Abstract
Childhood obesity is increasing in prevalence in the United States. Comorbid diseases once thought of as adult issues such as hypertension, diabetes, and dyslipidemia, are now being encountered in the pediatric population as a result of obesity. Primary prevention is still the most cost-effective approach to this growing problem. In terms of management, the treatment of obesity in children is not identical to that in adults. Thus far, the only accepted weight loss therapy for children are diet, exercise, modification of eating behaviors and family education. Further options for morbidly obese children include weight loss medications or surgery, regarding which long-term benefits are still under investigation.
Keywords: adolescent, anti-obesity agents, body weight, child, diet therapy, pediatrics, obesity, orlistat, sibutramine
INTRODUCTION
Recently, the prevalence of overweight and obese children and adolescents has increased dramatically. Chronic illnesses resulting from obesity that were once prevalent primarily among adults are now being encountered in children. Childhood obesity and its consequences later in life render the several years of research and advances in cardiovascular medicine counterproductive. In addition, hospital costs from obesity-related diseases in the young have increased from $35 million per year (0.43% of hospital costs) in 1979–1981 to $127 million per year (1.79% of total costs) during 1997–1999.1 For these reasons, healthcare practitioners need to focus on education and therapies directed at preventing weight gain and managing weight in those children and adolescents who are overweight.
DEFINITIONS OF OVERWEIGHT
Obesity among adults is defined in terms of the body mass index (BMI). BMI is calculated as body weight (in kg) divided by height (in meters squared). Because there is an excellent correlation between calculated BMI and measured adiposity in adults, overweight in adults is defined as a BMI between 25 and 29.9 kg/m,2 and obesity is defined as a BMI ≥30 kg/m2.2 If an individual's BMI is ≥ 40 kg/m,2 the person is considered to be extremely obese.
A summary of BMI for age and weight status categories and the corresponding percentiles are shown in Table 1.3 Children 2 to 20 years of age are considered at risk for becoming overweight if their BMI is between the 85th and 95th percentiles. Overweight is defined as a BMI at or above the 95th percentile. In children, the equivalent of obesity is overweight. Avoidance of the term obesity is made because of social and psychological stigmata. In children less than 2 years of age, overweight is defined as a weight for length above the 95th percentile.2 A BMI calculator for children can be found on the Centers for Disease Control and Prevention (CDC) website at http://www.cdc.gov/nccdphp/dnpa/bmi/childrens_BMI/about_childrens_BMI.htm.3
Table 1.
BMI-for-age weight status categories and the corresponding percentiles
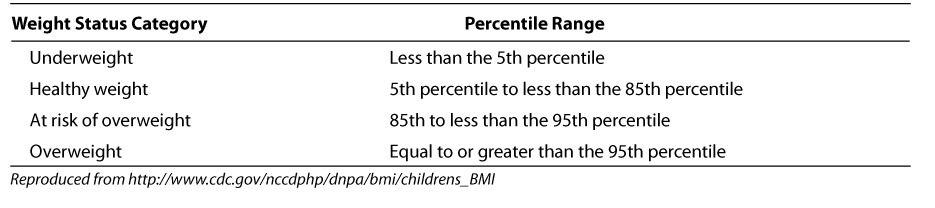
NORMAL BMI TREND IN CHILDREN
The use of BMI is slightly less reliable in children, whose BMI patterns change with age and gender.4,5 Recently, the CDC has released new growth charts accompanied by BMI charts for those who are 2 to 20 years of age.6 The BMI charts can be found at http://www.cdc.gov/nccdphp/dnpa/bmi/00binaries/bmi-tables.pdf and are reproduced in Figures 1 and 2. Figure 3 shows how to use these charts.3 These charts are age- and gender-specific, can be followed graphically through time, and have been published in English, Spanish, and French. A normal decline of adiposity and BMI occurs after 2 years of age. Adipose cell proliferation then resumes after 6 years of age. This period is called adiposity rebound. Thereafter, BMI normally increases from 8 years of age at 0.5 to 1 unit per year. An annual increase of more than 2 BMI units is considered a rapid increase.6,7
Figure 1.
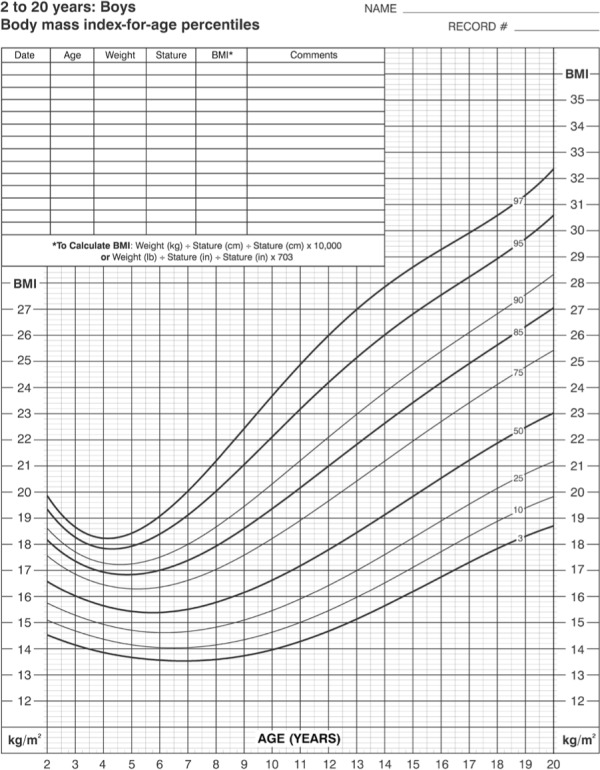
Body-mass-index for age growth chart for boys age 2–20 years.
Reproduced from: http://www.cdc.gov/nchs/data/nhanes/growthcharts/set2clinical/cj41l073.pdf
Figure 2.
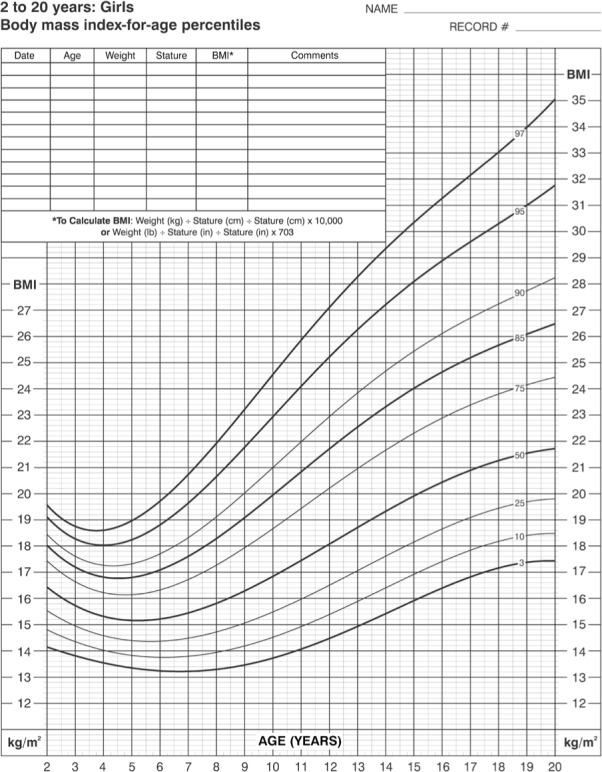
Body-mass-index for age growth chart for girls age 2–20 years.
Reproduced from: http://www.cdc.gov/nchs/data/nhanes/growthcharts/set2clinical/cj41l074.pdf
Figure 3.
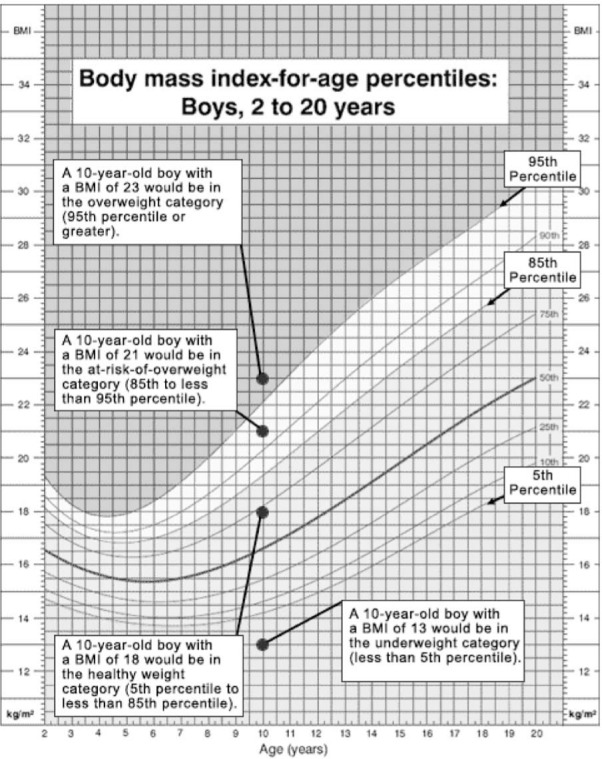
The following chart shows an example for a 10-year-old boy and a 15-year-old boy who both have a BMI-for-age of 23.
Reproduced from http://www.cdc.gov/nccdphp/dnpa/bmi/childrens_BMI/about_childrens_BMI.htm
Two children of different ages are plotted on the same growth chart to illustrate a point. Normally the measurement for only one child is plotted on a growth chart.
Overall, obesity affects 1 in every 5 children in the United States. In the United States, from 1999 to 2002, 16% of those 6 to 19 years old were overweight.8 Between 2003–2004, the prevalence of overweight increased to 19% among children 6 to 11 years of age and to 17% among adolescents 12 to 19 years of age.8 The prevalence rates, in children 6 to 19 years of age, have increased 2 to 3 times in the last two decades.
CRITICAL PERIODS IN THE DEVELOPMENT OF OBESITY
It is important to understand which children are likely to outgrow obesity and which are likely to be obese as adults. There are critical periods that can predict persistence of obesity: prenatal and intrauterine environmental exposure, adiposity rebound, and adolescence.
The thrifty phenotype concept was proposed to explain epidemiological evidence of metabolic programming based on the in utero environment.9 Small for Gestational Age (SGA) babies have an increased incidence of insulin resistance, type 2 diabetes mellitus (DM), obesity, and cardiovascular disease later in life. Poor nutrition in fetal life is detrimental to the development and function of pancreatic beta cells, and insulin-sensitive tissues may eventually become resistant to insulin over time. In a study in India on birth weight and insulin resistance, the highest risk of insulin resistance was among individuals who both had the lowest birth weight and who had achieved the highest weight gain at 8 years of age.10 Large for gestational age (LGA) babies are also impacted. It is speculated that a hyperglycemic environment may cause fetal adaptation to an excess of fuel, thus mediating insulin resistance in later life. A Danish study, adjusted for maternal factors, gestational age, and birth length, showed an increase in BMI at 18 to 26 years of age as birth weight increased from < 2.5kg to > 4.5kg.11 Also, infants of diabetic mothers have an increased risk of obesity (up to 50% prevalence) by 6 to 10 years of age.2 For instance, infants born to Pima Indian mothers who were diabetic during pregnancy were more likely to be obese during childhood than their siblings who were born before the manifestation of their mother's diabetes.12
Adiposity rebound is described as the period after 6 years of age when the BMI increases after a toddler pause. Longitudinal studies suggest that children whose BMI increased before 5 and a half years of age (early adiposity rebound) are more likely to be obese later in life.13,14 It is suggested that early adiposity rebound is a marker for generalized growth acceleration and cell hyperplasia. One study showed that adult obesity rates were higher in those with early adiposity rebound than in those with late adiposity rebound (25% versus 5%). The odds ratio for adult obesity associated with early versus late adiposity rebound is 6.0 (95% CI, 1.3–26.6).15
It is known that there is a normal tendency during adolescence for insulin resistance to occur, which is a cofactor for excessive weight gain during this period. The increase in BMI during this period can potentially persist through adulthood. One study addressed the predictive value of BMI during the adolescent period for being overweight at 35 years of age. The prediction is excellent at 18 years of age, good at 13 years of age, and moderate at < 13 years of age. Among 18-year olds with a BMI > 60th percentile, there is a 34% probability for males and 37% probability for females of becoming overweight at 35 years of age. Based on the model the authors proposed, a BMI of 95th percentile at 13 years of age for both boys and girls has a 40% to 80% probability of becoming overweight at 35 years of age.16
OBESITY: NATURE OR NURTURE?
Definitely there are interactions between genetics and the environment that influence the degree of adiposity, but how much of obesity is genetic and how much of it is environmental and socioeconomic related? Over the last 30 years, the gene pool has undergone only minimal change, while the prevalence of obesity has significantly increased. Thus, experts attribute the epidemic of obesity mainly to environmental and socioeconomic factors and not to genetics.
Nature
Parental obesity is a strong predictor of a child's subsequent obesity as an adult. The odds ratio for a young child to develop obesity as an adult is 3 if one parent is obese and 10 if both are obese.17 A study by Whitaker and colleagues reported that 64% of children who were obese during childhood and had normal-weight parents became obese adults. If one or both parents were obese and the child was also obese, the incidence increased to 79%.18 Overall, parental obesity increases the risk of obesity two to threefold in children of all ages.
Certain genetic syndromes can predispose a child to obesity such as Prader-Willi Syndrome and Bardet-Biedl Syndrome. Genetic mutations of receptors and neuropeptides involved with the regulation of satiety, hunger, lipogenesis, and lipolysis have been discovered recently. All of these conditions will help refine the understanding of obesity and may lead to more effective treatments.
Nurture
The environment is hypothesized to play a large role in the increase in prevalence of childhood obesity. Children are not as active as they were years ago. A national transportation survey reported that walking and bicycling decreased by 40% between 1977 and 1995 among children 5 to 15 years of age.19 Youth participation in physical education decreased from 42% to 29% between 1991 and 1999.20 Television has greatly impacted daily activities of children. In one study, children who watch > 4 hours of television daily had significantly greater BMI than those watching < 2 hours per day.21 Among adolescents 12 to 17 years old, there is a 2% increase in the prevalence of obesity for each extra hour of television.22
The impact of dietary patterns on obesity, in infants and children, has also been evaluated. Limited evidence showed breastfeeding prevents later obesity. It is postulated that breastfeeding leads to a better internal control of energy intake by the child. A longitudinal cohort study compared children breastfed less than 3 months or bottle-fed from birth with children breastfed more than 3 months. Both groups were followed over 9 periods between birth and 6 years of age. Breastfed and bottle-fed children began diverging in BMI and triceps skin folds at 3 months of age. By 6 months, a higher proportion of children bottle-fed or breastfed less than 3 months had BMI exceeding the 90th percentile and skin-fold thickness exceeding 97th percentile. By four years of age, the prevalence of obesity nearly doubled among bottle-fed infants.23 A meta-analysis was recently done to compare the duration of breastfeeding and the risk of being overweight later in life. The duration of breastfeeding was inversely associated with the risk of overweight (regression coefficient=0.94, 95%: 0.89, 0.98). Each month of breastfeeding was associated with a 4% decrease in risk of overweight in later life (OR=0.96/month of breastfeeding, 95% CI: 0.94, 0.98). It was concluded that exclusive or partial breastfeeding for at least 9 months leads to a greater decrease in risk of being overweight in later life.24
Several studies have shown that infants and toddlers are not only overeating, but are not eating the right foods at very early ages.25–27 Their mean energy intake exceeds the dietary requirements by 10% at 4 to 6 months of age, by 23% at 7 to 11 months of age, and by 31% at 1 to 2 years of age. By 19 to 24 months of age, 10% toddlers routinely consumed candy, 23% consumed sodas, 27% consumed salty snacks, and 27% consumed hot dogs, sausages, or cold cuts. Specific amounts were not specified. Data from the Department of Agriculture showed that among school-aged children, only 35% consumed fruits daily and 45% consumed vegetables daily.28 Among children 6 to 13 years of age, consumption of more than 12 ounces per day of excessively sweetened drink was associated with lower milk consumption, lower protein and calcium intake, higher daily caloric intake, and greater weight gain.29 A cluster, randomized control trial of 644 children, ages 7 to 11 years of age, was done to determine if an educational intervention aimed at reducing intake of sweetened carbonated drinks can prevent excessive weight gain in children.30 Consumption of sweetened carbonated drinks over 3 days decreased by 0.6 glasses or 150 mL in the intervention group and by 0.2 glasses or 50 mL in the control group (mean difference 0.7; 95% CI, 0.1–1.3). After 12 months, the percent of overweight children increased by 7.5% in the control group and decreased by 0.2% in the intervention group (mean difference 7.7%; 95% CI, 2.2–13.1%).30
Although there is inadequate data to establish a definite causality, greater maternal control of a child's diet and eating habits measured at 5 years of age was associated with more eating in the absence of hunger at later ages (7–9 years of age). In addition, overweight girls who were exposed to highly restrictive diet at 5 years of age appeared to have the greatest susceptibility to the effects of maternal restriction. 31 A high degree of maternal control may be beneficial for toddlers; however, it may be detrimental for older children. Finally, in our current fast-paced culture where both parents work outside the home in most families, it is worth mentioning that the absence of family meals has been associated with less consumption of fruits and vegetables. Although there are no data to adequately support these, the higher prevalence of obesity among low-income families may be attributed to the following: lack of safe place for physical activity, lack of access to healthful food choices, low cognitive stimulation affecting parental food choices and feeding styles, and increasing prices of healthy foods such as fruits and vegetables.
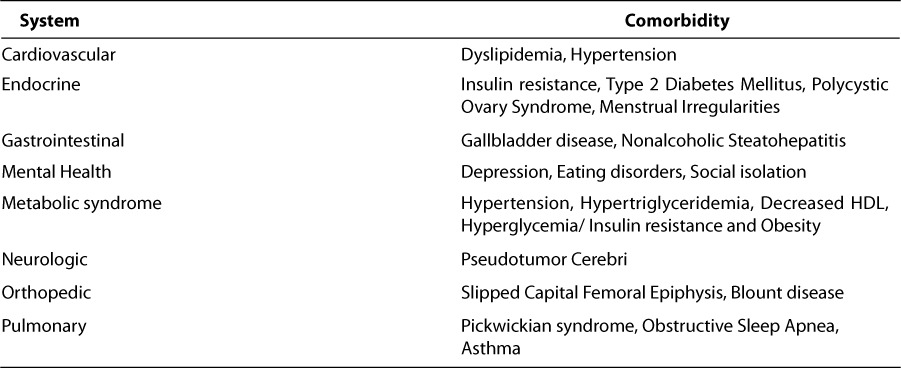
COMORBIDITIES OF CHILDHOOD OBESITY
Many of the adverse effects of childhood obesity are not evident until later in adulthood. However, recently, increasing numbers of children are manifesting metabolic, cardiovascular, and physiologic consequences of childhood obesity (Table 2). The Bogalusa Heart study was a cohort study that involved 9167 children 5 to 17 years of age; the children were examined in 7 cross-sectional studies between 1993–1994.32 The study evaluated the relationship of a BMI > 95th percentile with such cardiac risk factors as hypertension, increased LDL, low HDL and high fasting insulin levels. Among these factors, an elevated BMI > 95th percentile has an odds ratio of up to 12.6 of having an elevated fasting insulin an odds ratio of 7.1 of having hypertriglyceridemia. Measures of obesity are weakly correlated to the levels of total cholesterol and LDL but more strongly correlated to the levels of insulin, triglycerides, HDL and systolic blood pressure. The study found that nearly 58% of overweight children have one cardiovascular risk factor (dyslipidemia, hypertension or hyperinsulinemia). By routinely using weight status (i.e., overweight) as a screening tool during a physical examination, one can identify 50% of schoolchildren with two or more cardiovascular risk factors.32
Table 2.
Comorbidities of childhood obesity
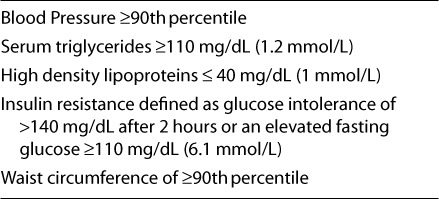
In 1988, Reaven and colleagues described the metabolic syndrome as a link between insulin resistance and hypertension, dyslipidemia, type 2 DM, and other metabolic abnormalities associated with increased risk of cardiovascular disease in adults. The criteria used to diagnose the metabolic syndrome in children and adolescents were modified from those of the National Cholesterol Education Program (NCEP) and the World Health Organization.33 The modified criteria of the Metabolic Syndrome can be found in Table 3. In order to make a diagnosis of the syndrome a person must have at least 3 of the 5 findings listed.33 Using the NCEP guidelines, the prevalence of metabolic syndrome among those 12 to 19 years of age is 4.2%.33 Among severely obese children, the prevalence is up to 49%.34 Of those with metabolic syndrome, 25% are at risk for overweight, and 74% are overweight. Cluster-tracking studies have shown that multiple cardiovascular risk factors in childhood persist 8 years later in 60% of cases.32
Table 3.
Modified criteria of the Metabolic Syndrome33
In adults, a central distribution of fat that causes an increased waist circumference is an excellent correlate of increased cardiovascular risk. Normative values for waist circumference for children were recently published for both boys and girls and are age, gender and ethnicity specific.35 Studies have shown that waist circumference provide information on coronary artery risk beyond that provided by BMI alone. These studies provide evidence that a combination of BMI and waist circumference should be used to evaluate elevated health risks in children.36,37 Infants and children with rapid weight gain and low linear growth as well as children with syndromic features, eating disorders, neurological or psychiatric problems, or those with complications of obesity should be referred to the respective specialist.
PRIMARY PREVENTION OF CHILDHOOD OBESITY
Although most of the metabolic complications of childhood obesity emerge during adolescence, guidelines suggest that intervention is warranted in the prevention and treatment of young children. A two-pronged approach that involves both prevention and treatment is suggested. Prevention may prove more beneficial than treatment of established obesity in adolescents and adults.38 Prevention is divided into diet, modification of eating behaviors, increased physical activity, and reduction of sedentary lifestyle.
Primary prevention can be done during all stages of life. In the fetal period, measures should be taken to prevent intrauterine growth retardation and to prevent growth that results in the characterization of babies as large for gestational age. During infancy, breastfeeding should be encouraged for at least 6 months per the American Academy of Pediatrics recommendation. Healthcare providers should also recognize early adiposity in young children. Television should not be encouraged, and when viewed, it should be limited to no more than two hours per day after 2 years of age. Intake of fruits and vegetables should be increased and portion control should be practiced. Sweetened beverages should be limited to 4–6 ounces per day in children less than 6 years of age and no more than 12 ounces per day in children older than 6 years of age. Low-fat milk can be offered after 2 years of age. Modification of food behaviors consists of setting the appropriate limits on food choices, avoiding the use of foods as punishment or reward, and encouraging children to eat only when hungry. Increased physical activity should be encouraged as the child ages. In general, children need 30 to 60 minutes of exercise per day at least 3 to 5 days a week.39–41 Structured and unstructured activities that are fun, developmentally appropriate, and tailored to the child's fitness level should be encouraged. Activities should emphasize the positives and involve the whole family.41
TREATMENT OPTIONS FOR OBESITY
Treatment goals should be simple, explicit, and measurable. They should restore the balance between energy intake and expenditure, stabilize the weight as the child grows, provide adequate nutrition for growth and development, and prevent and reverse long-term comorbidities. Numerous treatment options are available, but only a few are realistic options. Current treatment for obesity includes a variety of popular diets, weight loss medications, bariatric surgery, and most importantly, modification of eating behaviors.
Diets
Diets for children have advantages and disadvantages. The advantage is that they may provide structure for children and their families, resulting in a positive cycle. Conversely, they may foster restrictive practices and lead to a restriction-relapse cycle with subsequent higher weight gain. The more control the parent exerts over the child's diet, the less the child is able to regulate his or her own energy intake appropriately. With the restriction-relapse cycle, a child may go to extremes and develop excessive weight loss or weight gain when the restriction is lifted.42,43
A randomized controlled trial comparing low carbohydrate and high protein diet with low-fat diet for weight reduction among overweight adolescents was studied by Sondike et al.44 The low carbohydrate group (n = 16) consumed < 20 grams of carbohydrate per day for two weeks, then < 40 grams/day for ten weeks for a total of three months. The low-fat group (n = 14) consumed a diet with < 30% of calories from fat. The study showed that there was a 9.9 kg weight loss in the low carbohydrate group over 3 months compared to a 4.9 kg weight loss over 3 months in the low-fat diet group. LDL decreased in the low-fat group but not in the low carbohydrate group. The dropout rate among the low carbohydrate group was 25%, but none were related to side effects. However, the long-term effects and benefits of a low carbohydrate diet among children are still not well studied.44
The Traffic Light Diet was designed to maximize healthy food choices and encourage individual control of the diet.45 It classifies foods into green foods that can be eaten in unlimited quantities (non-fat or low-fat foods such as fruits and vegetables), red food that are eaten rarely (high fat foods such as butter and nuts), and yellow foods that are eaten with caution (low to moderate fat foods such as whole grain breads/pastas and beans). In combination with behavioral and exercise programs, this approach led to long-term weight loss in children.45
The South Beach Diet recommends an initial brief period of carbohydrate restriction. It is designed to encourage people to eat the “right” carbohydrates and the “right” fats. It measures foods based on the glycemic index, the degree at which a particular food contributes to increases in blood glucose and to weight gain.46 A small study among adolescents showed that the glycemic index can impact intake. The highest food intake after a meal occurred among adolescents who consumed a high-glycemic-index meal. However, larger studies are needed to examine the relationship of glycemic index to food in-take and subsequent obesity. In addition, there are no studies to support that a high glycemic index causes weight gain.43,47 Selection and implementation of an appropriate diet should occur under medical supervision. In general, healthy eating without strict dieting is still the mainstay of treatment for children.
Weight Loss Medication
Although sibutramine and orlistat are the only two weight loss medications approved by the Food and Drug Administration (FDA) for use in children, the long-term data and risks associated with their use are unknown in this population. Sibutramine (Meridia; Abbott Laboratories, Abbott Park, Illinois) is approved for obese individuals who are 16 years and older and have a BMI ≥ 30kg/m2 or a BMI ≥ 27kg/m2 accompanied by risk factors such as hypertension, diabetes, and dyslipidemia. Sibutramine suppresses the appetite by inhibiting the neuronal uptake of serotonin, norepinephrine, and dopamine (minimal).
Although the bioavailability of sibutramine is about 77%, it is rapidly absorbed after oral administration and with a time to maximum serum concentration of 1.2 hours.48 It undergoes first-pass metabolism in the liver and is metabolized by the cytochrome P450 (CYP) 3A4 isoenzyme to form two active metabolites (mono- and di-desmethyl metabolites; M1 and M2). These metabolites are then hydroxylated and conjugated to inactive metabolites that are renally eliminated. The parent compound and its two active metabolites are all highly protein bound (> 94%). Because sibutramine is a major substrate of the CYP 3A4 isoenzyme, it can be associated with numerous drug interactions that may alter sibutramine concentrations. Recommended initial dosing is 10 mg daily for four weeks, followed by a titration to 15 mg daily if needed.
Table 4.
Recommended criteria for bariatric surgery in adolescents57
Three clinical trials have evaluated sibutramine in the adolescent population.49–51 The first study was a one-year randomized, double-blind, placebo-controlled trial that was done by Berkowitz and colleagues.49 This work establish the efficacy and safety of 10mg of sibutramine among 82 obese adolescents, 13 to 17 years of age. Both groups received behavioral weight loss therapy and were recommended to consume a maximum of 1500 kcal/day, accompanied by exercise. Doses could be increased to 15mg daily. During the six-month phase, an 8.5% reduction of BMI was seen in the sibutramine group, and a 4.0% reduction was noted in the placebo group (P = .001). The placebo group was given the option to continue for 6 more months in an open-label trial with sibutramine. This group reduced their BMI by an additional 2.4%. For those in the original sibutramine group, a 0.2% reduction in BMI was found after an additional 6 months of therapy, showing that primary weight loss was achieved during the first months of medication therapy. The most common side effects were hypertension or tachycardia, which required discontinuation of sibutramine in six patients. Sixteen patients had their dose reduced to 10 mg, and seven additional patients had their dose decreased to 5 mg. Another double-blind randomized, placebo-controlled trial among 60 adolescents (14–17 years of age) was conducted to determine the safety and efficacy of sibutramine use in obese adolescents.50 The 24-week study was conducted in conjunction with diet and exercise. The mean BMI and weight loss in the intervention group was significantly greater at the end of six months (BMI: 3.6% vs. 0.9%, P < .0001; Weight: 10.3 kg vs. 2.4 kg, P < .001). No significant differences in blood pressure, heart rate, and echocardiographic parameters were seen in either group. Constipation occurred more in the sibutramine group; however, no patients discontinued the medication due to adverse effects.
To date, the largest trial of sibutramine was conducted in the United States in 498 adolescents, 12 to 16 years of age.51 Patients were included if they had a BMI that was at least 2 units more than the 95th percentile based on age and gender, but not greater than 44 kg/m2. Patients were excluded if they had cardiovascular disease (SBP > 130 mm Hg, DBP > 85 mm Hg, HR > 95 beats/min), DM, or major psychiatric disorders. They were also excluded if they had used weight loss medication or structured weight loss program for more than 2 weeks prior to study enrollment. Other contraindications included pregnancy, cigarette smoking, or use of medications contraindicated with sibutramine. This 12-month, multicenter, double-blind trial randomized patients to sibutramine 10 mg (n = 368) or placebo (n = 130) in a 3:1 fashion. All patients received behavior therapy at their study site. If a patient had not lost more than 10% of BMI after 6 months of therapy, the sibutramine dose was increased to 15 mg daily.
Baseline characteristics of the participants in the sibutramine trial were similar, and 52% of patients had dyslipidemia. By the sixth month, 48% of the sibutramine group required an increase in the dose to 15 mg. Overall, 76% of patients in the sibutramine group and 62% in the placebo group completed the study, P = .001. The average adherence rate was 89.1% in the sibutramine group and 83.9% in the placebo group. After 12 months, the estimated BMI mean change was –3.1 kg/m2 for sibutramine and –0.3 kg/m2 for placebo (P < .001). This difference was statistically significant at all study visits as well. Thirty-three percent of the sibutramine group no longer had a BMI greater than 2 units of the 95th percentile and 16.7% of patients on sibutramine had a BMI less than the 95th percentile at the end of the study. The estimated mean change in body weight was –6.5 ± 0.31kg and 1.9 ± 0.56 kg for sibutramine and placebo, respectively, P < .001. This difference was statistically significant from week 2 to the end of the study. Weight circumference also changed by –8.2 ± 0.49 cm in the sibutramine group versus –1.8 ± 0.86 cm in the placebo group, P < .001. Insulin levels, insulin sensitivity, fasting triglycerides, and HDL cholesterol improved with sibutramine therapy, P < .001. Serum glucose levels, LDL cholesterol, and total cholesterol did not show a statistically significant change; however, fasting lipid and glycemic variables clinically improved in patients who decreased their BMI by 5% or greater. The SBP, DBP, and HR showed statistical difference between the groups; however, clinical significance could be questioned. Mean absolute changes in SBP, DBP, and HR were 1.0 mm Hg, 1.7 mm Hg, and 2.5 beats/min, respectively. Growth and maturation did not differ between groups at the end of the study. Tachycardia occurred more frequently in the sibutramine group (12.5%) versus placebo (6.2%), P = .049. Dry mouth, constipation, dizziness, insomnia, and hypertension were seen more in the sibutramine group. Twenty-three patients in the sibutramine group and seven patients in the placebo group withdrew due to adverse events (P = .83). Five of the patients in the sibutramine group withdrew due to hypertension; however, this was not seen in the placebo group. This study proved sibutramine's efficacy with behavior therapy in very obese adolescents over a 1-year period. Long-term benefits and effects still need to be assessed.
Orlistat (Xenical; Hoffmann-La Roche Inc. Nutley, New Jersey) is a reversible gastric and pancreatic lipase inhibitor that results in fecal loss of dietary fats. It is minimally absorbed and works by exerting local action in the gastrointestinal tract.52 The onset of action is 24 to 48 hours, but can last up to 72 hours in duration. The major route of elimination is fecal excretion. It is currently approved for children older than 12 years of age with the same BMI restrictions as sibutramine. Recommended dosing is 120 mg threes times daily with meals. If a meal is skipped or does not contain fat, it is not recommended to administer the dose. If administered three times a day, orlistat inhibits absorption of approximately 30% of dietary fat.52
A 52-week controlled study randomized 539 obese adolescents to orlistat or placebo.53 Patients received a hypocaloric diet with 30% fat, behavior modification therapy, and recommendation for exercise. By the end of the study, the BMI in the orlistat group decreased by 0.55 versus 0.31 in the placebo group (P = .001). Over 25% of patients in the orlistat group had a BMI reduction of 5% or greater versus 15.7% in the placebo group. Body weight increased by 0.53 kg with orlistat and 3.14 kg with placebo (P < .001). No differences were found in serum cholesterol or glucose concentrations in either group. Gastrointestinal effects were the most common adverse events, but only led to 2% of the orlistat group discontinuing the medication. Vitamin supplementation may be recommended for patients taking orlistat due to the alteration in vitamin absorption.
Another trial evaluated the efficacy of orlistat in 40 adolescents (14–18 years of age).54 This double-blind study randomized patients to orlistat, 120 mg three times daily or placebo for 6 months. Patients were enrolled if their BMI was greater than the 85th percentile for age and gender, but were excluded if they had a secondary cause for obesity. Subjects were financially compensated for study visits and per kg of weight loss and educated on diet and exercise. Thirty-four patients completed the study. Patients in the orlistat group experienced a decrease in BMI by 1.3 ± 1.6 kg/m2 (P = .04), and those in the placebo group had a decrease of 0.8 ± 3 kg/m2 (P = .02). No statistical difference was found between the groups (P = .39). The change in both groups could be the result of diet and exercise education. No significant change in weight or fat percentage was found in the groups or between the groups. Medication compliance was similar in both groups. Compared to placebo, orlistat had statistically higher rates of soft stools, oily spotting, fatty or oily stools, oily evacuation, liquid stools, cramping, flatus with discharge, and fecal incontinence. No statistically significant difference of fat soluble vitamin measurements were identified between groups.
In January 2006, an FDA advisory committee recommended to sell orlistat as a non-prescription weight loss aid. The approval is based on two clinical trials in the overweight, adult population. The final FDA ruling occurred February 2007. Alli (orlistat; GlaxoSmithKline, Research Triangle Park, North Carolina) will be available in 60 mg capsules over-the-counter, with dosing recommendations for use up to three capsules daily with fatty meals.55 It is only indicated in patients 18 years of age and older when used with an exercise plan and a low-fat, calorie-reduced diet . The pharmaceutical company marketing this product has been given a pediatric assessment deferral from the FDA for use in children ages 12 to 17 years. A starter pack including 90 capsules, a capsule carrying case, and various healthy lifestyle reference guides will be available summer 2007. Bottles will also be available in 60, 90, and 120 counts. GlaxoSmithKline developed www.myalli.com for patients and healthcare providers to learn more about the product and weight loss plans.
Currently, there are no comparative trials of sibutramine and orlistat in the pediatric population. Adverse effects and compliance should be considered when choosing a therapy.
Surgery
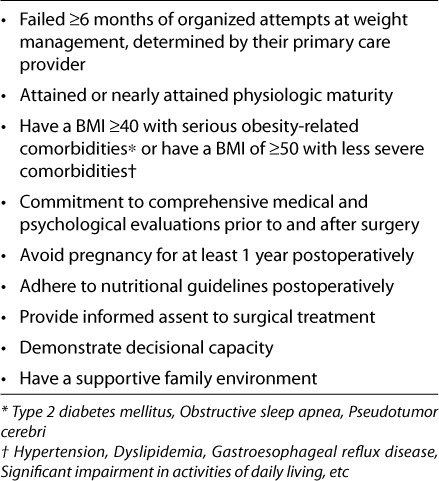
Bariatric surgery has been widely used in morbidly obese adults. There is very little experience related to surgical therapy in children. However, a recent study evaluated trends with bariatric surgery in adolescents.56 They reported bariatric surgeries more than tripled from 2000 to 2003. In-hospital charges accounted for > $23 million in 2003. Recommendations have been published to establish guidelines in the evaluation of adolescents for bariatric surgery, in an effort to reduce the risk of adverse medical and psychological outcomes and improve to compliance.57 Some have criticized these recommendations for being too conservative.58
Modification of Eating Behaviors
Epstein and colleagues conducted a study on the effect of behavior treatment on weight loss among obese parents and their overweight children.59 Behavior modification was more effective in children than in adults on the long-term basis. This 10-year study compared the number of parents and children who maintained at least a twenty percent weight loss at 6, 60, and 120 months. At each measurement over this 10-year period, children were more likely than their parents to have maintained at least a 20% weight loss. However, a decline in treatment response occurred in both groups from 6 months to 60 months. Thereafter, children tended to stabilize their relative weight change while adults continued to increase their overweight percentage in the time between 60 and 120 months.
A recent study by Wrotniak and Epstein evaluated the effectiveness of family-based behavioral weight control management. They found that the parental BMI change was a significant incremental predictor of child BMI change at 6 and 24 months. This study supports the inclusion of parents in programs for their children. Concurrent treatment of both parents and children is a cost-effective way of improving the health of all family members.60 Overall, success is still limited when using behavioral therapy alone in the treatment of obesity; behavioral therapy should be used with diet and exercise for the greatest impact.
CENTERS FOR DISEASE CONTROL RECOMMENDATIONS
The Centers for Disease Control (CDC) recently published its recommendations for weight loss and weight management in children.6 However, the recommendations are not evidence-based. For children 2 to 7 years of age, weight maintenance is advocated. Weight loss is only recommended for this age group if they have a BMI ≥ 95th percentile with complications. It is recommended that the child lose no more than one pound per month. For children 7 years of age and above, weight loss is recommended if they have a BMI in the 85th and 95th percentile range with complications or if they have a BMI ≥ 95th percentile with or without complications. For markedly over-weight children in this age group (BMI > 35) weight loss of 1 to 2 pounds per week may be warranted if acute health risks are evident. The goal for a child is an age-specific BMI less than the 85th percentile.
CONCLUSION
The prevalence of childhood obesity has greatly increased in the recent decade. Physicians caring for children are now encountering chronic illnesses resulting from obesity in children. Primary prevention is still the most cost-effective solution. Primary care physicians should routinely assess a child's BMI, evaluate the patterns of eating and activity, identify risk factors for obesity, and monitor comorbidities in any child deemed overweight. Pharmacists can assist in giving proper advice on other treatment options such as weight loss medications and in raising parental awareness on childhood obesity prevention, treatment, and morbidity. Every effort should always be made in educating both parents and their children on proper nutrition, modification of eating behaviors, and increased physical activity. In terms of advocacy, general public support should be raised for programs that support increased physical activity and proper nutrition in schools, day-cares and community centers. At the national level, policies should be supported that regulate the marketing of energy-dense foods with little nutritional value and for research that may help in the understanding and treatment of obesity. Medications are second-line agents for weight loss after therapeutic lifestyle changes have failed. Bariatric surgery, a last-line therapy, is only recommended for a select type of patient.
ABBREVIATIONS
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- FDA
Food and Drug Administration
- HDL
high-density lipoprotein
- HR
heart rate
- LDL
low-density lipoprotein
- LGA
large for gestational age
- NCEP
National Cholesterol Education Program
- SBP
systolic blood pressure
- SGA
small for gestational age
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Wang G, Dietz WH. Economic Burden of Obesity in youths 6 to 17 years: 1979–1999. Pediatrics. 2002;109:e81. doi: 10.1542/peds.109.5.e81. [DOI] [PubMed] [Google Scholar]
- 2.Styne DM. Childhood and adolescent obesity: prevalence and significance. Pediatr Clin North Am. 2001;48:823–847. doi: 10.1016/s0031-3955(05)70344-8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. BMI- Body Mass Index. August 26, 2006. Available at: www.cdc.gov/nccdphp/dnpa/bmi/childrens_BMI/about_childrens_BMI.htm Accessed April 8, 2007.
- 4.Hammer LD, Kraemer HC, Wilson DM. Standardized percentile curves of body mass-index for children and adolescents. Am J Dis Child. 1991;145:259–263. doi: 10.1001/archpedi.1991.02160030027015. et al. [DOI] [PubMed] [Google Scholar]
- 5.Pietrobelli A, Faith MS, Allison DB. Body mass index as a measure of adiposity in children and adolescents: a validation study. J Pediatr. 1998;132:204–210. doi: 10.1016/s0022-3476(98)70433-0. et al. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Overweight children and adolescents recommendations to screen, assess and manage. August 26, 2006. Available at: http://www.cdc.gov/nccdphp/dnpa/growthcharts/training/modules/module3/text/module3print.pdf Accessed April 8, 2007.
- 7.Himes JH, Dietz WH. Guidelines for over-weight in adolescent preventive service: recommendations from an expert committee. Am J Clin Nutr. 1994;59:307–316. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. Prevalence of overweight among children and adolescents: United States, 1999–2002, 2003–2004. Available at: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_child_03.htm Accessed April 8, 2007.
- 9.Miller J, Rosenbloom A, Silverstein J. Childhood obesity. J Clin Endocrinol Metab. 2004;89:4211–4218. doi: 10.1210/jc.2004-0284. [DOI] [PubMed] [Google Scholar]
- 10.Bavdekar A, Yajnik CS, Fall CHD. The insulin resistance syndrome in 8 year old Indian children: small at birth, big at eight years or both? Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen HT, Sabroe S, Rothman KJ. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ. 1997;314:1137. doi: 10.1136/bmj.315.7116.1137. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabelea D, Hanson RL, Lindsay RS. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 13.Rolland-Cachera MF, Deheeger M, Bellisle F. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39:129–135. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 14.Siervogel RM, Roche AF, Guo S. Patterns of change in weight/stature2 from 2 to 18 years: findings from long-term serial data for children in the Fels Longitudinal growth Study. Int J Obes. 1991;13:479–485. [PubMed] [Google Scholar]
- 15.Whitaker RC, Pepe MS, Wright JA. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:e5. doi: 10.1542/peds.101.3.e5. et.al. [DOI] [PubMed] [Google Scholar]
- 16.Guo SS, Chumlea WC. Tracking body mass index in children in relation to overweight in adulthood. AM J Clin Nutr. 1999;70(suppl):145S–8S. doi: 10.1093/ajcn/70.1.145s. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics (Committee on Nutrition) Prevention of pediatric overweight and obesity. Pediatrics. 2003;112:424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker RC, Wright JA, Pepe MS. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. et al. [DOI] [PubMed] [Google Scholar]
- 19.Nationwide Personal Transportation Survey. U.S. Department of Transportation, Federal Highway Administration, Research and Technical Support Center. Lantham, MD: Federal Highway Administration; 1997. [Google Scholar]
- 20.Centers for Disease Control and Prevention. Youth risk behavior surveillance—United States, 1999. Morbidity & Mortality Weekly Report. 2000;49(SS-5):1–94. [PubMed] [Google Scholar]
- 21.Andersen RE, Crespo CJ, Bartlett SJ. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. JAMA. 1998;279:938–942. doi: 10.1001/jama.279.12.938. et al. [DOI] [PubMed] [Google Scholar]
- 22.Dennison BA. Television watching and television in bedroom associated with overweight risk among low-income pre-school children. Pediatrics. 2002;109:1028–1035. doi: 10.1542/peds.109.6.1028. [DOI] [PubMed] [Google Scholar]
- 23.Bergmann KE, Bergmann RL, Von Kries R. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breastfeeding. Int J Obes Relat Metab Disord. 2003;27:162–172. doi: 10.1038/sj.ijo.802200. [DOI] [PubMed] [Google Scholar]
- 24.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Clin Epidemiol. 2005;162:397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 25.Ponza M, Devaney B, Ziegler P. Nutrient intakes and food choices of infants and toddlers participating in WIC. J Am Diet Assoc. 2004;104(suppl 1):s71–79. doi: 10.1016/j.jada.2003.10.018. et al. [DOI] [PubMed] [Google Scholar]
- 26.Skinner JD, Ziegler P, Pac S. Meal and snack patterns of infants and toddlers. J Am Diet Assoc. 2004;104(suppl 1):s65–70. doi: 10.1016/j.jada.2003.10.021. et al. [DOI] [PubMed] [Google Scholar]
- 27.Devaney B, Ziegler P, Pac S. Nutrient intakes of infants and toddlers. J Am Diet Assoc. 2004;104(suppl 1):s14–s21. doi: 10.1016/j.jada.2003.10.022. et al. [DOI] [PubMed] [Google Scholar]
- 28.Gleason P, Suitor C. Children's diets in the mid-1990s: dietary intake and its relationship with school meal participation. Alexandria (VA): US Department of Agriculture; 2001. Report No.: CN-01-CD1. [Google Scholar]
- 29.Mrdjenovic G, Levitsky D. Nutritional and energetic consequences of sweetened drink consumption 6 to 13 year old children. J Pediatr. 2003;142:604–610. doi: 10.1067/mpd.2003.200. [DOI] [PubMed] [Google Scholar]
- 30.James J. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomized controlled trial. BMJ. 2004;328(7450):1237–1239. doi: 10.1136/bmj.38077.458438.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birch LL, Fischer Jo, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls eating in the absence of hunger. Am J Clin Nutr. 2003;78:215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman DS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa heart Study. Pediatrics. 1999;103(6 Pt 1):1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 33.Cook S, Weitzman M, Auinger P. Prevalence of metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. et al. [DOI] [PubMed] [Google Scholar]
- 34.Weiss R, Dziura J, Burgert TS. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. et al. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez J, Redden D, Petrobelli A. Waist circumference percentiles in nationally representative samples of African-American, European American and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. et al. [DOI] [PubMed] [Google Scholar]
- 36.Kahn HS, Imperatore G, Cheng YJ. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J Pediatr. 2005;146:482–488. doi: 10.1016/j.jpeds.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Bacha F, Gungor N. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148:188–194. doi: 10.1016/j.jpeds.2005.10.001. et al. [DOI] [PubMed] [Google Scholar]
- 38.Speiser PW, Rudolph MCJ, Anhalt H. Consensus statement: Childhood obesity. J Clin Endocr Metab. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. et al. [DOI] [PubMed] [Google Scholar]
- 39.Strong WB, Malina RM, Blimkie JR. Physical activity recommendations for school-age youth. J Pediatr. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. et al. [DOI] [PubMed] [Google Scholar]
- 40.Dietz WH. Physical activity recommendations: where do we go from here? J Pediatr. 2005;146:719–720. doi: 10.1016/j.jpeds.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Small E. Physical Activity Basics. Presented at the conference “Fact, Fiction or Truth of Pediatric Obesity” sponsored by the American Academy of Pediatrics October 12, 2004. Available at: http://www.aap.org/peds-21/Peds21Obesity.pdf Accessed April 8, 2007.
- 42.Field AE, Austin SB, Taylor CB. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics. 2003;112:900–926. doi: 10.1542/peds.112.4.900. et al. [DOI] [PubMed] [Google Scholar]
- 43.Krebs N. Role of alternative therapies and fad diets in pediatric obesity. Presented at the conference “Fact, fiction or Truth of Pediatric Obesity” sponsored by the American Academy of Pediatrics October 12, 2004. Available at http://www.aap.org/peds-21/Peds21Obesity.pdf Accessed April 8, 2007.
- 44.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142:253–258. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 45.Schneider MB, Brill SR. Obesity in children and adolescents. Pediatr Rev. 2005;26:155–162. doi: 10.1542/pir.26-5-155. [DOI] [PubMed] [Google Scholar]
- 46.Agatson A. The South Beach Diet: The Delicious, Doctor-designed, Fool Proofed plan for Fast and Healthy Weight Loss. New York, NY: Random House; 2003. [Google Scholar]
- 47.Ludwig DS, Majzoub JA, Al-Zahrani A. High glycemic index foods, overeating and obesity. Pediatrics. 1999;103:e26. doi: 10.1542/peds.103.3.e26. et al. [DOI] [PubMed] [Google Scholar]
- 48.Meridia [package insert] North Chicago, IL: Abbott Laboratories; 2006. [Google Scholar]
- 49.Berkowitz RI, Wadden TA, Tershakovec AM. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289:1805–1812. doi: 10.1001/jama.289.14.1805. et al. [DOI] [PubMed] [Google Scholar]
- 50.Godoy-Matos A, Carraro L, Vieira A. Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2005;90:1460–1465. doi: 10.1210/jc.2004-0263. et al. [DOI] [PubMed] [Google Scholar]
- 51.Berkowitz RI, Fujioka K, Daniels SR. Sibutramine Adolescent Study Group. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145:81–90. doi: 10.7326/0003-4819-145-2-200607180-00005. et al. [DOI] [PubMed] [Google Scholar]
- 52.Xenical [package insert] Nutley, NJ: Roche Laboratories; 2007. [Google Scholar]
- 53.Chanoine JP, Hampl S, Jensen C. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–2883. doi: 10.1001/jama.293.23.2873. et al. [DOI] [PubMed] [Google Scholar]
- 54.Maahs D, de Serna DG, Kolotkin RL. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18–28. doi: 10.4158/EP.12.1.18. et al. [DOI] [PubMed] [Google Scholar]
- 55.FDA/Center for Drug Evaluation and Research. Orlistat OTC (marketed as alli) Information. Available at: http://www.fda.gov/cder/drug/infopage/orlistat_otc/index.htm Accessed April 8, 2007.
- 56.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome. Arch Pediatr Adolesc Med. 2007;161:217–21. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 57.Inge TH, Krebs N, Garcia V. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. doi: 10.1542/peds.114.1.217. et al. [DOI] [PubMed] [Google Scholar]
- 58.Wittgrove AC, Buchwald H, Sugerman H. American Society for Bariatric Surgery. Surgery for severely obese adolescents: further insight from the American Society for Bariatric Surgery. Pediatrics. 2004;114:253–254. doi: 10.1542/peds.114.1.253. e al. [DOI] [PubMed] [Google Scholar]
- 59.Epstein LH, Valoski A, Wing RR. Ten year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264:2519–2523. et al. [PubMed] [Google Scholar]
- 60.Wrotniak B, Epstein LH, Paluch RA. Parent weight change as a predictor of child weight change in family-based behavior obesity treatment. Arch Pediatr Adolesc Med. 2004;158:342–347. doi: 10.1001/archpedi.158.4.342. et al. [DOI] [PubMed] [Google Scholar]


