Abstract
Cyclooxygenase inhibitors have proven efficacy in the treatment of patent ductus arteriosus (PDA). Intravenous indomethacin has been the only approved treatment for PDA available in the United States for the past 20 years. The armamentarium has recently been expanded with the approval of intravenous ibuprofen lysine in 2006. Ibuprofen lysine has been used for years in Europe, and the author reviews the extensive published literature. This review addresses common questions about ibuprofen lysine, summarizes the available literature, and discusses the data submitted to the Food and Drug Administration (FDA) in support of its approval. Three major trials served as the approval basis for the safety and efficacy of ibuprofen lysine. The author has summarized these studies and, where appropriate, presents pooled results from additional analyses that have not been previously published. Many practical questions regarding the drug, including dosing, administration, and storage are addressed. The results of recently completed but unpublished tests on stability and compatibility with commonly used drugs in the neonatal setting are also reviewed. Ibuprofen lysine now represents an alternative pharmacological option to surgery for the treatment of PDA.
Keywords: intravenous ibuprofen lysine, nonsteroidal anti-inflammatory drug, patent ductus arteriosus
INTRODUCTION
Patent ductus arteriosus (PDA) is a condition seen in premature infants with an incidence as high as 60% in infants of less than 28 weeks gestational age (GA).1 Approximately 45% of neonates weighing less than 1,750 g and nearly 80% of those weighing less than 1,000 g present with a PDA.2 Spontaneous closure can occur in up to 40% of premature neonates, but up to 70% of those weighing less than 1,000 g or of less than 28 weeks gestational age require intervention due to significant hemodynamic symptoms.3
Cyclooxygenase inhibitors have become the mainstay of pharmacologic therapy for a PDA because normal closure of the ductus is primarily mediated by homeostatic down-regulation of prostaglandins. Intravenous (IV) indomethacin (Indocin IV, Ovation Pharmaceuticals, Deerfield, IL) has been the sole therapy approved by the Food and Drug Administration (FDA) for the treatment of PDA during the last 20 years. IV ibuprofen, which has been used in various forms in other countries, was first demonstrated to be effective for ductal closure in animals in 1979.4 Since that time, ibuprofen lysine has been well studied in humans and was approved for use in the United States (US) in 2006 (NeoProfen, Ovation Pharmaceuticals, Deerfield, IL). It is currently FDA-approved for the closure of a clinically significant PDA in premature infants who weigh between 500 g and 1,500 g and who are no more than 32 weeks GA, when usual medical management has been ineffective.5
The following summary reviews the most commonly asked questions about ibuprofen lysine in the management of PDA. With a focus on clinical and practical applications, this summary is meant to help health care practitioners better understand the place of ibuprofen lysine in the armamentarium of treatment strategies for PDA.
Question 1. What clinical studies were submitted to gain FDA approval of ibuprofen lysine?
IV ibuprofen lysine was approved under the Orphan Drug Act in April 2006. The sponsor submitted results of 3 controlled clinical trials in support of efficacy and safety. Two of the trials were reanalyses of completed trials from Europe.6,7 One was a randomized, prospective, active-controlled multicenter PDA treatment trial.6 and the second a double-blind, randomized, placebo-controlled PDA prophylaxis trial.7 The data from these 2 trials were fully adjudicated and validated before submission to the FDA.
In order to estimate a placebo or no-treatment effect, the active-controlled trial submission included a post-hoc group of infants who were not treated.6 This trial was also re-examined for the primary outcome of percentage of patients requiring rescue so that its results could be pooled with a third pivotal clinical trial.1,8
The pivotal trial, a multicenter, randomized, double-blind, placebo-controlled study, served as the primary data source for the safety and efficacy evaluation of ibuprofen lysine. This study enrolled 136 infants of less than 30 weeks GA, with birth weights between 500 g and 1000 g.1,8 Infants were randomized to receive either a 3-treatment course of IV ibuprofen lysine or placebo. This study was designed to determine whether early treatment with ibuprofen lysine would reduce the need for rescue therapy (i.e., indomethacin or surgery) in infants at risk for a symptomatic PDA. Given that it would be unethical to perform a placebo-controlled study including infants with symptomatic PDA, asymptomatic premature infants with echocardiographically-documented ductal shunting were randomly assigned to placebo or ibuprofen lysine and followed for the need to treat (rescue) a symptomatic PDA. In these 3 trials a total of 357 infants received ibuprofen lysine, 349 received placebo or no treatment, and 73 received indomethacin.
Question 2. How did the populations from the major ibuprofen lysine studies compare?
In each of the trials, there was no difference in demographic or baseline characteristics within the study groups. The ibuprofen-treated infants in the pivotal trial had a significantly younger GA (26.1 weeks) vs. those in the active-controlled study (29.1 weeks, P < .001) and vs. those in the prevention study (28.1 weeks, P < .001).6,7,8 Birth weights for the ibuprofen-treated infants were also significantly lower in the pivotal trial (798.5 g) vs. the active-controlled study (1,230 g, P < .001) and vs. the prevention study (1,048 g, P < .001). When the 2 treatment groups were pooled, there were similar percentages of males in the placebo/no-treatment group and the ibuprofen lysine group (56% vs. 52%).7,8
Question 3. How did the efficacy compare among the major ibuprofen PDA trials?
In the pivotal trial, ibuprofen lysine was significantly more effective than placebo for the treatment of PDA. A statistically significantly lower proportion of infants treated with ibuprofen lysine required rescue, died, or dropped out vs. those receiving placebo (30.9% vs. 53%, P = .005).8,9 Similar results were obtained in the re-analysis of the active-controlled treatment study in the ibuprofen-treated vs. the no-treatment group (34% vs. 50%, P = .01).6,9 When these results are pooled, a significantly lower proportion of infants who received ibuprofen lysine required rescue treatment, died, or dropped out of the study prior to study day 14 compared to those who received placebo or no treatment (32.4% vs. 51.9%, P < .01).6,8,9
No placebo-controlled trials with ibuprofen for the treatment of PDA have been published; however, the pooled rescue rate from the 2 treatment trials reviewed above is similar (P = .13) to the percentage of infants (25%) receiving a second course of ibuprofen treatment (surrogate for rescue) in 2 separately published active-controlled treatment trials.10,11
Question 4. Were there any differences in response based on age or weight in these trials?
In regression analyses performed in the pivotal trial, no correlations were found between GA or weight and rescue rate. Therefore, ibuprofen lysine can be said to be effective in all age and weight groups studied. Furthermore, pivotal trial results indicate that in patients less than 28 weeks GA and weighing less than 750 g, significantly more infants required rescue with placebo (11/22) than with ibuprofen lysine (5/22, P = .045).8,9 There were no statistically significant differences (P > .05) in rescue rates between those who started ibuprofen lysine at less than 24 hours of age (21%), 24 to 48 hours of age (28%), or greater than 48 hours of age (23%).
Table 1 provides further detail of the pooled data from the 2 treatment trials by GA categories and rescue rates.6,8,9 This analysis demonstrates comparable efficacy across all age groups studied. Table 2 provides detail on specific weight categories and rescue rates as observed in the pooled data from the 2 treatment trials.6,8,9 The overall rescue rate was significantly lower with ibuprofen (and indomethacin) vs. placebo, and most individual weight categories showed significantly lower rescue rates with ibuprofen as well (individual probability values are listed in Table 2). There were too few patients in the lower (400–700g) and higher (> 1,600 g) weight ranges for meaningful analyses.
Table 1.
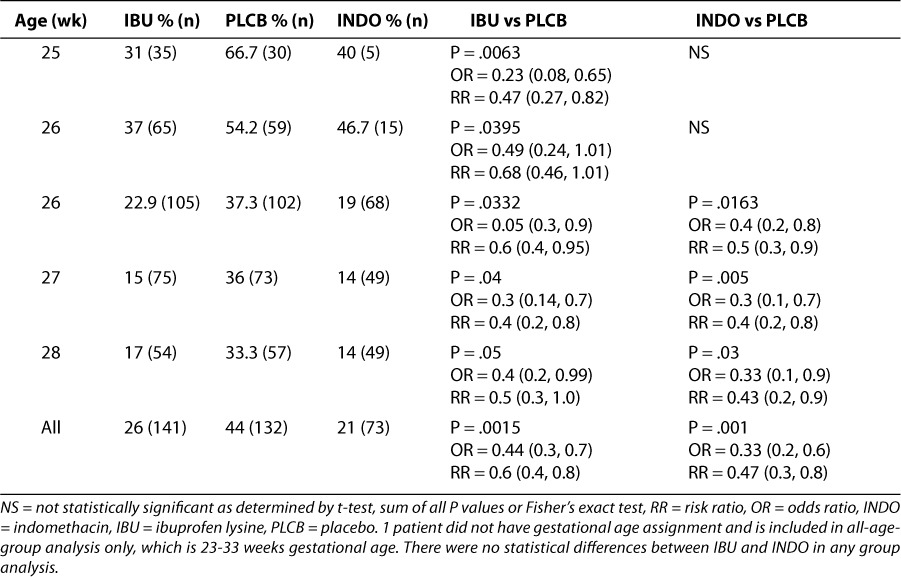
Table 2.
PDA rescue rate by birth weight in pooled treatment trials6,8,9
Question 5. Is ibuprofen lysine effective for the prevention of a PDA?
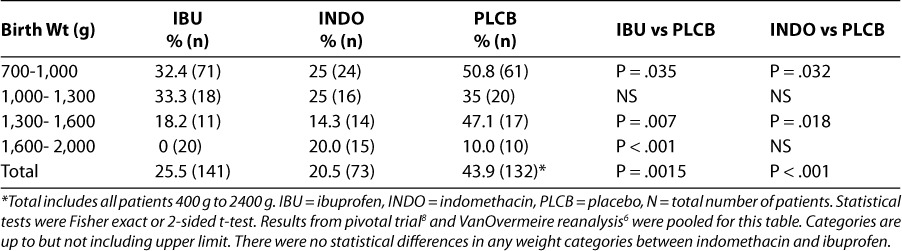
Five controlled and 4 uncontrolled published trials have investigated IV ibuprofen for the prevention of PDA.7,12–19 The 5 controlled studies were analyzed in an attempt to calculate an early treatment benefit (Table 3). The pooled results from these studies indicate a statistically significant favorable difference in closure of PDA at day 3 (84% vs. 57%, P < .0001), need for rescue treatment (7% vs. 33%, P < .0001), and need for surgery (1.6% vs. 5.3%, P = .008) with ibuprofen lysine compared to placebo or control group. Based on this analysis, approximately 30% of the untreated group would be expected to benefit from prophylactic therapy with ibuprofen, where benefit is defined as a closed PDA by day 3 or lack of need for rescue therapy or surgery (Table 3).
Table 3.
Prophylactic benefit of ibuprofen in PDA7,12–15
A Cochrane meta-analysis evaluated ibuprofen for the prevention of PDA in 2003 and found a closure rate at day 3 of 84% for ibuprofen and 55% for placebo.20 An update of this analysis published in 2006 concluded there was a statistically significant decrease in the incidence of PDA on day 3 in the ibuprofen group (P < .00001, RR = 0.37, NNT = 3), in the need for rescue treatment with cyclooxygenase inhibitors (P < .00001, RR = 0.17, NNT = 4), and in the need for surgical ligation (P = .02, RR = 0.34, NNT = 25).21 The review authors found that in comparison to placebo or no treatment there was a significant increase in serum creatinine levels on day 3 in the ibuprofen-treated patients, with no differences in mortality, intraventricular hemorrhage, chronic lung disease, necrotizing enterocolitis, or gastrointestinal hemorrhage or perforation. The serum creatinine rise occurred in only 1 study,13 which was stopped prematurely because of hypoxemia reported in 3 of 65 infants who received ibuprofen. The formulation used in this study (ibuprofen trishydroxyamino-methane or THAM [Pedea, Orphan Europe S.a.r.l., Paris, France]) is different from the formulation available in the US (ibuprofen lysine). The Cochrane meta-analysis authors concluded that ibuprofen reduces the incidence of PDA, the need for rescue therapy, and surgical closure; however, with the high spontaneous rate of closure (60%) in the target infant population, they did not recommend exposing a large proportion of infants to prophylactic therapy.21
Question 6. Can ibuprofen lysine be used for intraventricular hemorrhage (IVH) prevention?
Previous studies and systematic reviews have confirmed that another non steroidal anti-inflammatory drug (NSAID), indomethacin, when used prophylactically for PDA, can reduce the incidence of IVH.22,23 The long-term sequelae on neurodevelopment, however, have not been shown to be negatively or positively affected.23 Ohlsson has argued that the positive effects of indomethacin on short-term reduction of IVH may be outweighed by its longer-term effects on reducing cerebral blood flow.24
Ibuprofen has been shown to have neuroprotective effects in animal models and to enhance cerebral autoregulation without affecting cerebral blood flow, cerebral metabolism or intestinal or renal hemodynamics.25–29 Mosca et al. have demonstrated in premature infants that, unlike indomethacin, ibuprofen widens the upper limit of autoregulated cerebral blood flow.17,30
A number of studies have captured the onset incidence of IVH with intravenous cyclooxygenase inhibitor therapy; however, most of these trials were not designed specifically to study IVH, and many of them excluded infants who had IVH.21,31 In a systematic review of trials comparing indomethacin with ibuprofen, whether for prevention or treatment of a PDA, no differences were noted in the onset incidence of IVH between indomethacin and ibuprofen or between ibuprofen and placebo.21,31
Two randomized, placebo-controlled studies of ibuprofen lysine that used IVH as a primary outcome measure in premature infants with PDA reported no statistically significant difference between the placebo and ibuprofen groups in regard to the incidence of any stage of IVH.7,15 No controlled trial has compared ibuprofen lysine to indomethacin with IVH as the primary outcome variable. In 3 trials in which this was a secondary measure, no difference was seen in the incidence of IVH (11% with ibuprofen vs. 13% with indomethacin, P = .87).6,10,32 Lago et al. noted that the incidence of IVH in both the indomethacin and ibuprofen groups was lower than in the overall population within their unit.10 In a study comparing treatments for PDA, the incidence of progressive IVH was similar in patients randomized to indomethacin and ibuprofen.6 Eight patients in the indomethacin group progressed at least 1 grade category, and 5 patients in the ibuprofen group progressed from grade 0 to 1 or greater (P = .38).6
As has been pointed out by Van Overmeire et al., the efficacy of PDA closure is not correlated with improved IVH outcomes, and other pharmacologic effects of cyclooxygenase inhibitors may play a greater role in more favorable neurodevelopmental outcomes.7 Others have concluded that although ibuprofen has an improved safety profile, especially in regard to renal and gastrointestinal function, the lack of demonstrated efficacy in IVH prophylaxis makes its use in this setting unsubstantiated.1 With no other alternative for IVH prophylaxis, the risks and benefits of IV indomethacin should be considered.1
Question 7. What were the most common side effects seen with ibuprofen lysine in the clinical trials?
The most common side effects reported in the pivotal trial are summarized in Table 4.5,8,9 No statistically significant differences between treatment groups were observed in the incidence of serious adverse events in the pivotal study or when the results were pooled from the 2 treatment studies submitted to the FDA.6,8,9 In addition, no serious adverse events were reported in a substantially different percentage of infants receiving ibuprofen lysine or placebo (i.e., difference of at least 5 percentage points), including outcomes of special interest such as retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), periventricular leukomalacia (PVL), and death. No statistically significant differences between treatment groups were observed for mean cumulative urine output during the studies.
Table 4.
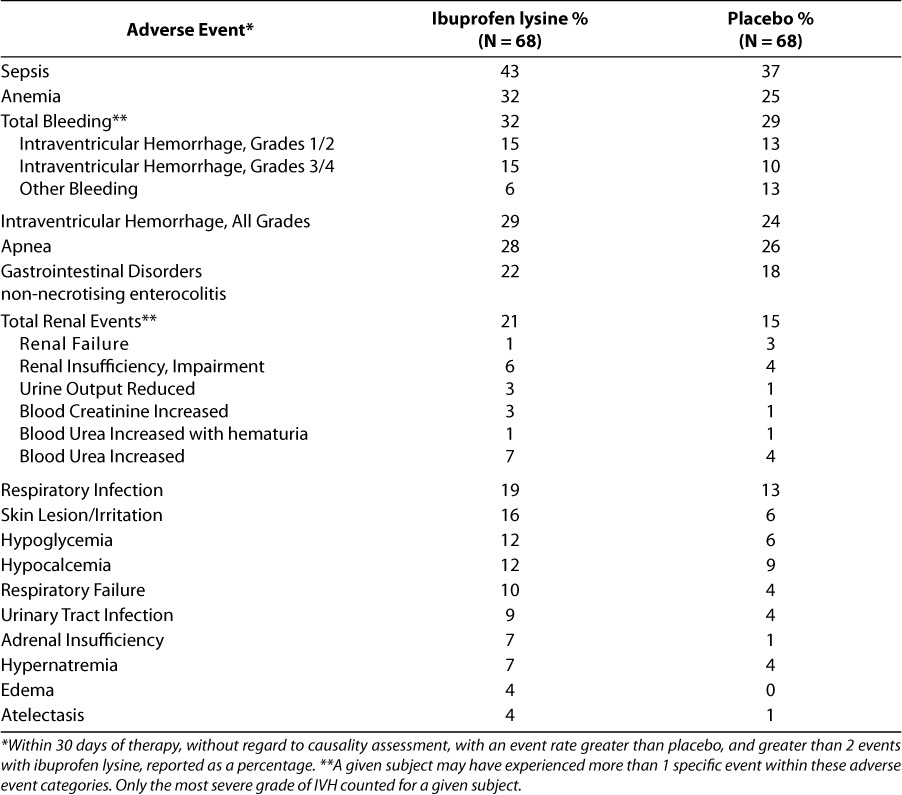
In an analysis of the combined treatment studies, significantly fewer “common significant but not serious treatment emergent adverse effects” were reported in infants receiving ibuprofen than in infants receiving placebo (79% vs. 92%, P ≤ .05).6,8,9 This was primarily driven by a smaller number of cases of “hyperbilirubinemia neonatal,” anemia, sepsis, jaundice, respiratory distress, and necrotizing enterocolitis (NEC) reported with ibuprofen lysine. Hyperbilirubinemia, jaundice, and NEC were individually statistically significantly different from the placebo/no-treatment group (P < .05).6,8,9
In all trials submitted to the FDA, there were no statistically significant differences between treatment groups in renal function, as measured by mean change in blood urea nitrogen (BUN) from baseline to worst value and last value.6,7,8 However, a higher percentage of infants in the ibuprofen lysine group (7.4%) than in the placebo group (4.4 %) had an increase in BUN values from a less severe baseline value, although this was not statistically significant (P = .22).7,8
In the pivotal trial, mean urinary output as measured over days of life was significantly higher in the ibuprofen group than in the placebo group on day 9 of life (P = .019).5,8,9 This probably represented the deleterious action of the rescue medication (indomethacin) within the placebo group because there were no differences noted prior to day 9, and fluid intake and diuretic use were not different within the study groups. There were no differences between placebo and ibuprofen lysine in BUN during any study day. Differences in serum creatinine were noted on study day 3 (1.03 mg/dL vs. 0.98 mg/dL) and study day 4 (1.07 mg/dL vs. 0.94 mg/dL), with slightly higher values noted among the ibuprofen-lysine-treated infants (P = .02 in both). The findings that the ibuprofen group had a slightly higher starting serum creatinine than the placebo group (1.03 vs. 1.01) on the first day of therapy and that values on study days 5 and 6 were not significantly different indicate that these serum creatinine changes are clinically insignificant.5,8,9
Additional support for this conclusion comes from pooling the data from the 2 treatment trials, which indicates that there was no statistically significant difference in the ibuprofen-lysine-treated infants and the placebo group in regard to change from baseline to worst serum creatinine value (0.19 and 0.24, respectively).5,6,8,9 Furthermore, there was a significantly higher serum creatinine change from baseline to last value in the placebo group compared to the ibuprofen group (0.03 versus −0.06, P = .0164).5,6,8,9
In the active-controlled study,6 148 infants with evidence of PDA were randomized to ibuprofen lysine or indomethacin. Of the infants who experienced treatment-emergent serious adverse events, there was a statistically significant difference (P = .018) between the ibuprofen and indomethacin treatment groups favoring ibuprofen, with regard to the highest relationship of study drug to the serious adverse event (possibly related: 16.7% ibuprofen vs. 23.3% indomethacin; probably not related: 11.1% ibuprofen vs. 36.7% indomethacin; not related: 72.2% ibuprofen vs. 40% indomethacin).
In all trials submitted to the FDA, 47 infants (placebo/no treatment 2.3%, ibuprofen lysine 8.9%, and indomethacin 9.58%) experienced a total of 58 adverse events that were considered possibly or probably related to the study drug.6–9 Although these studies were not powered to address this issue and the number of events was small in each group, there was a statistically significant difference in the number of infants reported to have possibly experienced a drug-related adverse event in the treatment group (ibuprofen lysine or indomethacin) vs. the placebo or no-treatment group (P < .01). The most common events reported were IVH (all grades) and NEC/perforation, which represented over 50% of events. However, none of the individual events deemed to be drug related were statistically significantly more frequent in either of the treatment groups vs. the placebo/no-treatment group.6–9
In the PDA prevention study7, 433 infants were randomized to ibuprofen or placebo within 6 hours of birth. Statistically significant differences were reported in the percentage of infants experiencing apnea (ibuprofen less than placebo, P < .05) and hypotension (ibuprofen greater than placebo, P < .05). These events were captured without regard to presumed causality.
Of the events considered possibly or probably related to study drug, 22 infants experienced 29 treatment-emergent serious adverse events.7 The most commonly reported events were IVH and perforation. Perforation occurred more often in the ibuprofen group, although at a low frequency (2.3% vs. 0%, P = .03).7 Reports of IVH were not statistically significantly different (P = .3) between the ibuprofen lysine-treated patients (2.3%) and those receiving placebo (1%).7
In conclusion, the percentage of infants in whom the study drug was discontinued due to adverse events was small (2.5% in both ibuprofen and placebo groups and 4.1% in the indomethacin group) and did not differ between groups.6–9 In pooled data from these 3 trials submitted to the FDA, no individual event classified as possibly or probably drug related was reported statistically more often in the treatment group compared to the placebo/no-treatment group.6–9
Question 8. Are there contraindications to the use of ibuprofen lysine?
Ibuprofen lysine should generally not be used in patients proven or suspected to have infection (in whom it may mask the signs and symptoms); congenital heart disease (such as pulmonary atresia, severe tetralogy of Fallot, or severe coarctation of the aorta), in whom patency of the PDA is necessary for blood flow; thrombocytopenia, coagulation defects or bleeding disorders, including NEC or IVH; or significant renal impairment.5 As noted by others in this supplement, ibuprofen can displace bilirubin from its binding site like other highly protein-bound drugs. Although the plasma ibuprofen levels required to displace bilirubin are not normally achieved under the recommended dosing, ibuprofen lysine should be used with caution in patients with elevated total bilirubin.5 In an analysis of the safety data submitted to the FDA, there was no evidence of significant elevation of total or direct bilirubin in the ibuprofen lysine-treated patients.6–9
Question 9. Are there any significant drug-drug or drug-disease interactions?
There have been no well-controlled reports of drug interactions with ibuprofen lysine in neonates. All non-steroidal anti-inflammatory drugs (NSAIDs) have the potential to increase the gastrointestinal toxicity of steroids. Peltoniemi et al. stopped a trial of hydrocortisone in preterm newborns because of gastrointestinal perforations in the treatment group, which were attributed to combined toxicity of NSAIDs and the steriod.33 Three of the 4 infants in that study who experienced perforations had received NSAIDs, whereas none in the control group had. In a retrospective study of preterm newborns, amikacin's serum half-life was reported to be significantly lengthened and its clearance reduced by ibuprofen lysine.34
In the clinical trials conducted by the sponsor, no significant drug-drug or drug-disease interactions were observed.9 The efficacy of ibuprofen lysine in infants with PDA was demonstrated regardless of maternal steroid use, concomitant medications received by the infant from birth up to and including 7 days into therapy, or maternal medications received within 72 hours of delivery. No significant drug-demographic interactions were observed with ibuprofen lysine. The efficacy of ibuprofen lysine was demonstrated regardless of sex, birth weight, GA, or delivery type (vaginal or cesarean section). Efficacy was demonstrated regardless of maternal complications (spontaneous or nonspontaneous labor), use of high frequency oscillatory ventilation, or significant weight loss.
Ojala et al. have proposed in a case-control study that continuous exposure to heparin for maintaining catheter patency may decrease the efficacy of indomethacin in closing a PDA.35 They have postulated that the vasodilatory effects of heparin, such as increased nitric oxide release, may make the ductus more resistant to cyclooxygenase inhibition.35
Visual drug compatibility and turbidity evaluations with ibuprofen lysine (10 mg/mL) conducted by the sponsor indicate that the following drugs commonly used in preterm infants are likely not compatible with IV ibuprofen lysine: amikacin, caffeine, dobutamine, isoproterenol, midazolam, vancomycin, vecuronium, total parenteral nutrition (TPN) electrolytes, TrophAmine (10% amino acid injection, B. Braun, Bethlehem, PA), and neat morphine 50 mg/mL.9 Drugs that appear compatible with ibuprofen lysine are ceftazidime 200 mg/mL in 5% dextrose in water (D5W) or normal saline (NS), epinephrine 8 mg/mL-0.1 mg/mL in D5W, furosemide 10 mg/mL, heparin lock flush 100 U/mL in D5W, neat heparin lock flush 10 U/mL and 100 U/mL, regular insulin 0.1 U/mL and 1 U/mL in NS, morphine sulfate 0.5 mg/mL in D5w or NS, phenobarbital 130 mg/mL or 30 mg/mL in D5W or NS, potassium chloride 2 mEq/mL, and sodium bicarbonate 1 mEq/mL. Dopamine 3.2 mg/mL in D5W was initially compatible but demonstrated precipitation at 4 hours.9 IntraLipid 10%, which is a milky white opaque solution, remained so during the test, and therefore its compatibility with ibuprofen lysine could not be determined either visually or by turbidity measurement.9
Question 10. What are the optimal dosing, administration, and storage conditions for IV ibuprofen lysine?
Commercially available ibuprofen lysine is provided in packages of 3 clear, sterile, preservative-free, single-use vials, each containing 2 mL of pH-neutral solution. The vial is made of a USP Type 1 borosilicate with ammonium sulfate-treated tubing. The drug vial stoppers, which consist of butyl rubber, are latex free. Each milliliter contains 17.1 mg of ibuprofen l-lysine, which is equivalent to 10 mg/mL of ibuprofen.5
Storage
Prior to administration, ibuprofen lysine should be stored at 20–25°C (68–77°F).5 The drug should be protected from light and stored in the carton until the contents have been used. Natural light exposure has been reported to decrease the concentration of ibuprofen by as much as 7% over 15 days.36
Stability
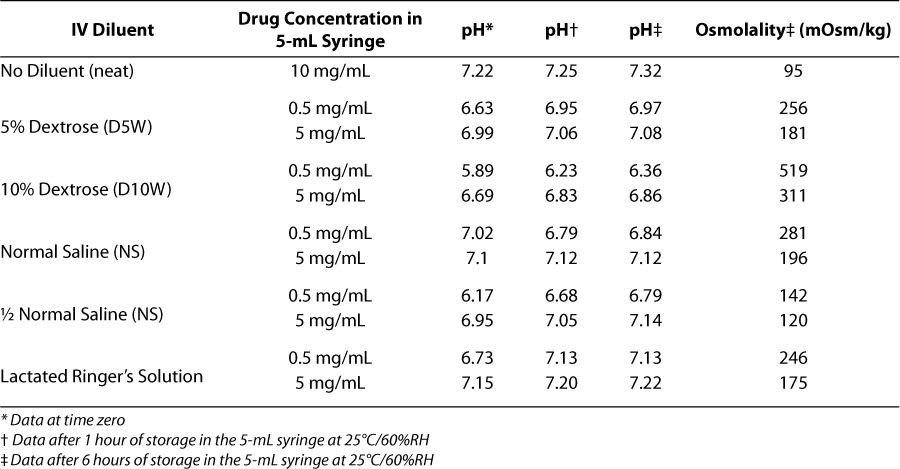
Extemporaneously compounded IV ibuprofen lysine solutions have been found to be stable for up to 15 days when protected from light.36 These studies carried out in Argentina were performed with ibuprofen lysine at different pH and concentrations than the commercially available preparation in the US. Stability studies conducted by the US sponsor indicate that, when it is diluted with commonly used IV fluids, ibuprofen lysine is stable in 60% relative humidity for up to 12 hours when stored in 5-mL syringes at concentrations of 0.5 mg/mL and 5 mg/mL at temperatures of 5°C to 25°C.9 Results from these studies indicate that no significant change in visual appearance, pH, osmolality, or chromatographic purity was observed during the study period. Typical values for pH and osmolality after 1 hour are included in Table 5.9
Table 5.
pH and osmolaltiy data for ibuprofen lysine in diluents9
Administration, Dosing, and Treatment Course
A course of therapy for the treatment of PDA is 3 doses of ibuprofen lysine administered intravenously (administration via an umbilical arterial line has not been evaluated). An initial dose of 10 mg/kg should be followed by 2 doses of 5 mg/kg each after 24 hours and 48 hours.5 Ibuprofen lysine can be diluted for ease of administration. All doses should be based on birth weight. Ibuprofen lysine should be infused continuously over a period of 15 minutes and should be administered via the IV port that is nearest the insertion site. If anuria or marked oliguria (urinary output < 0.6 mL/kg/hr) is evident at the scheduled time of the second or third dose of ibuprofen lysine, no additional dosage should be given until laboratory studies indicate that renal function has returned to normal.5 If during continued medical management the ductus arteriosus fails to close or re-opens, then a second course of ibuprofen lysine, alternative pharmacological therapy, or surgery may be necessary.5
Question 11. In those who do not respond to a first course of therapy, what percentage respond to a second course? Are there differences in response to a second course of therapy with ibuprofen vs. indomethacin?
Three controlled trials comparing ibuprofen with indomethacin for the treatment of PDA have reported response rates to a second course of therapy.6,10,11 In these trials, 15 of 37 indo-methacin-treated patients (41%) and 16 of 41 ibuprofen-treated patients (39%) experienced closure after not responding to the first course. The difference between the groups was not statistically significantly different (P = 1.0). The total closure rate (first plus second course closure rate) in the largest of the 3 trials was 82% for indomethacin and 86% for ibuprofen.10
Question 12. How do the prices of indomethacin and ibuprofen lysine compare?
Both products are available in the US as single-dose vials in packages of 3 and are almost identical in acquisition price.37 When one calculates the cost of a therapy, variables other than acquisition price should be kept in mind, such as cost avoidance, costs of differential side effects and resources needed to administer the therapy and monitor the patient's response to therapy. As is pointed out by others in this supplement, avoiding 1 surgical PDA treatment through pharmacotherapy can produce significant savings to the health care system. Some have concluded that the lower risk of oliguria is likely to support cost savings and produce an overall cost benefit with ibuprofen.1 The author is not aware of any published trials on the pharmacoeconomics of cyclooxygenase inhibitor therapy for the treatment of a PDA.
Two economic studies of indomethacin for PDA prophylaxis have reported differing results.38,39 A decision analysis model based on literature sources found a favorable cost-effectiveness ratio, but this analysis was completed before the publication of the large, recently completed Trial of Indomethacin Prophylaxis in Preterms (TIPP), which found no difference in the primary outcome measure.39 An economic analysis of TIPP did not provide economic support for the use of indomethacin prophylaxis in extremely low birth weight infants.39
This finding is further supported by recent findings from TIPP investigators indicating that, although indomethacin prophylaxis can prevent some surgical ligations and associated long-term neurosensory impairment, the prophylactic benefit is small and may be offset by a small adverse long-term effect.40 In addition, the authors of a large case-control retrospective study comparing indomethacin as “expectant” or prophylactic PDA therapy concluded that prophylaxis offered no advantages over “expectant” therapy and exposed a significant number of infants to potentially serious side effects.41 On the other hand, a number of studies have demonstrated that indomethacin, when given for the treatment of a confirmed PDA, can significantly reduce the need for surgical ligation.42 In the only controlled, randomized, comparative PDA treatment study published, surgically treated patients spent on average 6 more days in the hospital compared to cyclooxygenase inhibitor treated infants (P = .02).43 It may be concluded that these studies provide support for the economic benefit of early, but not prophylactic, treatment of a PDA with cyclooxygenase inhibitory therapy, but formal pharmacoeconomic analyses are needed to confirm this conclusion.
CONCLUSIONS
Commercially available ibuprofen lysine is the first new cyclooxygenase inhibitor approved in the US for the treatment of PDA in 20 years. Ibuprofen lysine is officially indicated for the closure of a clinically significant PDA in premature infants weighing between 500 g and 1500 g, who are no more than 32 weeks GA when usual medical management has been ineffective. Recommended doses (based upon birth weight) include an initial dose of 10 mg/kg followed by 2 doses of 5 mg/kg each, after 24 hours and 48 hours.
This paper has reviewed common clinical and practical questions regarding this US commercially available product. Intravenous ibuprofen lysine was approved based upon 3 placebo- or active-controlled clinical trials involving 357 infants who received the product. Pivotal trial results demonstrate this product to be a significantly more effective treatment for a “silent PDA” and equivalent to placebo with respect to serious adverse events, including ROP, BPD, PVL, and death than the alternative. There were no statistically significant differences or changes in treatment group vs. placebo with respect to renal function. Significant differences in short-term serum creatinine changes and oliguria have been reported favoring ibuprofen vs. indomethacin. PDA prophylaxis studies have shown ibuprofen to be effective, although its efficacy for IVH prophylaxis has not been demonstrated. No significant drug-drug interactions were observed in clinical trials, although clinically significant interactions with some medications used in the neonatal setting either have been reported or can be predicted, particularly with amikacin.
Commercially available ibuprofen lysine is provided in single-use vials, which should be protected from light and stored in the carton at 20–25°C (68–77°F) until used. Neonatal practitioners can mix the product with commonly used IV fluids, if needed, and stability has been observed for up to 12 hours when stored in 5-mL syringes at 0.5 mg/mL and 5 mg/mL at temperatures from 5°C to 25°C. The drug has differential compatibility with a number of commonly administered drugs in the neonatal setting.
Limited pharmacoeconomic evaluations have been completed on cyclooxygenase inhibitor therapies in the treatment or prophylaxis of PDA, but these therapies have a clear advantage in cost effectiveness over surgery.
ACKNOWLEDGMENTS
The author would like to thank Robert J Holt, PharmD, MBA, for providing all necessary information, stability data and calculations.
ABBREVIATIONS
- BPD
bronchopulmonary dysplasia
- BUN
blood urea nitrogen
- D5W
5% dextrose in water
- FDA
Food and Drug Administration
- GA
gestational age
- IV
intravenous
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NNT
number needed to treat
- NS
normal saline
- NSAID
nonsteroidal anti-inflammatory drug
- PDA
patent ductus arteriosus
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
- RR
risk ratio
- TIPP
Trial of Indomethacin Prophylaxis in Preterms
- US
United States
Footnotes
DISCLOSURE Dr. Van Overmeire participated as an investigator in the pivotal ibuprofen trial and received travel support to attend investigator meetings concerning this trial.
He also spoke on scientific meetings (topics: hemodynamics, patent ductus arteriosus, ibuprofen and indomethacin use in neonates) but received no payments from industry for that. It is however probable that industry co-sponsored some scientific meetings on which Dr. Van Overmeire was invited by the independent scientific organizing committee. On some occasions, the organizing congress secretariat reimbursed his accommodation and travel costs.
REFERENCES
- 1.Lee CKK, Meekins MD. Review of the clinical trial information for intravenous ibuprofen for the treatment and prophylaxis of PDA. eNeonatal Review. 2007;4:1–8. [Google Scholar]
- 2.Gomella TL, Cunningham MD, Eyal FG, editors. Neonatalogy: Management, Procedures, On-call Problems, Diseases and Drugs. 4th ed. Stamford, CT: Appleton & Lange; 1999. et al., eds. [Google Scholar]
- 3.Hermes-DeSantis ER, Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26(Suppl):S14–S18. doi: 10.1038/sj.jp.7211465. [DOI] [PubMed] [Google Scholar]
- 4.Coceani F, White E, Bodach E, Olley PM. Age dependent changes in the response of the lamb ductus arteriosus to oxygen and ibuprofen. Can J Physiol Pharmacol. 1979;57:825–831. doi: 10.1139/y79-126. [DOI] [PubMed] [Google Scholar]
- 5.Ovation Pharmaceuticals. NeoProfen (ibuprofen lysine) package insert. Deerfield, IL: May 2006. [Google Scholar]
- 6.Van Overmeire B, Smets K, Lecoutere D. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. et al. [DOI] [PubMed] [Google Scholar]
- 7.Van Overmeire B, Allegaert K, Casaer A. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1945–1949. doi: 10.1016/S0140-6736(04)17477-1. et al. [DOI] [PubMed] [Google Scholar]
- 8.Aranda JV. Multicentre randomized double-blind placebo controlled trial of ibuprofen L-lysine intravenous solution (IV ibuprofen) in premature infants for the early treatment of patent ductus arteriosus (PDA) Pediatric Academic Societies Annual Meeting. May 14–17, 2005. Washington, DC.
- 9.Data on file. Ovation Pharmaceuticals. Deerfield, IL: 2006. [Google Scholar]
- 10.Lago P, Bettiol T, Salvadori S. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomized controlled trial. Eur J Pediatr. 2002;161:202–207. doi: 10.1007/s00431-002-0915-y. et al. [DOI] [PubMed] [Google Scholar]
- 11.Van Overmeire B, Follens I, Hartmann S. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed. 1997;76:F179–F184. doi: 10.1136/fn.76.3.f179. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Carolis M, Romagnoli C, Polimeni V. Prophylactic ibuprofen therapy of patent ductus arteriosus in preterm infants. Eur J Pediatr. 2000;159:364–368. doi: 10.1007/s004310051288. et al. [DOI] [PubMed] [Google Scholar]
- 13.Gournay V, Roze JC, Kuster A. Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double blind, placebo-controlled trial. Lancet. 2004;364:1939–1944. doi: 10.1016/S0140-6736(04)17476-X. et al. [DOI] [PubMed] [Google Scholar]
- 14.Dani C, Bertini G, Reali MF. Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatr. 2000;89:1369–1374. doi: 10.1080/080352500300002598. et al. [DOI] [PubMed] [Google Scholar]
- 15.Dani C, Bertini G, Pezzati M. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among pre-term infants: a multicenter, randomized study. Pediatrics. 2005;115:1529–1535. doi: 10.1542/peds.2004-1178. et al. [DOI] [PubMed] [Google Scholar]
- 16.Varvarigou A, Bardin C, Beharry K. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA. 1996;275:539–544. et al. [PubMed] [Google Scholar]
- 17.Mosca F, Bray M, Colnaghi MR. Cerebral vasoreactivity to arterial carbon dioxide tension in preterm infants: the effect of ibuprofen. J Pediatr. 1999;135:644–646. doi: 10.1016/s0022-3476(99)70065-x. et al. [DOI] [PubMed] [Google Scholar]
- 18.Romagnoli C, De Carolis MP, Papacci P. Effects of prophylactic ibuprofen on cerebral and renal hemodynamics in very preterm neonates. Clin Pharmacol Ther. 2000;67:676–683. doi: 10.1067/mcp.2000.107048. et al. [DOI] [PubMed] [Google Scholar]
- 19.Bray M, Stucchi I, Fumagalli M. Blood withdrawal and infusion via umbilical catheters: effect on cerebral perfusion and influence of ibuprofen. Biol Neonate. 2003;84:187–193. doi: 10.1159/000072301. et al. [DOI] [PubMed] [Google Scholar]
- 20.Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2003;(2):CD004213. doi: 10.1002/14651858.CD004213. [DOI] [PubMed] [Google Scholar]
- 21.Shah SS, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;(1):CD004213. doi: 10.1002/14651858.CD004213.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt B, Davis P, Modderman D. Long-term effect of indomethacin prophylaxis in extremely-low-birth-weight infants. N Eng J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. et al. [DOI] [PubMed] [Google Scholar]
- 23.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2002;(3):CD000174. doi: 10.1002/14651858.CD000174. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsson A. Back to the drawing board. Pediatr Res. 2000;47:4–5. doi: 10.1203/00006450-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Chemtob S, Beharry K, Rex J. Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets. Stroke. 1990;21:777–784. doi: 10.1161/01.str.21.5.777. et al. [DOI] [PubMed] [Google Scholar]
- 26.Pellicer A, Aparicio M, Cabanas F. Effect of the cyclo-oxygenase blocker ibuprofen on cerebral blood volume and cerebral blood flow during normocarbia and hypercarbia in newborn piglets. Acta Paediatr. 1999;88:82–88. doi: 10.1080/08035259950170664. et al. [DOI] [PubMed] [Google Scholar]
- 27.Grosfeld JL, Kamman K, Gross K. Comparative effects of indomethacin, prostaglandin E1, and ibuprofen on bowel ischemia. J Pediatr Surg. 1983;18:738–742. doi: 10.1016/s0022-3468(83)80015-3. et al. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan BS, Restraino I, Raval DS. Renal failure in the neonate associated with in utero exposure to non-steroidal anti-inflammatory agents. Pediatr Nephrol. 1994;8:700–704. doi: 10.1007/BF00869093. et al. [DOI] [PubMed] [Google Scholar]
- 29.Hardy P, Peri KG, Lahaie I. Increased nitric oxide synthesis and action preclude choroidal vasoconstriction to hyperoxia in newborn piglets. Circ Res. 1996;79:504–511. doi: 10.1161/01.res.79.3.504. et al. [DOI] [PubMed] [Google Scholar]
- 30.Mosca F, Bray M, Lattanzio M. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ducuts arteriosus. J Pediatr. 1997;131:549–554. doi: 10.1016/s0022-3476(97)70060-x. et al. [DOI] [PubMed] [Google Scholar]
- 31.Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2005;(4):CD003481. doi: 10.1002/14651858.CD003481.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Su PH, Chen JY, Su CM. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatr Int. 2003;45:665–670. doi: 10.1111/j.1442-200x.2003.01797.x. et al. [DOI] [PubMed] [Google Scholar]
- 33.Peltoniemi O, Kari MA, Heinonen K. Pretreatment cortisol values may predict responses to hydrocortisone administration for the prevention of bronchopulmonary dysplasia in high-risk infants. J Pediatr. 2005;146:632–637. doi: 10.1016/j.jpeds.2004.12.040. et al. [DOI] [PubMed] [Google Scholar]
- 34.Allegaert K, Cossey V, Langhendries JP. Effects of co-administration of ibuprofen-lysine on the pharmacokinetics of amikacin in preterm infants during the first days of life. Biol Neonate. 2004;86:207–211. doi: 10.1159/000079618. et al. [DOI] [PubMed] [Google Scholar]
- 35.Ojala TH, Lehtonen L. A preliminary report – Heparin counteracts indomethacin effect on ductus arteriosus in very low birthweight infants. Pediatr Crit Care Med. 2007;8:1–3. doi: 10.1097/01.pcc.0000270203.07648.8e. [DOI] [PubMed] [Google Scholar]
- 36.Volonte MG, Valora PD, Cingolani A. Stability of ibuprofen in injection solutions. Am J Health Syst Pharm. 2005;62:630–633. doi: 10.1093/ajhp/62.6.630. et al. [DOI] [PubMed] [Google Scholar]
- 37.Poon G. Ibuprofen lysine (NeoProfen) for the treatment of patent ductus arteriosus. Proc Bayl Univ Med Cent. 2007;20:83–85. doi: 10.1080/08998280.2007.11928244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moya MP, Goldberg RN. Cost-effectiveness of prophylactic indomethacin in very-low-birth-weight infants. Ann Pharmacother. 2002;36:218–224. doi: 10.1345/aph.10347. [DOI] [PubMed] [Google Scholar]
- 39.Zupancic JA, Richardson DK, O'Brien BJ. Retrospective economic evaluation of a controlled trial of indomethacin prophylaxis for patent ductus arteriosus in premature infants. Early Hum Dev. 2006;82:97–103. doi: 10.1016/j.earlhumdev.2006.01.004. et al. [DOI] [PubMed] [Google Scholar]
- 40.Kabra NS, Schmidt B, Roberts RS. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: Results from the trial of indomethacin prophylaxis in preterms. J Pediatr. 2007;150:229–234. doi: 10.1016/j.jpeds.2006.11.039. et al. [DOI] [PubMed] [Google Scholar]
- 41.Cordero I, Nankervis, DeLooze K, Giannone PJ. Indomethacin prophylaxis or expectant treatment of patent ductus arteriosus in extremely low birth weight infants? J Perinatol. 2007;27:158–163. doi: 10.1038/sj.jp.7211659. [DOI] [PubMed] [Google Scholar]
- 42.Shah S. Should a prolonged or short course of indomethacin be used in preterm infants to treat patent ductus arteriosus? Arch Dis Child. 2003;88:1132–1133. doi: 10.1136/adc.88.12.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gersony WM, Peckham GJ. Ellison RC, et al. Effects of indomethacin in premature infants with patent ductus arteriosus: Results of a national collaborative study. J Pediatr. 1983;102:895–906. doi: 10.1016/s0022-3476(83)80022-5. [DOI] [PubMed] [Google Scholar]


