This column is designed to address a specific pharmacotherapeutic issue in the pediatric patient. Typically, the commentary will be in response to an article that will have appeared in a prominent pediatric journal, and the results are deserving of being reinforced or challenged. We hope to provoke thought and controversy through the opinions presented in these commentaries. The article prompting this editorial appeared in the May 2007 issue of Pediatrics, and was entitled “Therapeutic Drug Monitoring for Caffeine in Preterm Neonates: An Unnecessary Exercise?”
Caffeine is widely regarded as the drug of choice for apnea of prematurity.1,2 This is largely based on its efficacy and relatively wide therapeutic index.1,2 A recent article by Natarajan et al. concluded that monitoring caffeine serum concentrations is not necessary when caffeine is used for apnea of prematurity.3 The premise for their conclusions is based on results from a retrospective review of 101 neonates who received 5 to 8 mg/kg/day caffeine for apnea of prematurity. The authors concluded that because 94% of measured serum caffeine concentrations were within the “therapeutic range” (5.1 to 20 mg/L [2.5 to 10 mmol/L]) there was no need to routinely monitor serum concentrations.
Is it sufficient to know that caffeine serum concentrations are in the “therapeutic range” when a patient is experiencing an increase in apnea events, or when concentrations are above the “therapeutic range” if toxicity is observed? The answer lies, in part, in the way clinicians use therapeutic drug monitoring (TDM) in the management of apnea. In a recent review, the conclusion by Natarajan et al. that monitoring serum caffeine concentrations is not necessary presumes that the therapeutic range for caffeine is between 5 and 20 mg/L (2.5 to 10 mmol/L) and that within this range all responses are equal.1 The use of TDM to merely achieve standard “therapeutic ranges” has been challenged, especially in neonates, because clinical data describing pharmacodynamic response curves are often not available, leaving one to extrapolate from other populations or make up values as the basis for therapeutic ranges.4–6
Despite the large number of concentrations between 5 and 20 mg/L (2.5 to 10 mmol/L), Natarajan et al.3 reported that caffeine concentration-per-dose (mg/kg) ratios were from less than 1 up to 5, reflecting more than fivefold variability. When these authors plotted multiple serum caffeine concentration-to-dose ratios over time, the values changed in unpredictable directions, sometimes increasing and sometimes decreasing. Thus, in any group of neonates, the relationship between apnea control and caffeine serum concentrations can be expected to change with time. Furthermore, the unpredictable change in direction and magnitude of caffeine values makes estimating this change from prior caffeine dose and concentration pairs difficult.
Apnea is defined in several ways, but a fairly common definition is apnea lasting longer than 20 seconds, or longer than 10 seconds when accompanied by bradycardia or oxygen desaturation below 80 percent. Desaturation events lasting less than 10 seconds may also be interpreted as significant apnea by some clinicians.7 Apnea of prematurity is by definition a diagnosis of exclusion, and typically requires that the clinician feels confident in excluding such common etiologies as infection, anemia, cardiac causes such as patent ductus arteriosus, neurologic causes such as intraventricular hemorrhage or seizures, gastrointestinal reflux, and insufficient oxygen supplementation. Thus, when caffeine is used, it is with the assumed or proven elimination of the other etiologies, or to prevent apnea episodes after extubation. When a neonate treated with caffeine develops breakthrough apnea, a practitioner will often begin a sepsis workup and evaluation for anemia, and perhaps other actions such as additional laboratory testing, placing the infant on antibiotics, nasal continuous positive airway pressure, and occasionally mechanical ventilation. The severity of episodes that causes a clinician to make these interventions is variable, since the link between apnea and adverse neonatal outcomes is unproven.7 On the other hand, selected animal studies and intuition cause most clinicians to believe that apnea with bradycardia or desaturations is not good for neonates and is probably harmful.
In the clinical environment, there remain important confounders for the target response and the target serum concentration of caffeine. Nursing documentation of apnea using typical apnea monitoring devices has been proven to miss over 50% of apnea events longer than 30 seconds.8,9 Thus, usual clinical monitoring that combines leads for chest wall movement, heart rate recording, and oximetry is insufficient to truly ensure adequate measurement of apnea frequency to assess control. Similar issues arise when weighing the effect of caffeine on events that may be viewed as apnea equivalents, such as bradycardia or desaturations. Consequently, in the clinical arena, some events that do not provoke the medical team to make additional interventions could be acceptable clinically, and perhaps provide the best measure of desired caffeine response. The number of these events in a given day may fluctuate due to numerous factors that are unrelated to the serum caffeine concentrations.
It can be important to know the serum caffeine concentration at which a desired response occurred in a specific patient. For example, in the event of breakthrough apnea, bradycardia, or desaturations without other obvious disease-related etiologies, comparing current caffeine serum concentrations to those previously associated with efficacy allows one to select appropriate loading and maintenance doses of caffeine that will rapidly achieve and sustain the known effective concentration. If an adjustment in caffeine dosage resolves the patient's signs and symptoms, expensive and invasive tests or procedures that are used to evaluate other causes of apnea may be avoided.
Tachycardia can occur in neonates for numerous reasons, including worse respiratory distress, fluid overload, and pain or agitation. If a patient with tachycardia has a caffeine concentration below 20 mg/L (10 mmol/L), one would be inclined to attribute the tachycardia to another cause. Conversely, the elevated heart rate might be attributed to caffeine if the serum concentration were above 20 mg/L (10 mmol/L). If caffeine doses were held when it actually was helping to manage increasing respiratory distress, the patient might be unnecessarily intubated. Most clinicians would agree that mechanical ventilation is likely more toxic than methylxanthines to both short- and long-term outcomes. However, if routine TDM documents that the same caffeine serum concentration was not associated previously with tachycardia, the clinician might be prompted to investigate alternative etiologies.
Failure to identify the “critical caffeine concentration” for a patient (the concentration at which apnea control is acceptable and no toxicity is present) may cause a practitioner to misinterpret the caffeine concentration when clinical circumstances are changing. We have defined pharmacodynamic curves for caffeine efficacy (based on an acceptable or optimal response) and toxicity (based on tachycardia) for our institution as an aid for our expectations (Figure).
Figure.
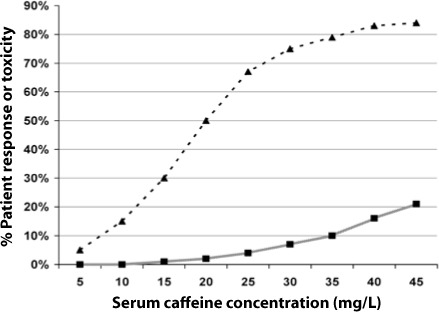
Pharmacodynamic curves for caffeine efficacy based on an acceptable or optimal response, and lack of toxicity. Pharmacodynamic profile is based on clinical experience in 268 neonates over a 3-year period. Using this curve, a reduction of caffeine concentration from 20 mg/L (10 mmol/L) to 10 mg/L (5 mmol/L) would result in loss of clinical response in 35% of the potential responders, despite both concentrations being in the “therapeutic” range.
▴ = Clinical response (apnea, or bradycardia, and/or oxygen desaturation)
▪ = Toxicity (tachycardia)
The use of caffeine in neonates meets some important criteria used to justify routine TDM for other drugs. These include: a wide range of drug clearances resulting in a several-fold range of drug concentrations; changing drug clearances related to clinical events or as the patient ages; difficulty in separating changing caffeine concentrations from other etiologies of increasing apnea, bradycardia, or oxygen desaturation events, or signs of caffeine toxicity, resulting in expensive laboratory tests and work-up; potentially aggressive or toxic interventions as a result of therapeutic failure, in this case being the use of mechanical ventilation.
The usefulness of a caffeine serum concentration would depend on how the clinician employed TDM. For example, in the study by Natarajan et al. a serum caffeine concentration would not be routinely obtained, but would be monitored only if toxicity was noted, or if breakthrough apnea occurred.3 The caffeine concentration would be used to confirm a clinical suspicion. This approach to monitoring may create a cycle where the decision to assess a serum concentration is based on expectations that are created by an artificial “therapeutic range.” Although the target range of 5 to 20 mg/L (2.5 to 10 mmol/L) was noted in the Natarajan study,3 a recent review by the same authors1 suggested that a range of 8 to 40 mg/L (4 to 20 mmol/L) may be more appropriate. The higher target range is more consistent with our own experience (Figure).
It is reasonable for expert opinion to disagree on issues such as the need for TDM when patients have responded well to a medication. It depends, in part, on how the practitioner clinically applies TDM. If the intent is to simply target a prescribed therapeutic range, limiting caffeine serum concentration monitoring to the presence of clinical symptoms or toxicity may be reasonable. If the purpose of TDM is to individualize caffeine dosage in order to meet each patient's clinical needs by obtaining the best possible response, optimum dosing can only be accomplished through routine monitoring of serum caffeine concentrations and documentation of the “critical caffeine concentration” for that patient. It is this author's contention that the caffeine “therapeutic range” has not been properly designed and adherence to a concocted range is detrimental. Furthermore, documentation of the effective caffeine serum concentration that is unique to individual patients can help avoid unnecessary laboratory tests and interventions. Individualized serum concentration monitoring in patients receiving caffeine is essential to optimal care of critically ill neonates or other populations with changing physiology, and should not be compromised for simplicity or perceived cost savings.
ABBREVIATIONS
- TDM
therapeutic drug monitoring
Footnotes
DISCLOSURE The author declares no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Natarajan G, Lulic-Botica M, Aranda JV. Clinical pharmacology of caffeine in the newborn. NeoReviews. 2007;8:e-14–e221. [Google Scholar]
- 2.Comer AM, Perry CM, Figgitt DP. Caffeine citrate: a review of its use in apnoea of prematurity. Paediatr Drugs. 2001;3:61–79. doi: 10.2165/00128072-200103010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Natarajan G, Lulic-Botica M, Thomas R, Aranda JV. Therapeutic drug monitoring for caffeine in preterm infants: an unnecessary exercise? Pediatrics. 2007;119:936–940. doi: 10.1542/peds.2006-2986. [DOI] [PubMed] [Google Scholar]
- 4.Gal P, Gilman JT. Concerns about the Food and Drug Administration guidelines for neonatal theophylline dosing. Ther Drug Monit. 1986;8:1–3. [PubMed] [Google Scholar]
- 5.Brown GR, Miyata M, McCormack JP. Drug concentration monitoring: an approach to rational use. Clin Pharmacokinet. 1993;24:187–194. doi: 10.2165/00003088-199324030-00001. [DOI] [PubMed] [Google Scholar]
- 6.Gal P. Therapeutic drug monitoring in neonates: problems and issues. Drug Intell Clin Pharm. 1988;22:317–323. doi: 10.1177/106002808802200411. [DOI] [PubMed] [Google Scholar]
- 7.Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from apnea-of-prematurity group. Pediatrics. 2006;117:S-7–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- 8.Muttitt SC, Finer NN, Tierney AJ, Rossmann J. Neonatal apnea: Diagnosis by nurses versus computer. Pediatrics. 1988;82:713–720. [PubMed] [Google Scholar]
- 9.Southall DP, Levitt GA, Richards JM. Undetected episodes of prolonged apnea and severe bradycardia in preterm infants. Pediatrics. 1983;72:541–551. et al. [PubMed] [Google Scholar]


