., Henderson C., Watts J., Wollscheid B., Cooper J. A. (2009). Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J. Cell Biol. 186, 99–111 10.1083/jcb.200812160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Jung J. J., Inamdar S. M., Brown C. O., Goel A., Choudhury A. (2011a). Endothelial cell migration on fibronectin is regulated by syntaxin 6-mediated alpha5beta1 integrin recycling. J. Biol. Chem. 286, 36749–36761 10.1074/jbc.M111.260828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S., Askari J. A., Humphries M. J., Bulleid N. J. (2011b). Divalent cations regulate the folding and activation status of integrins during their intracellular trafficking. J. Cell Sci. 124, 1672–1680 10.1242/jcs.084483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale K. J., Offenhäuser C., Whittaker S. P., Estrella R. P., Murray R. Z. (2010). Recycling endosome membrane incorporation into the leading edge regulates lamellipodia formation and macrophage migration. Traffic 11, 1370–1379 10.1111/j.1600-0854.2010.01094.x [DOI] [PubMed] [Google Scholar]
- Wary K. K., Mariotti A., Zurzolo C., Giancotti F. G. (1998). A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625–634 10.1016/S0092-8674(00)81604-9 [DOI] [PubMed] [Google Scholar]
- Watson R. T., Pessin J. E. (2000). Functional cooperation of two independent targeting domains in syntaxin 6 is required for its efficient localization in the trans-golgi network of 3T3L1 adipocytes. J. Biol. Chem. 275, 1261–1268 10.1074/jbc.275.2.1261 [DOI] [PubMed] [Google Scholar]
- Watson R. T., Hou J. C., Pessin J. E. (2008). Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors. J. Cell Sci. 121, 1243–1251 10.1242/jcs.017517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. J., Parsons J. T., Horwitz A. F. (2002). Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat. Cell Biol. 4, E97–E100 10.1038/ncb0402-e97 [DOI] [PubMed] [Google Scholar]
- Wendler F., Tooze S. (2001). Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic 2, 606–611 10.1034/j.1600-0854.2001.20903.x [DOI] [PubMed] [Google Scholar]
- Wendler F., Page L., Urbé S., Tooze S. A. (2001). Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol. Biol. Cell 12, 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A. J., White D. P., Caswell P. T., Norman J. C. (2004). PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 23, 2531–2543 10.1038/sj.emboj.7600267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M. A., Modzelewska K., Kwong L., Keely P. J. (2004). Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 1692, 103–119 10.1016/j.bbamcr.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Wurmbach E., Chen Y. B., Khitrov G., Zhang W., Roayaie S., Schwartz M., Fiel I., Thung S., Mazzaferro V., Bruix J.et al. (2007). Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 45, 938–947 10.1002/hep.21622 [DOI] [PubMed] [Google Scholar]
- Ye H., Yu T., Temam S., Ziober B. L., Wang J., Schwartz J. L., Mao L., Wong D. T., Zhou X. (2008). Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics 9, 69 10.1186/1471-2164-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. O., Shin S., Mercurio A. M. (2005). Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 65, 2761–2769 10.1158/0008-5472.CAN-04-4122 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shu L., Chen X. (2008). Syntaxin 6, a regulator of the protein trafficking machinery and a target of the p53 family, is required for cell adhesion and survival. J. Biol. Chem. 283, 30689–30698 10.1074/jbc.M801711200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Guan J. L. (2009). Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 28, 35–49 10.1007/s10555-008-9165-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.