Abstract
OBJECTIVE Pancreatic enzyme products were available before the 1938 passage of the Federal Food, Drug, and Cosmetic Act and have to date been marketed without required safety and efficacy testing. Despite a lack of demonstrated bioequivalence, they are often substituted for each other without physician or patient consent or monitoring. We investigated the in vitro variability of key performance parameters among a representative group of currently available pancreatic enzyme formulations.
MATERIALS AND METHODS Three “branded” preparations (Creon 20 Minimicrospheres, Pancrease MT 20, Ultrase MT 20) and 3 “generic” formulations (Pangestyme CN-20, Pancrelipase 20,000 URL, and Lipram CR 20) were evaluated in vitro for physical parameters of the capsules, actual vs. labeled enzyme activity, resistance of the enteric coating to simulated gastric acid, and kinetics of simulated duodenal lipase release. All products were labeled as providing 20,000 units of lipase activity per capsule.
RESULTS All products varied considerably in the percentage relationship between actual and labeled lipase activity. Actual lipase activity exceeded 165% of the label claim in 4 batches of the Pangestyme product and 1 batch of the Lipram product. All batches of the Creon, Lipram, Ultrase, and Pancrease products were found to have residual lipase activity above 80% of their baseline measurements after testing in simulated gastric acid; residual lipase activity varied significantly among batches of the Pangestyme product and was only 1% for the Pancrelipase product. The Creon and Lipram products demonstrated effective protection by the enteric coating at pH <6.0 and rapid release of enzymatic activity at pH ≥6.0. The Pangestyme and Pancrelipase products showed substantial activity of released enzymes already at pH 5.0. Release kinetics were inconsistent between batches for the Ultrase and Pancrease products.
CONCLUSION This study confirms the existence of “branded”-to-“generic,” product-to-product, and batch-to-batch variability among representative pancreatic enzyme formulations with pharmaceutically equivalent labels. The results confirm current cautions regarding pharmacy substitution of pancreatic enzyme products and support the announcement by the US Food and Drug Administration, made subsequent to this study, that as of April 2008 approved new drug applications will be required in order to ensure the quality, potency, and stability of these products.
Keywords: bioequivalence, cystic fibrosis, exocrine pancreatic insufficiency, microencapsulation, pancreatic extracts, pancrelipase, steatorrhea
INTRODUCTION
Pancreatic enzyme products are prescribed for the treatment of exocrine pancreatic insufficiency, as is often associated with cystic fibrosis (CF), chronic pancreatitis, ductal obstruction of the pancreas or common bile duct (e.g., from neoplastic disease), or surgical procedures such as pancreatectomy or gastrointestinal bypass surgery (e.g., Billroth II gastroenterostomy). The pancreas secretes digestive enzymes (lipase, protease, and amylase) into the proximal duodenal lumen, where they facilitate the hydrolysis of macronutrients. Although extra-pancreatic sources of amylase and protease contribute to the digestion of carbohydrates and protein, respectively, nonpancreatic endogenous sources of lipase contribute relatively little to the digestion of lipids.1 Thus, patients with untreated exocrine pancreatic insufficiency typically have difficulty digesting fat and suffer symptoms of both maldigestion and malnutrition, with deficiencies of essential fatty acids and fat-soluble vitamins, weight loss, cramping, flatulence, bloating, and greasy, foul-smelling, loose stools (steatorrhea). For patients with CF, inadequate treatment may have serious consequences;2 good nutritional status has been directly correlated with good lung function.3
By convention, pancreatic enzyme products are labeled according to the amount of lipase they contain. All pancreatic enzyme products also contain protease and amylase, but the labeled and actual amounts of these 2 enzymes may differ from product to product even when labeled lipase amounts are the same. Older pancreatic enzyme formulations are based on pancreatin, a substance obtained from the pancreas of the hog or ox and defined by the United States Pharmacopeia (USP) as containing not less than 25 USP units of amylase activity, 2.0 USP units of lipase activity, and 25 USP units of protease activity per mg.4 (One USP unit of enzyme activity is defined as the amount of a substance that decomposes a given substrate at a specified rate under standard USP assay conditions.) Newer pancreatic enzyme formulations are based on pancrelipase, a more potent extract from the hog pancreas, which the USP defines as containing not less than 24 USP units of lipase activity, 100 USP units of amylase activity, and 100 USP units of protease activity per mg. The USP definition does not express upper limits of enzyme activity per mg.4 International application of these definitions is somewhat confusing, because in Europe all exocrine pancreatic extracts are described as “pancreatin” (the term “pancrelipase” is not typically used in Europe).
For exogenous enzyme supplementation to be effective, it is crucial that as much as possible of the dose reaches the proximal small intestine at the same time as the chyme that is to be digested. Lipase is the most sensitive of the enzymes to the effects of both pepsin and acid and is irreversibly inactivated at a pH of 4.0 or lower.5 Early exogenous preparations consisting of tablets or encapsulated powder were not protected against such inactivation in the stomach, and as little as 8% of ingested lipase and 22% of protease actually reached the duodenum.2,5 It was thus necessary to administer orally up to 5 to 10 times as much lipase as was required for intraluminal digestion, limiting compliance with therapy.1 In the 1970s, the development of enteric coatings and of microsphere and microtablet formulations made it possible to protect the enzymes for passage through the stomach, get them into the duodenum simultaneously with the chyme (particles with a diameter of 4 mm or greater have been found to remain in the stomach until the interdigestive phase6), and have them release when the intestinal pH is most conducive to their activity. Partly as a result of the improvements in delivery of exogenous enzyme formulations, prognosis has been improved for patients with pancreatic insufficiency, especially those with CF. (From 1970 to 2000, the median survival of CF patients increased from 14 years to over 30 years.7 More effective lipase supplementation has allowed CF patients to shift from the previously recommended low-fat, high-protein program to a diet high in fat as well as protein, making it possible for them to meet high energy needs while absorbing essential fatty acids and fat-soluble vitamins.) Subsequent refinements in microencapsulation technology facilitated increases in lipase content and more efficient dosing.8
Previous studies have suggested that pancreatic enzyme products vary in terms of actual enzyme content and in vitro response to simulated gastric and duodenal conditions.5,9–11 Because pancreatic enzymes are sensitive proteins and liable to inactivation, capsules are routinely overfilled to ensure that potency will not drop below label claims before the end of shelf life.2 The USP standard has evolved in recognition of these circumstances. According to the USP standard that became official on May 1, 2007, pancrelipase capsules should not contain less than 90% nor more than 150% of the labeled lipase activity, and “delayed-release” (that is, enteric-coated) capsules should not contain less than 90% nor more than 165% of the labeled lipase activity. (For amylase and protease activity, the USP only sets a lower limit of 90% of labeled activity.)4
Because pancreatic enzyme products were available before the passage of the 1938 Federal Food, Drug, and Cosmetic Act, they have historically been marketed in the United States without any requirements for safety and efficacy testing. There is no current enzyme product marketed in the United States with a new drug application (NDA) approved by the Food and Drug Administration (FDA).12 The efficacy of some of the most widely prescribed products has been documented with in vitro and in vivo data.13–17 But many preparations have come to market lacking supportive experimental or clinical data; thus clinicians have little objective information to guide their use or to predict variations in patient response when different products are used.
Citing concerns about the significant differences in bioavailability among pancreatic enzyme products and consequent instances of serious under- and overdosing,8,18–23 the FDA in 1995 ruled that “pancreatic insufficiency drug products” could no longer be marketed on an over-the-counter (OTC) basis and expressed its intention to require NDAs or abbreviated new drug applications (ANDAs) for all prescription pancreatic insufficiency products.24 The FDA attributed the differences in bioavailability to the wide range of enzyme activity in the products, the variety of dosage forms marketed, and “the apparent uneven quality of the enteric coatings, . . . dependent on the manufacturing process.” “Preclearance” of each product is necessary, said the FDA, “in order to standardize enzyme bioactivity” and to avoid “serious safety problems resulting from too little or too much enzyme supplementation.”24 On April 28, 2004, the FDA formally announced the NDA requirement for exocrine pancreatic insufficiency drug products, with the stipulation that because the drugs are “medically necessary,” manufacturers may continue to market current products without an approved application for the next 4 years.12
Because none of the currently available pancreatic enzyme products has been approved by the FDA or listed in the FDA publication Approved Drug Products with Therapeutic Equivalence Evaluations (referred to as the “Orange Book”),25 there are no true reference products with respect to which any other products can claim to be “therapeutic equivalents” or true generics. We use the term “branded” to refer to pancreatic enzyme products introduced to the market at an earlier time, during or previous to the early 1990s. We use the term “generic” to refer to pancreatic enzyme products introduced to the market subsequent to the introduction of the “branded” products and labeled the same as specific “branded” products.
Despite the statements in the 1995 FDA ruling about OTC pancreatic enzyme products,24 the 2004 FDA notice of the NDA requirement,12 and warnings from the Cystic Fibrosis Foundation (CFF)7 and others,8,18,26,27 pancreatic enzyme products continue to be substituted as if they are approved therapeutic equivalents,28,29 and since 1995, new unapproved “generic” formulations have been introduced as prescription (rather than OTC) drugs.30 The CFF has helped to educate physicians, pharmacists, patients, and the FDA about the lack of justification for and the dangers of pancreatic enzyme substitution.29,31,32 The 2004 FDA notice cites correspondence from the CFF reporting adverse events and treatment failures — including abdominal pain, intestinal obstruction, increased incidence of steatorrhea, increased episodes of rectal prolapse, and increased number of stools — apparently associated with the practice of substituting drugs that are not bioequivalent.12,29
The current in vitro study was intended to provide information regarding the variability of key performance parameters that may affect bioequivalence and clinical outcomes. A representative group of currently available pancreatic enzyme products was used. The study was designed to allow investigation of inter-lot conformity as well as product-to-product and “branded”-to-“generic” consistency.
MATERIALS
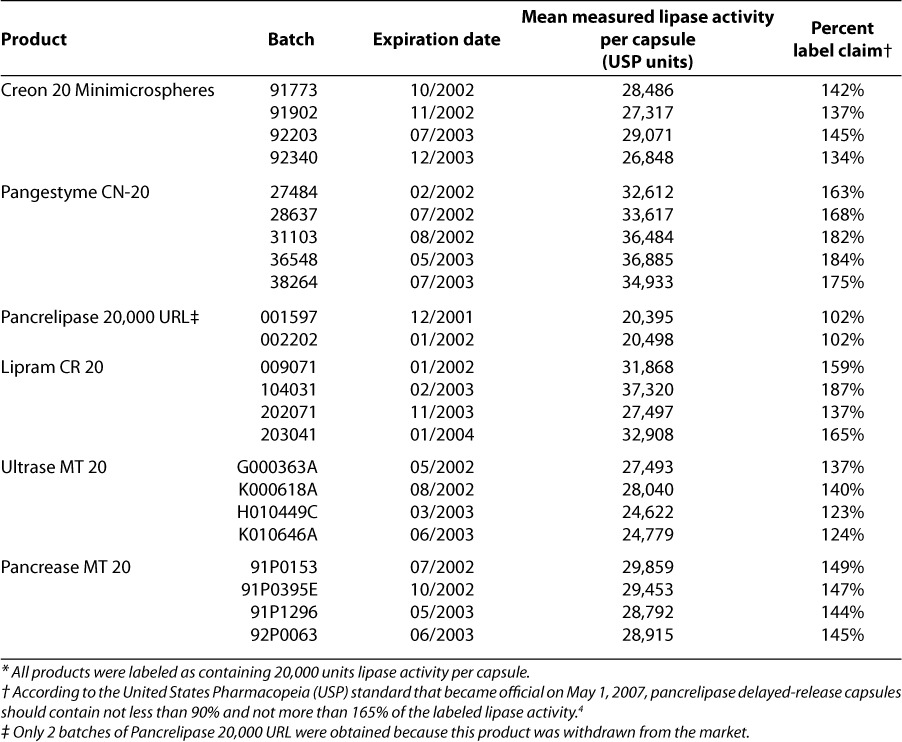
Three “branded” enteric-coated microencapsulated pancreatic enzyme preparations (Creon 20 Minimicrospheres, Pancrease MT 20, Ultrase MT 20) and 3 “generic” formulations (Pangestyme CN-20, Pancrelipase 20,000 URL, Lipram CR 20) were evaluated (Table 1). At least 4 separate batches were obtained for each product tested except Pancrelipase 20,000 URL; only 2 batches of Pancrelipase 20,000 URL were obtained because this product was withdrawn from the market. The labeling on all 6 products claimed 20,000 units of lipase activity per capsule. All products were purchased from a retail pharmacy and shipped to Solvay Pharmaceuticals GmbH in Hannover, Germany, for testing. The first 2 batches were received in August 2001 and analyzed during September and November of the same year. The second 2 batches were received in May and June of 2002 and analyzed within a 2-month period. All samples were maintained in the laboratory environment at a room temperature throughout the entire testing period and were tested well before the expiration dates identified on their packaging (Table 2).
Table 1.
Products Investigated in In Vitro Study of Enteric-Coated Microencapsulated Pancreatic Enzymes

Table 2.
Variability of Measured Lipase Activity per Capsule Relative to Label Claim*
METHODS
Physical parameters of enzyme capsules
For representative batches of each product formulation, average weight of capsule content was determined, the distribution of microsphere/microtablet sizes was assessed, and the average number of microspheres/microtablets per capsule was calculated.
Actual enzymatic activity
Baseline lipase, amylase, and free and total protease activity levels were determined for each sample in accordance with procedures specified by the Fédération Internationale Pharmaceutique (FIP) and the European Pharmacopoeia (PhEur). Results were converted from FIP/PhEur units to USP units as follows: for lipase, 1 FIP/PhEur unit = 1 USP unit; for amylase, 1 FIP/PhEur unit = 4.15 USP units; and for protease, 1 FIP/PhEur unit = 62.5 USP units.33 Actual enzymatic activity was compared as a percentage of label claims. All assays of baseline enzymatic activity were performed at least in duplicate.
Resistance of enteric coating to simulated gastric acid
Product samples were agitated in a disintegration apparatus in a solution of 0.1 N hydrochloric acid (pH 1) at 37°C for 120 minutes to simulate gastric conditions. Then the undissolved portion of the samples was separated from the solution, and the residual lipase activity was determined and reported as percentage of the actual lipase activity determined in the first part of the study and as percentage of label claim. All tests of the resistance of the enteric coating to simulated gastric acid were performed at least in triplicate.
Kinetics of simulated duodenal lipase release
New product samples were agitated in a disintegration apparatus designed to simulate intestinal peristalsis for 60 minutes at 37°C at pH 5.0. Then the pH was adjusted to 6.0, and the test was continued for an additional 60 minutes. Samples were withdrawn every 15 minutes for determination of the activity of the lipase that had been released from the enteric coating. The results at each time point were reported as percentage of the actual lipase activity determined in the first part of the study and as percentage of label claim. All tests of lipase release kinetics were performed at least in triplicate.
RESULTS
Physical parameters of enzyme capsules
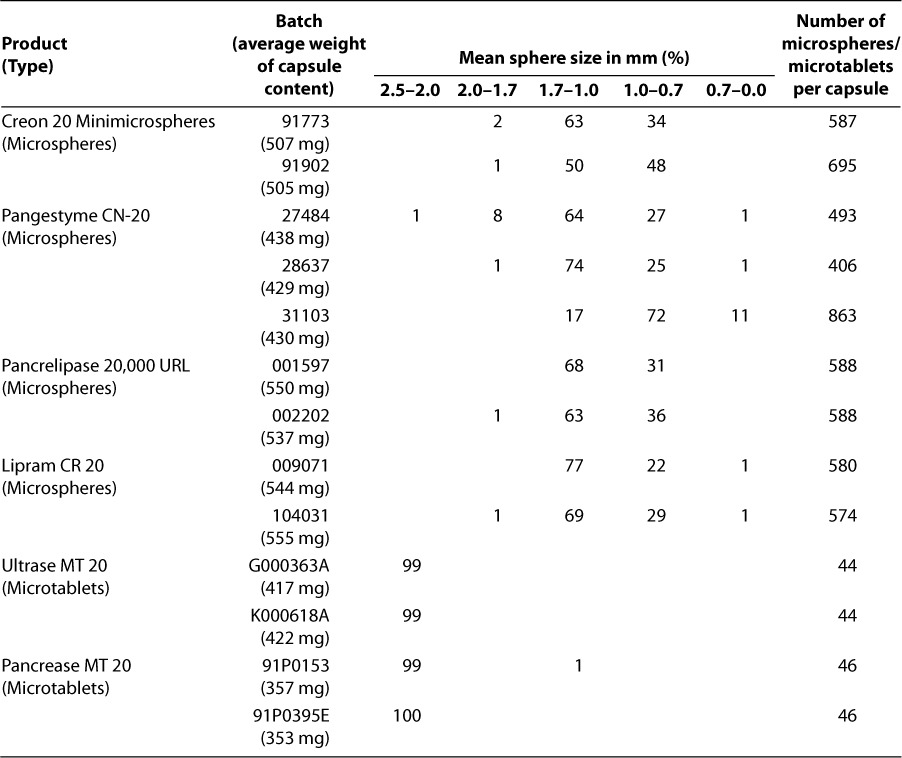
For the 2 “branded” products with capsules composed of microtablets (Ultrase MT 20 and Pancrease MT 20), the microparticle size was significantly larger, there was less heterogeneity of microparticle sizes within each capsule, and the average number of microparticles per capsule was significantly lower than for the other 4 products with capsules composed of microspheres (Table 3).
Table 3.
Physical Parameters of Pancreatic Enzyme Capsules in Representative Batches
Actual enzymatic activity
There was considerable variation among the products in the percentage relationship between actual and labeled lipase activity, with values ranging from 102% for Pancrelipase 20,000 URL to 187% for Lipram CR 20 (Table 2). However, for each product, the values were fairly consistent between batches; the values were least consistent between batches for 2 of the “generic” products—Pangestyme CN-20 (values ranging from 163% to 184%) and Lipram CR 20 (values ranging from 137% to 187%). The percentage-of-label-claim values were over 120% for all batches of all products, except for the 2 batches of Pancrelipase 20,000 URL, which were barely over 100%. Inspection of Table 2 shows that lipase activity exceeded the USP-specified upper percentage limit for pancrelipase delayed-release capsules (165% of label claim) in 4 out of 5 batches of Pangestyme CN-20 and 1 out of 4 batches of Lipram CR 20, while values above 180% of label claims were found for 2 batches of Pangestyme CN-20 (191% and 184%) and 1 batch of Lipram CR 20 (187%). Values for the 3 “branded” products (Creon 20 Minimicrospheres, Pancrease MT 20, and Ultrase MT 20) were consistently under 165% of label claims.
Resistance of enteric coating to simulated gastric acid
Creon 20 Minimicrospheres, Lipram CR 20, Ultrase MT 20, and Pancrease MT 20 were found to have residual lipase activity, after testing for resistance of their enteric coatings to simulated gastric acid, above 80% of their baseline measurements (Table 4, Figure 1). This finding indicated that their enteric coatings provided sufficient resistance to gastric acid to protect the enzymes even under conditions of extreme acidity. Two of the “generic” products, however, showed a marked reduction in lipase activity: After 2 hours of incubation at pH 1.0, the samples of Pancrelipase 20,000 URL were found to contain only 1% of their original level of lipase activity, and post-incubation analysis of Pangestyme CN-20 samples revealed extreme variability among batches, with residual lipase activity ranging from 9% to 75% of pre-incubation levels in the 5 batches tested. Residual lipase activity as a percentage of actual original activity was very consistent between batches for Creon 20 Minimicrospheres, Lipram CR 20, Ultrase MT 20, and Pancrease MT 20.
Table 4.
Resistance of Enteric Coating to Simulated Gastric Juice: Residual Lipase Activity after Incubation of Products for 120 Minutes at pH 1.0
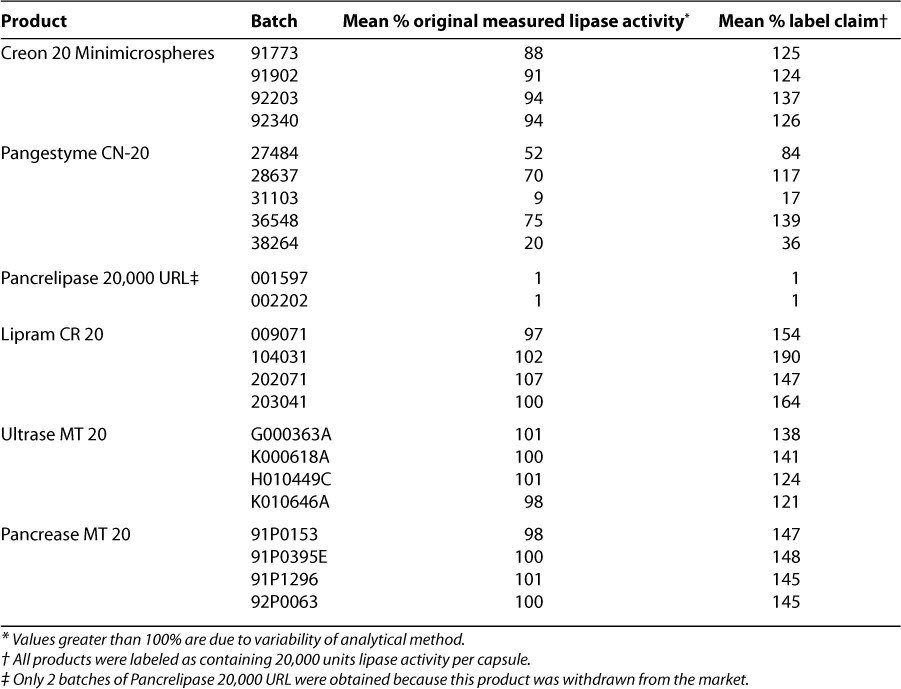
Figure 1.
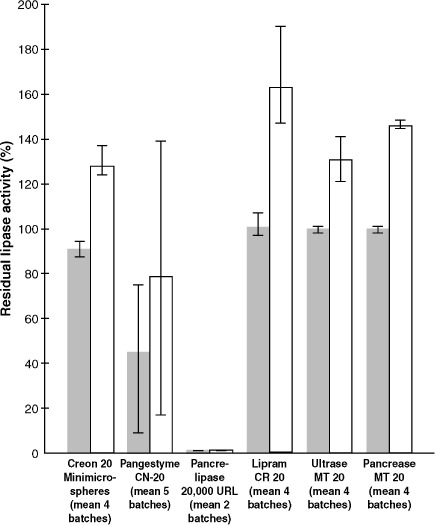
Residual lipase activity, average of per-capsule means for all batches of product, after incubation of samples for 120 minutes at pH 1.0 in a solution of 0.1 N hydrochloric acid (pH 1.0) at 37°C to simulate gastric conditions. Expressed as percentage of actual measured lipase activity ( ) at the start of the study before the gastric simulation and as percentage of the label claim (20,000 USP units of lipase) (□).
) at the start of the study before the gastric simulation and as percentage of the label claim (20,000 USP units of lipase) (□).
Kinetics of simulated duodenal lipase release
Testing designed to assess the kinetics of enzyme release under simulated duodenal conditions showed substantial differences among products in terms of the pH at which enzymes were released as well as consistency of performance. Analysis of samples taken after 60 minutes at pH 5.0 (Table 5, Figure 2) revealed only minimal release for Creon 20 Minimicrospheres and Lipram CR 20. In contrast, the activity levels for released enzymes were well over half of the actual original activity for Pangestyme CN-20 (72% to 78%) and Pancrelipase 20,000 URL (61% and 68%) after 60 minutes at pH 5.0. For the 2 remaining products, Ultrase MT 20 and Pancrease MT 20, the activity levels for enzymes released after 60 minutes at pH 5.0 were highly variable, ranging from 15% to 75% of original activity for Ultrase MT 20 and from 16% to 69% of original activity for Pancrease MT 20.
Table 5.
Released Lipase Activity after Incubation of Products for 60 Minutes at pH 5.0 and for 15 and 30 minutes at pH 6.0
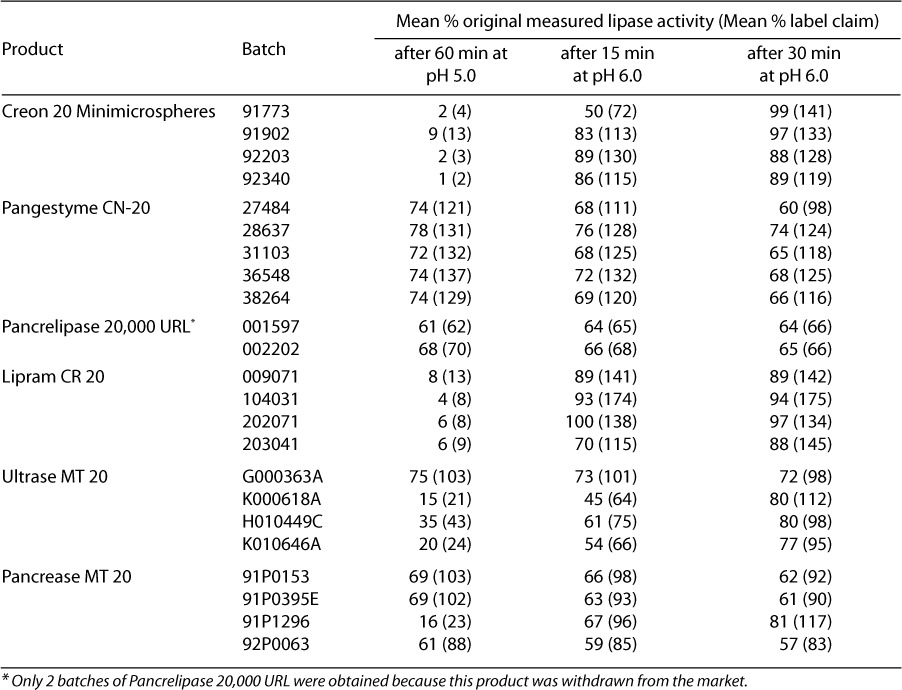
Figure 2.
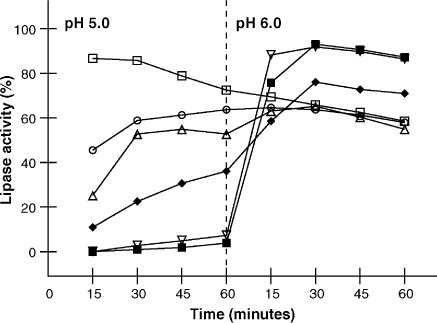
Kinetics of enzyme release for Pangestyme CN-20 (□), Pancrelipase 20,000 URL (○), Lipram CR 20 (∇), Creon 20 Minimicrospheres (▪), Pancrease MT 20 (▵), and Ultrase MT 20 (♦). Released lipase activity is expressed as percentage of actual measured lipase activity at baseline. Fresh product samples were agitated in a disintegration apparatus designed to simulate intestinal peristalsis for 60 minutes at 37°C at pH 5.0. Then the pH was adjusted to 6.0 and the test was continued for an additional 60 minutes. Samples were withdrawn every 15 minutes for the determination of the activity of the lipase released from the enteric coating at each time point. Plotted values are the means for all batches tested for each product (n = 4, except n = 5 for Pangestyme CN-20 and n = 2 for Pancrelipase 20,000 URL; Pancrelipase 20,000 URL was withdrawn from the market).
After 30 minutes at pH 6.0, the samples from all of the batches of Creon 20 Minimicrospheres and Lipram CR 20 as well as those from 2 batches of Ultrase MT 20 and 1 batch of Pancrease MT 20 showed a release of enzymes to 80% or more of the initially assessed activity levels. Enzyme release kinetics of Lipram CR 20 and Creon 20 Minimicrospheres were almost identical, with release activity values generally increasing from pH 5.0 to 6.0. In contrast, Pangestyme CN-20, Pancrelipase 20,000 URL, and 3 batches of Pancrease MT 20 showed a continuous decrease in released lipase activity after 60 minutes at pH 5.0.
DISCUSSION
The results of this in vitro study support and extend previous findings of variability among pancreatic enzyme products used for the treatment of exocrine pancreatic insufficiency.2,8–11,34–37 In contrast with the materials in some enzyme studies,2,9,11,37 the medications involved here all claimed to be pharmaceutically equivalent (at least with respect to lipase activity) enteric-coated microencapsulated products. While other studies also demonstrated that such proven bioavailability-affecting parameters as actual enzyme content, acid resistance, and dissolution characteristics varied between brands or between “branded” and “generic” products, our research further reveals inconsistencies among the separate batches of particular products, giving the clinician one more potential explanation when shifts in patient response to therapy are observed.
Patients and physicians typically arrive at an optimal regimen based on a series of trials. In a December 2000 letter to the FDA, Robert C. Stern, MD, explained that although some patients do well on different brands, for others one brand is “noticeably more effective” than others and that once the optimal brand has been discovered, patients typically remain stable on that product “for many years.” He observed that “generic” substitution has greatly complicated the differential diagnosis of patients who complain that their enzymes “don't work.”26 Substitution may lead to inadvertent overdosing or underdosing due to variation in the extent of overfilling for the product substituted after the first product has been successfully titrated or due to dosage escalation based on the perceived need for more lipolytic activity following substitution of a product that is less protective against gastric acid and/or less efficient in releasing enzymes in the duodenum at the right time. The possibility of inadvertent overdosing or misadvised dosage escalation is of concern given the association noted previously by some researchers between long-term ingestion of excessive doses of microencapsulated pancreatic enzyme products and development of fibrosing colonopathy in CF patients,19–23,28,38–41 possibly attributable to the excess enzyme and/or to a particular enteric-coating excipient.
O'Hare and colleagues studied labeled versus actual lipase content in 6 pancreatic enzyme supplements from 3 pharmaceutical companies (Cilag, Duphar, and Merck) and found that the activity of all the preparations exceeded label claims, in some cases by more than 100%.36 They concluded that “clinicians need to be aware of the extent of possible intrinsic variation between individual prescriptions before attempting to define possible dose/symptom relationships.” In a study published in 1994, Kraisinger and colleagues reported in vitro activity of 16 pancreatic enzyme products marketed in the United States at that time.2 Only 1 of the 16 was underfilled (Enzymase-16 [Econolab, Westland, Michigan], a “generic” for Pancrease MT 16); the maximum degree of overfilling was 150% (Pancrease MT 16).
An Australian study compared in vitro assessment of enzyme activity as well as in vivo performance for 3 pancreatic enzyme products (3 batches of Pancrease [Janssen-Cilag], 4 batches of Cotazym Forte [Organon], and 1 batch of Promylin HL 16 [US CFF/Pharmaceutical Delivery Systems, Creve Coeur, Missouri]).37 The randomized crossover trial (N = 9) did not reveal any statistically significant differences in efficacy among the 3 products, and the authors concluded that they were therapeutically equivalent. In vitro testing revealed variation in enzyme activity that correlated with time since manufacture, leading the authors to suggest that clinicians might monitor nutrient absorption when patients receive products from different batch numbers or enzymes that have been stored for prolonged periods.
While enzymes can be expected to lose potency over time, review of our data suggests that factors other than simply the proximity of the expiration date may be responsible for the variation between products and between batches in terms of actual contents (Table 2). For each of the products tested, the batch with the highest actual potency (as a percentage of the label claim) was not the batch with the latest expiration date, and the instances of greatest overfilling were found in batches with earlier expiration dates.
It is important that the enteric coating be able to withstand strong acidity because gastric acid secretion is increased for some patients with pancreatic insufficiency.42,43 The tendency of “generic” preparations to have ineffective enteric coatings, along with the consequences for young CF patients, was brought to our attention in publications by Hendeles in the early 1990s.8,18 The values for 2 of the “generic” products in the current study, Pangestyme CN-20 and Pancrelipase 20,000 URL (Table 4), suggest that patients receiving these drugs might suffer similar treatment failures. In speculating about the implications of our findings, it is important to bear in mind that our study included a separate stage for analysis of the effects of simulated gastric acid on the enteric coating. In contrast, the assay described by the USP for effectiveness of the enteric coating employs the same sample for the gastric acid simulation and the test of simulated duodenal enzyme release from the coating.4 In order that we might independently assess acid resistance and dissolution and facilitate comparison of release kinetics, our simulated duodenal dissolution condition used enzymes that had not been exposed to acid. Thus, the values in the 65% range for lipase activity for Pancrelipase 20,000 URL released during simulated duodenal conditions (Table 5) should be interpreted in the context of the 1% values for residual lipase activity after exposure to simulated gastric acid (Table 4). In clinical practice, the availability of enzymes for duodenal release might be severely reduced in a product so susceptible to gastric degradation. (In fact, only 2 batches of Pancrelipase 20,000 URL were obtained for our study because this product was withdrawn from the market.)
The case of Pangestyme CN-20 illustrates the potential for exposure to excessive doses of pancreatic enzymes based on unappreciated batch-to-batch inconsistency of the enteric coating. After exposure to simulated gastric acid, the residual lipase activity in the 5 batches of Pangestyme ranged from 9% to 75% of the original measured lipase activity (Table 4)—more than an 8-fold difference in terms of the enzyme that would be available to a patient in the duodenum. For a patient receiving Pangestyme from batch 31103 with only 9% post-gastric-acid residual lipase activity, a clinician might respond to the poor therapeutic effect of the drug by increasing the dose. The adjusted dose would be excessive if the patient then started receiving Pangestyme from a batch with far greater post-gastric-acid residual lipase activity (such as batch 36548). The great variation between the batches in terms of the effectiveness of the enteric coating would not be known by the physician, the pharmacist, or the patient.
The simulated duodenal study was also distinct from the assay described by the USP for evaluation of effectiveness of the enteric coating4 in that we tested the activity of released enzymes from the simulated duodenal medium not just at one time point (after 30 minutes at pH 6.0 as in the USP assay) but at 15-minute intervals throughout 120 minutes of incubation —60 minutes at pH 5.0 and 60 minutes at pH 6.0. This protocol allowed us to chart the kinetics of enzyme release (Table 5, Figure 2).
The bioactivity of enteric-coated microencapsulated enzyme products in the duodenum is determined by patient variables such as gastric and duodenal pH as well as by factors related to the galenical formulation of the products. These factors include the average particle size, the range of particle size, and the composition of the enteric coating, which must protect the enzymes from degradation in the stomach and then dissolve rapidly when the pH is adequate for digestion in the duodenum. Because absorption of fat is far slower than lipolysis,44 it is important that the enzymes be active as early as possible in the transit of food through the small intestine; if lipolysis is substantially shifted distally, fat absorption will be diminished.44 However, patients with pancreatic insufficiency, particularly those with CF, tend to have more difficulty than others in maintaining a relatively alkaline pH in the duodenum.5,41,45,46 Empiric testing of various exogenous preparations may be necessary to find the one with the dissolution profile that works best for the intestinal pH profile of the particular patient. But based on (1) the concern that dissolution of the coating near or below pH 5.0 could be followed by a drop in pH and hence lipase destruction and (2) the consideration that digestive capacity increases as the release of lipase occurs closer to the optimal pH 8.0,5,47 it seems reasonable to target pH 6.0 (the pH of the USP assay) as an ideal point of dissolution for the enteric coating. In our study, Creon 20 Minimicrospheres and Lipram CR 20 most closely approximated these generally desirable kinetics. After 30 minutes at pH 6.0, the samples from all of the batches of Creon 20 Minimicrospheres and Lipram CR 20 showed a release of enzymes that amounted to 88% or more of the initially assessed activity.
Of possible relevance for bioequivalence is the distinction between microtablets (all microencapsulated granules are the same size) and microspheres (the microencapsulated granules are of heterogeneous sizes) (Table 3).41 As noted, 2 of the microsphere products we tested (the “branded” Creon 20 Minimicrospheres and the “generic” Lipram) exhibited a different kinetics of enzyme release in simulated duodenal conditions compared to the 2 “branded” products consisting of microtablets (Pancrease and Ultrase) (Table 5, Figure 2).
The Orange Book criteria for “therapeutic equivalence” include “pharmaceutical equivalence” and “bioequivalence.” “Generic” pancreatic enzyme products may well be labeled as pharmaceutical equivalents: that is, with respect to the “innovator” drugs for which they are substituted, their labels may state that they contain the same active ingredient(s), that they are of the same dosage form, that their route of administration is the same, and that they are identical in strength or concentration.25 Indeed, labeled pharmaceutical equivalence is apparently the sole criterion upon which marketed databases “link” or cross reference “branded” drug products with “generic” drug products. But even among the “innovator” or “branded” drugs, bioequivalence has not been demonstrated. For drug products such as exogenous enzymes that are not intended to be absorbed into the bloodstream, bioequivalence cannot be demonstrated by the kind of area-under-the-curve (AUC) measurements of blood levels over time that pharmacists are accustomed to assessing. For such drugs, the FDA states that bioequivalence may sometimes be demonstrated by in vitro studies and sometimes through comparative clinical trials or pharmacodynamic studies.25 In vitro performance of exogenous pancreatic enzymes has been shown to correlate with in vivo efficacy in the treatment of steatorrhea.48 In the notice of April 28, 2004, on exocrine pancreatic insufficiency drug products, the FDA advised that given the significant differences among marketed pancreatic enzyme products, standardization of enzyme bioactivity is needed. At the same time, the FDA expressed the view that these products are “currently not likely to be appropriate subjects for ANDAs.” For an ANDA to be approved, it would have to be demonstrated that the drug in question contains the same active ingredient(s) as an approved reference listed drug; according to the FDA, “it is unlikely that currently available physiochemical and biological analytical tools would be able to demonstrate that the active ingredients in pancreatic extract products from 2 different manufacturers are the same.”12
Our in vitro study clearly demonstrates variability among representative pancreatic enzyme products labeled as pharmaceutically equivalent—in terms of off-the-shelf potency, lot-to-lot consistency, resistance of the enteric coatings to simulated gastric acid, and simulated duodenal lipase release. The variability was noted not only between “generic” products but also among the “branded” products. These findings may explain the serious variation that has been reported in clinical outcomes (for CF and other patients) with these formulations while underscoring the need to be consistent with product delivery. Until 2008, when all pancreatic exocrine insufficiency products will be required to have an approved NDA (or ANDA) from the FDA, unregulated substitution of inequivalent drugs for those prescribed will remain a possibility. There are no approved pancreatic insufficiency drugs in the United States; there are no approved therapeutic equivalents, and formulary claims regarding the acceptability of substitutes (whether “branded” or “generic”) are misleading. Despite label claims that the same enzymatic activities are present, it cannot be assumed that patients will obtain equivalent therapeutic effect from different pancreatic enzyme products. To maintain consistency of therapeutic response, these products should not be used interchangeably, except under the informed direction of the prescribing physician and with close monitoring. It is crucial that physicians and patients be able to rely on the quality of these products, since enzyme replacement therapy, in combination with aggressive attention to nutrition, must provide critical support for digestion and improved outcomes for CF and other patients.
ACKNOWLEDGMENTS
Study support provided by Solvay Pharmaceuticals.
ABBREVIATIONS
- ANDA
abbreviated new drug applications
- CF
cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- FDA
Food and Drug Administration
- FIP
Fédération Internationale Pharmaceutique
- NDA
new drug application
- OTC
over-the-counter
- PhEur
European Pharmacopoeia
- USP
United States Pharmacopeia
Footnotes
DISCLOSURE Robert J. Kuhn, PharmD, has previously received support in the form of honoraria from Solvay Pharmaceuticals and Axcan Pharma.
REFERENCES
- 1.Layer P, Keller J. Pancreatic enzymes: Secretion and luminal nutrient digestion in health and disease. J Clin Gastroenterol. 1999;28:3–10. doi: 10.1097/00004836-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kraisinger M, Hochhaus G, Stecenko A. Clinical pharmacology of pancreatic enzymes in patients with cystic fibrosis and in vitro performance of microencapsulated enzyme preparations. J Clin Pharmacol. 1994;34:158–166. doi: 10.1002/j.1552-4604.1994.tb03981.x. et al. [DOI] [PubMed] [Google Scholar]
- 3.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax. 2002;57:596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The United States Pharmacopeia, 30th revision, and the National Formulary. 25th edition. Rockville, Md: United States Pharmacopeial Convention; 2007. pp. 2850–2854. [Google Scholar]
- 5.Di Magno EP, Malegalada JR, Go VLW, Moertel CG. Fate of orally ingested enzymes in pancreatic insufficiency: Comparison of two dosage schedules. N Engl J Med. 1977;297:854–858. doi: 10.1056/NEJM197706092962304. [DOI] [PubMed] [Google Scholar]
- 6.Meyer JH, Elashoff J, Porter-Fink V. Human postprandial emptying of 1–3-millimeter spheres. Gastroenterology. 1988;94:1315–1325. doi: 10.1016/0016-5085(88)90669-5. et al. [DOI] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Foundation. Why pancreatic enzyme preparations are not interchangeable in the treatment of pancreatic insufficiency of cystic fibrosis (white paper) Bethesda, Md: Cystic Fibrosis Foundation; Accessed online in PDF format at http://www.cff.org/legislative_action/special_topics/ on February 23, 2006. [Google Scholar]
- 8.Hendeles L, Dorf A, Stecenko A, Weinberger M. Treatment failure after substitution of generic pancrelipase capsules. JAMA. 1990;263:2459–2461. [PubMed] [Google Scholar]
- 9.Whitehead AM. Study to compare the enzyme activity, acid resistance and dissolution characteristics of currently available pancreatic enzyme preparations. Pharm Weekbl Sci. 1988;10:12–16. doi: 10.1007/BF01966429. [DOI] [PubMed] [Google Scholar]
- 10.Littlewood JM, Kelleher J, Walters MP, Johnson AW. In vivo and in vitro studies of microsphere pancreatic supplements. J Pediatr Gastroenterol Nutr. 1988;7(suppl 1):S22–S29. doi: 10.1097/00005176-198811001-00006. [DOI] [PubMed] [Google Scholar]
- 11.Walters MP, Littlewood JM. Pancreatin preparations used in the treatment of cystic fibrosis—lipase content and in vitro release. Aliment Pharmacol Ther. 1996;10:433–440. doi: 10.1111/j.0953-0673.1996.00433.x. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Exocrine pancreatic insufficiency drug products (notice) Federal Register. 2004;69(82):23410–23414. [Google Scholar]
- 13.DeYoung JL. Development of pancreatic enzyme microsphere technology and US findings with Pancrease in the treatment of chronic pancreatitis. Int J Pancreatol. 1989;5(suppl):31–36. [PubMed] [Google Scholar]
- 14.Brady MS, Rickard K, Yu PL, Eigen H. Effectiveness and safety of small vs large doses of enteric coated pancreatic enzymes in reducing steatorrhea in children with cystic fibrosis: A prospective randomized study. Pediatr Pulmonol. 1991;10:79–85. doi: 10.1002/ppul.1950100208. [DOI] [PubMed] [Google Scholar]
- 15.Halm U, Loser C, Lohr M. A double-blind, randomized multicentre, crossover study to prove equivalence of pancreatin minimicrospheres versus microspheres in exocrine pancreatic insufficiency. Aliment Pharmacol Ther. 1999;13:951–957. doi: 10.1046/j.1365-2036.1999.00566.x. et al. [DOI] [PubMed] [Google Scholar]
- 16.Santini B, Antonelli M, Battistini A. Comparison of two enteric-coated microsphere preparations in the treatment of pancreatic exocrine insufficiency caused by cystic fibrosis. Dig Liver Dis. 2000;32:406–411. doi: 10.1016/s1590-8658(00)80261-3. et al. [DOI] [PubMed] [Google Scholar]
- 17.Stern RC, Eisenberg JD, Wagener JS. A comparison of the efficacy and tolerance of pancrelipase and placebo in the treatment of steatorrhea in cystic fibrosis patients with clinical exocrine pancreatic insufficiency. Am J Gastroenterol. 2000;95:1932–1938. doi: 10.1111/j.1572-0241.2000.02244.x. et al. [DOI] [PubMed] [Google Scholar]
- 18.Hendeles L, Hochhaus G, Kazerounian S. Generic and alternative brand-name pharmaceutical equivalents: Select with caution. Am J Hosp Pharm. 1993;50:323–329. [PubMed] [Google Scholar]
- 19.Freiman JP, FitzSimmons SC. Colonic strictures in patients with cystic fibrosis: results of a survey of 114 cystic fibrosis care centers in the United States. J Pediatr Gastroenterol Nutr. 1996;22:153–156. doi: 10.1097/00005176-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Smyth RL, van Velzen D, Smyth AR. Strictures of ascending colon in cystic fibrosis and high-strength pancreatic enzymes. Lancet. 1994;343(8889):85–86. doi: 10.1016/s0140-6736(94)90817-6. et al. [DOI] [PubMed] [Google Scholar]
- 21.Oades PJ, Bush A, Ong PS, Brereton J. High-strength pancreatic enzyme supplements and large-bowel stricture in cystic fibrosis [letter] Lancet. 1994;343(8889):109. [PubMed] [Google Scholar]
- 22.Campbell CA, Forrest J, Musgrove C. High-strength pancreatic enzyme supplements and large-bowel stricture in cystic fibrosis [letter] Lancet. 1994;343(8889):109–110. [PubMed] [Google Scholar]
- 23.Taylor CJ, Dodge JA. High-strength pancreatic enzyme supplements and large-bowel stricture in cystic fibrosis [letter] Lancet. 1994;343(8889):110. [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Exocrine pancreatic insufficiency drug products for over-the-counter human use (final rule) Federal Register. 1995;60:20162–20165. [Google Scholar]
- 25.US Food and Drug Administration. Approved Drug Products with Therapeutic Equivalence Evaluations. 27th edition. Accessed online at http://www.fda.gov/cder/orange/obannual.pdf on August 9, 2007.
- 26.Letter dated December 17, 2000, from Robert C. Stern, University Hospitals of Cleveland, to Dockets Management Branch, US Food and Drug Administration
- 27.Schibli S, Durie PR, Tullis ED. Proper usage of pancreatic enzymes. Curr Opin Pulm Med. 2002;8:542–546. doi: 10.1097/00063198-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Campbell PW. Illegal generic pancreatic enzymes. White paper dated November 28, 2000; accessed online at http://rx4cf.org/campbell.htm on August 9, 2007.
- 29.Letter dated February 14, 2001, from PW Campbell III, Cystic Fibrosis Foundation, to L. Talarico, US Food and Drug Administration. Accessed online in PDF format at http://www.fda.gov/ohrms/dockets/dailys/04/may04/050704/03n-0205-bkg0001-toc.htm on February 23, 2006.
- 30.Letter dated June 24, 2003, from Henry A. Waxman, United States House of Representatives, to Tommy G. Thompson, Secretary of Health and Human Services
- 31.Take the time to check your enzymes (brochure) Bethesda, Md: Cystic Fibrosis Foundation; 2002. [Google Scholar]
- 32.Letter dated July 30, 2003, from Robert J. Beall, Cystic Fibrosis Foundation, to Mark McClellan, US Food and Drug Administration. Accessed online in PDF format at http://www.cff.org/legislative_action/special_topics/ on February 23, 2006.
- 33.Sweetman S, editor. Martindale: the complete drug reference. 30th ed. London: Pharmaceutical Press; 1999. [Google Scholar]
- 34.Fatmi AA, Johnson JA. An in vitro comparative evaluation of pancreatic enzyme preparations. Drug Development and Industrial Pharmacy. 1988;14:1429–1438. [Google Scholar]
- 35.Beverley DW, Kelleher J, MacDonald A. Comparison of four pancreatic extracts in cystic fibrosis. Arch Dis Child. 1987;62:564–568. doi: 10.1136/adc.62.6.564. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Hare MM, McMaster C, Dodge JA. Stated versus actual lipase activity in pancreatic enzyme supplements: implications for clinical use. J Pediatr Gastroenterol Nutr. 1995;21:59–63. doi: 10.1097/00005176-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Thomson M, Clague A, Cleghorn GJ, Shepherd RW. Comparative in vitro and in vivo studies of enteric-coated pancrelipase preparations for pancreatic insufficiency. J Pediatr Gastroenterol Nutr. 1993;17:407–413. doi: 10.1097/00005176-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 38.FitzSimmons SC, Burkhart GA, Borowitz D. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283–1289. doi: 10.1056/NEJM199705013361803. et al. [DOI] [PubMed] [Google Scholar]
- 39.Smyth RL, Ashby D, O'Hea U. Fibrosing colonopathy in cystic fibrosis: results of a case-control study. Lancet. 1995;346(8985):1247–1251. doi: 10.1016/s0140-6736(95)91860-4. et al. [DOI] [PubMed] [Google Scholar]
- 40.Stevens JC, Maguiness KM, Hollingsworth J. Pancreatic enzyme supplementation in cystic fibrosis patients before and after fibrosing colonopathy. J Pediatr Gastroenterol Nutr. 1998;26:80–84. doi: 10.1097/00005176-199801000-00014. et al. [DOI] [PubMed] [Google Scholar]
- 41.Borowitz DS, Grand RJ, Durie PR. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. J Pediatrics. 1995;127:681–684. doi: 10.1016/s0022-3476(95)70153-2. [DOI] [PubMed] [Google Scholar]
- 42.Bruno MJ, Haverkort EB, Tytgat GNJ, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: Implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol. 1995;90:1383–1393. [PubMed] [Google Scholar]
- 43.Cox KL, Isenberg JN, Ament ME. Gastric acid hypersecretion in cystic fibrosis. J Pediatr Gastroenterol. 1982;1:559–565. doi: 10.1097/00005176-198212000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Meyer JH, Lake R, Elashoff JD. Postcibal gastric emptying of pancreatin pellets. Dig Dis Sci. 2001;40:1846–1852. doi: 10.1023/a:1010666510755. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn RJ, Horn L. Pancreatic enzyme therapy in patients with cystic fibrosis: The high dose lipase issue. Pediatr Nurs. 1994;20:623–624. [PubMed] [Google Scholar]
- 46.Robinson PJ, Smith AL, Sly PD. Duodenal pH in cystic fibrosis and its relationship to fat malabsorption. Dig Dis Sci. 1990;35:1299–1304. doi: 10.1007/BF01536423. [DOI] [PubMed] [Google Scholar]
- 47.Dressman JB, Shtohryn LV, Diokno D. Effects of product formulation on in vitro activity of pancreatic enzymes. Am J Hosp Pharm. 1985;42:2502–2506. [PubMed] [Google Scholar]
- 48.Graham DY. Enzyme replacement therapy of exocrine pancreatic insufficiency in man. N Engl J Med. 1977;296:1314–1317. doi: 10.1056/NEJM197706092962303. [DOI] [PubMed] [Google Scholar]


