Abstract
Background
Many studies have investigated the association between the angiotensin-converting enzyme (ACE) gene insertion/deletion (I/D) polymorphism and risk of ischemic stroke. However, the evidence is inadequate to draw robust conclusions because most studies were generally small and conducted in heterogeneous populations. To shed light on these inconclusive findings, we conducted a large meta-analysis of studies relating the ACE I/D polymorphism to the risk of ischemic stroke.
Methods
Relevant studies were identified by searching PubMed and Embase through February 2012 and by reviewing the references of retrieved articles. We included studies that reported odds ratio (OR) with 95% confidence interval (CI) for the association between this polymorphism and ischemic stroke risk.
Results
Fifty independent publications, with 10 070 stroke cases and 22 103 controls, were included. The results indicated that the DD homozygote carriers had a 37% higher risk of ischemic stroke when compared with the homozygotes II and heterozygote ID [odds ratio (OR) = 1.37, 95% confidence interval (CI): 1.22–1.53]. Subgroup analyses indicated that this higher risk was more pronounced among Asians, hospital-based studies, and small vessel disease (SVD). Potential publication bias may exist, but correction for this bias using a formal statistical method did not materially alter the combined risk estimate.
Conclusion
The results of our meta-analysis indicate that the D allele of ACE I/D polymorphism is a low-penetrance susceptibility marker of ischemic stroke.
Introduction
Stroke is a common neurological disease and a leading cause of death and long-term disability worldwide [1]. Strong evidence from genetic association studies indicates that genetic predisposition, in addition to such recognized risk factors as hypertension, smoking, diabetes, obesity, and advanced age, contributes to the development of stroke [2]. Identification and characterization of genetic variations that play such a role may allow improved prognostication, therapy, and prevention.
The renin-angiotensin system (RAS) is a hormonal signaling mechanism implicated in the atherosclerosis and regulation of blood pressure [3]. Angiotensin-converting enzyme (ACE), a key enzyme in the RAS, plays important roles in vascular remodeling, atherosclerosis, and ischemic stroke [4]–[6]. It catalyses the conversion of inactive angiotensin I to active angiotensin II, which is known to be involved in vascular hypertrophy, vasoconstriction, and atherosclerotic processes [7]. The human ACE gene is located on chromosome 17q23, where an insertion/deletion polymorphism (I/D, dbSNP rs4646994) in intron 16 has been identified [8]. This polymorphism is based on the presence (insertion, I) or absence (deletion, D) of a 287-bp DNA fragment. The D allele of this polymorphism has been associated with elevated serum ACE level in a codominant pattern and has been investigated as a potential susceptibility factor for ischemic stroke. A large number of studies have reported the association between the I/D polymorphism of ACE gene and the risk of ischemic stroke, but the results were inconclusive [9]–[12]. The association between this polymorphism with ischemic stroke risk has attracted widespread attention in recent years and has been a research focus. However, each of these studies typically contained a few subjects and therefore was neither adequate nor sufficiently informative to clearly demonstrate an association. Moreover, these studies varied markedly by including different populations, sampling strategies, genotyping procedures, and quality control. Previously published meta-analyses reported significant associations between ACE I/D and risk of ischemic stroke [13]–[19]. However, it remains unclear whether ethnicity, stroke subtype, subject source, and gender could affect the associations. Since then, additional many studies with a large sample size about this polymorphism on ischemic stroke risk have been reported. Subgroup analyses performed by ethnicity, stroke subtype, subject source, and gender were also possible now.
Therefore, we present herein the results of a large meta-analysis of published data investigating the association between ACE I/D and ischemic stroke for various genetic contrasts, in which we explored the between-studies heterogeneity and the existence of potential bias.
Materials and Methods
Literature Search and Selection
Two online electronic databases (PubMed and Embase) were searched for eligible articles through February 2012. The search was limited to English language full-text papers. Abstract, review or editorials were not included. The medical subject headings and terms used for the search were: stroke, brain infarction, and cerebrovascular disease in combination with ACE, angiotensin-converting enzyme, polymorphism, genotype, gene, or mutation. The references of all identified publications were searched for any additional studies, and the related articles option in PubMed was used to search for further potentially relevant articles.
Studies included in our meta-analysis have to meet the following criteria: (1) hypothesis-driven studies specific for ACE I/D polymorphism and provided cases of ischemic stroke (large-artery atherosclerosis, cardioembolic stroke, small-vessel stroke, or other determined and undetermined causes) and control subjects (population- or hospital-based controls), (2) had neuroimaging (CT or MRI) confirmation of an ischemic stroke diagnosis, (3) evaluation of ACE I/D polymorphism and ischemic stroke risk and (4) sufficient data for examining an odds ratio (OR) with 95% confidence interval (CI). Studies were excluded if: (1) patients were under 18 years of age, or (2) original genotype data was not reported. For duplicate publications, the smaller dataset was discarded.
Data Extraction
Two investigators (Z.Z. and G.X.) independently extracted data and reached a consensus on all of the items. For each study, the following characteristics were collected: the first author's last name, year of publication, country of origin, ethnicity, matching conditions, numbers of genotyped cases and controls, the genotype distribution of cases and controls for the ACE I/D polymorphism, source of control groups (population- or hospital-based controls), and genotyping methods. Different ethnic descents were categorized as European, Asian and African.
Statistical Analysis
For each study, we first examined whether the genotype distribution in controls was consistent with Hardy-Weinberg equilibrium (HWE) by χ2 test. To measure the strength of genetic association for ACE I/D polymorphism, the ORs, together with the 95% CIs were calculated. The statistical significance of the summary OR was determined with the Z test, and P<0.05 was considered as statistically significant. We first estimated the risks of the ID and DD genotypes on strokes, compared with the wild-type II homozygote, and then evaluated the risks of (ID/DD) vs II and DD vs (ID/II) on strokes, assuming dominant and recessive effects of the variant D allele, respectively. We also estimated the risks of D allele vs A allele. In addition, stratified analyses were performed by ethnicity, source of controls, subtype, gender, and HWE.
Heterogeneity among studies was assessed with the Q-test and I 2 statistics [20]. If there was no significant heterogeneity, the fixed-effects model (the Mantel-Haenszel method) was used to estimate the summary OR. Otherwise, the random-effects model (the DerSimonian and Laird method) was adopted. We conducted stratified analyses to explore possible explanations for heterogeneity and to test the robustness of the association.
Cumulative and recursive cumulative meta-analysis were performed to provide a framework for updating a genetic effect from all studies and to measure how much the genetic effect changes as evidence accumulates [21], [22]. Therefore, cumulative meta-analysis demonstrates the trend in risk effect, and recursive cumulative meta-analysis indicates the stability in risk effect.
We also performed sensitivity analyses to evaluate the stability of the results. A single study involved in the meta-analysis was deleted each time to reflect the influence of the individual data to the pooled ORs. For assessment of potential publication bias, we used funnel plots and Egger's linear regression test. Moreover, we performed the Duval and Tweedie nonparametric trim and fill procedure to further assess potential effects of publication bias [23]. This method considers the possibility of hypothetical missing studies, and recalculates a pooled estimate. All analyses were done with STATA version 11.0 (StataCorp, College Station, TX).
Results
Literature Search
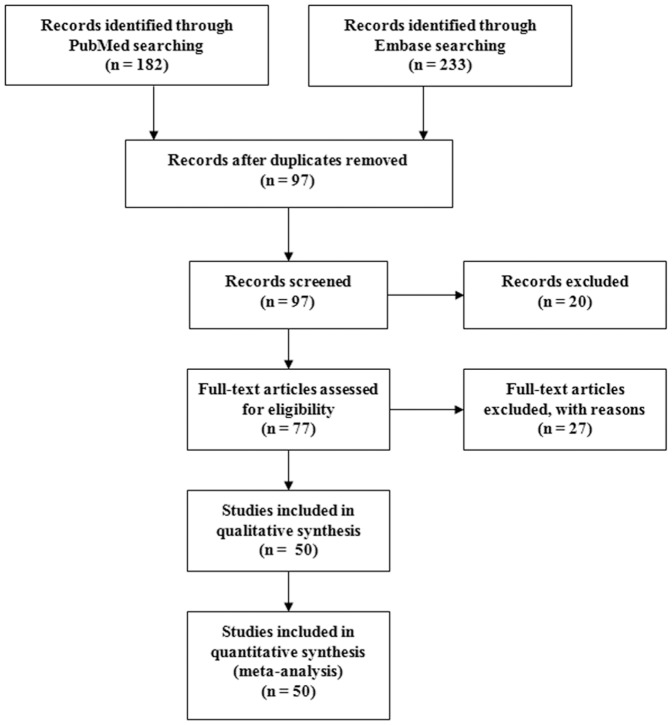
A flow diagram of the literature search is shown in Figure 1. Total searches yielded 415 entries. Of these, 318 studies were excluded after reading the title or abstract because of obvious irrelevance to our study aim. Ninety-seven studies appeared to be potentially relevant for inclusion in our study. Twenty studies were excluded because of overlapping cases or their data were not extractable. Seventy-seven full-text articles were reviewed. Twenty-seven studies were further excluded for the following reasons: abstract, review or editorials (n = 22); no control population (n = 4); or children (n = 1). Therefore, a total of 50 studies met the inclusion criteria [9]–[12], [24]–[69].
Figure 1. Flow diagram of the literature search.
Study Characteristics
The characteristics of included studies are summarized in Table 1. The 50 included studies were published between 1994 and 2011 and comprised a total of 10 070 cases and 22 103 controls. There were 26 studies of Asian descendents, 23 studies of European descendents, and 1 study of African descendents. Of the 50 studies, 42 studies used frequency-matched controls to the cases by the age or sex. A classic polymerase chain reaction assay was performed in all of the 50 studies; however, only 20 (40%) studies mentioned quality control on genotyping. The genotype distributions among the controls of all studies were in agreement with HWE except for eight studies.
Table 1. Main characteristics of selected studies.
| First author | Year | Country | Ethnicity | Sample size | Matching criteria | Genotyping method | Quality control | |
| case | control | |||||||
| Sharma [9] | 1994 | UK | European | 100 | 73 | Age and sex | PCR | No |
| Ueda [24] | 1995 | UK | European | 488 | 188 | Age and sex | PCR | Yes |
| Catto [25] | 1996 | UK | European | 406 | 215 | Age and sex | PCR | Yes |
| Margaglione [27] | 1996 | Italy | European | 101 | 109 | Sex | PCR | No |
| Kario [26] | 1996 | Japan | Asian | 138 | 104 | Age and sex | PCR | Yes |
| Agerholm-Larsen [28] | 1997 | Denmark | European | 452 | 9038 | Age | PCR | Yes |
| Nakata [30] | 1997 | Japan | Asian | 55 | 61 | Age and sex | PCR | No |
| Doi [29] | 1997 | Japan | Asian | 181 | 271 | Age and sex | PCR | Yes |
| Seino [32] | 1998 | Japan | Asian | 26 | 28 | Age and sex | PCR | No |
| Pfohl [31] | 1998 | Germany | European | 91 | 297 | – | PCR | Yes |
| Shen [33] | 1998 | China | Asian | 44 | 62 | Age and sex | PCR | No |
| Xu [34] | 1998 | China | Asian | 65 | 117 | Age | PCR | No |
| Kostulas [35] | 1999 | Sweden | European | 96 | 93 | Age and sex | PCR | No |
| Notsu [10] | 1999 | Japan | Asian | 175 | 213 | Sex | PCR | Yes |
| Zee [11] | 1999 | USA | European | 338 | 338 | Age | PCR | Yes |
| Lin [36] | 2000 | China | Asian | 306 | 300 | Age and sex | PCR | Yes |
| Wei [37] | 2000 | China | Asian | 87 | 257 | Age | PCR | No |
| Zhang [39] | 2001 | China | Asian | 165 | 106 | Age and sex | PCR | No |
| Zhang [38] | 2001 | China | Asian | 74 | 72 | Sex | PCR | No |
| Ohkubo [40] | 2002 | Japan | Asian | 69 | 294 | Age and sex | PCR | No |
| Szolnoki [41] | 2003 | Hungary | European | 867 | 743 | Age and sex | PCR | No |
| UM [42] | 2003 | Korea | Asian | 208 | 636 | Age and sex | PCR | No |
| Yuan [43] | 2003 | China | Asian | 122 | 1229 | Age | PCR | No |
| Zhang [47] | 2004 | Japan | Asian | 151 | 150 | Age and sex | PCR | Yes |
| Karagiannis [44] | 2004 | Greece | European | 100 | 100 | Age and sex | PCR | Yes |
| Wang [46] | 2004 | China | Asian | 46 | 43 | – | PCR | No |
| Rubattu [45] | 2004 | Italy | European | 215 | 236 | Age | PCR | Yes |
| Brenner [48] | 2005 | France | European | 459 | 459 | Age and sex | PCR | Yes |
| Pera [51] | 2006 | Poland | European | 368 | 556 | Age and sex | PCR | No |
| Dikmen [49] | 2006 | Turkey | European | 141 | 50 | – | PCR | No |
| Gao [50] | 2006 | China | Asian | 100 | 100 | Age and sex | PCR | Yes |
| Tuncer [52] | 2006 | Turkey | European | 108 | 79 | Age and sex | PCR | No |
| Tseng [58] | 2007 | China | Asian | 92 | 780 | – | PCR | Yes |
| Gormley [54] | 2007 | UK | European | 299 | 600 | Age and sex | PCR | No |
| Li [56] | 2007 | China | Asian | 454 | 334 | Sex | PCR | No |
| Lalouschek [55] | 2007 | Austria | European | 450 | 817 | – | PCR | Yes |
| Polupanov [57] | 2007 | Kirghiz | Asian | 69 | 64 | Age | PCR | No |
| Gawel [53] | 2007 | Poland | European | 66 | 45 | Age | PCR | No |
| Hong [59] | 2008 | Korea | Asian | 232 | 225 | Age and sex | PCR | No |
| Munshi [61] | 2008 | India | Asian | 162 | 150 | Age and sex | PCR | No |
| Mollsten [60] | 2008 | Sweden | European | 222 | 542 | Age and sex | PCR | Yes |
| Tascilar [64] | 2009 | Turkey | European | 157 | 85 | – | PCR | No |
| Saidi [63] | 2009 | Tunisia | African | 228 | 323 | Age and sex | PCR | No |
| Celiker [62] | 2009 | Turkey | European | 162 | 107 | – | PCR | No |
| Li [66] | 2010 | China | Asian | 76 | 311 | Age and sex | PCR | Yes |
| Domingues-Montanari [65] | 2010 | Spain | European | 519 | 540 | – | PCR | No |
| Kalita [12] | 2011 | India | Asian | 193 | 188 | Age and sex | PCR | Yes |
| Chutinet [67] | 2011 | Thailand | Asian | 141 | 167 | Age | PCR | No |
| Markoula [69] | 2011 | Greece | European | 176 | 178 | Age and sex | PCR | Yes |
| Indrajaya [68] | 2011 | Indonesia | Asian | 30 | 30 | Age and sex | PCR | No |
Quantitative Synthesis
Overall, the variant genotypes of ACE I/D polymorphism were associated with a significantly higher risk of ischemic stroke in different genetic models when all the eligible studies were pooled into the meta-analysis. As shown in Table 2, the variant heterozygote ID and homozygote DD, were associated with a significantly higher risk of ischemic stroke in a dose-response manner, compared with the wild-type homozygote II (OR = 1.16, 95% CI: 1.06–1.26 for ID and 1.54, 1.34–1.78 for DD; P trend<0.001). In addition, significant main effects were also observed in dominant model, recessive model and allele contrast model (OR = 1.29, 95% CI: 1.17–1.43, OR = 1.37, 95% CI: 1.22–1.53 and OR = 1.27, 95% CI: 1.17–1.37, respectively).
Table 2. Stratification analyses of the ACE I/D polymorphism on stroke risk.
| Variables | n | ID vs II | DD vs II | DD/ID vs II (dominant) | DD vs ID/II (recessive) | D vs I allele | |||||
| OR (95% CI) | P * | OR (95% CI) | P * | OR (95% CI) | P * | OR (95% CI) | P * | OR (95% CI) | P * | ||
| Total | 50 | 1.16 (1.06–1.26) | 0.017 | 1.54 (1.34–1.78) | <0.001 | 1.29 (1.17–1.43) | <0.001 | 1.37 (1.22–1.53) | <0.001 | 1.27 (1.17–1.37) | <0.001 |
| Ethnicities | |||||||||||
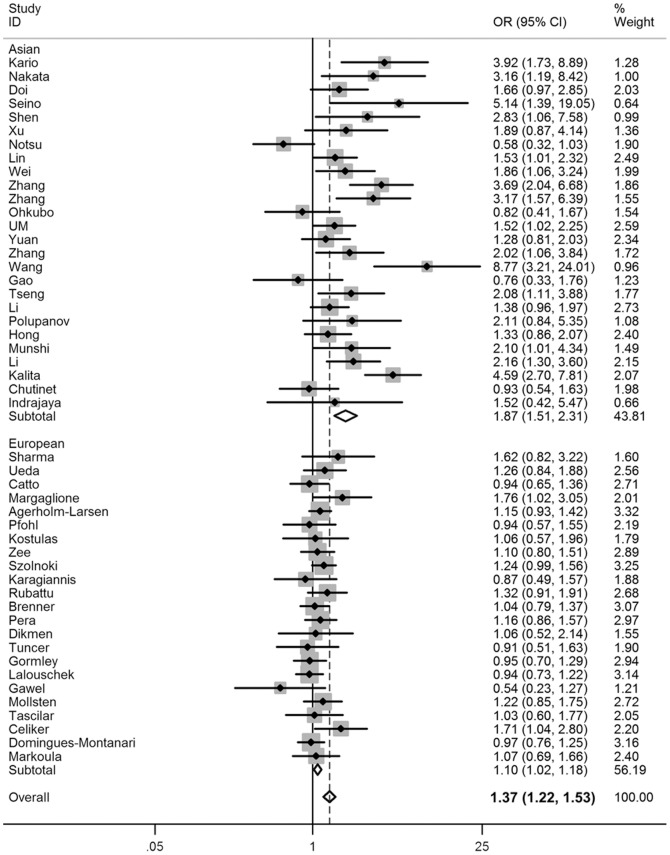
| Asian | 26 | 1.30 (1.12–1.50) | 0.010 | 2.20 (1.70–2.84) | <0.001 | 1.54 (1.30–1.82) | <0.001 | 1.87 (1.51–2.31) | <0.001 | 1.52 (1.33–1.75) | 0.185 |
| European | 23 | 1.03 (0.94–1.12) | 0.555 | 1.12 (1.01–1.23) | 0.627 | 1.06 (0.98–1.15) | 0.502 | 1.10 (1.02–1.18) | 0.684 | 1.06 (1.01–1.12) | 0.465 |
| Source of control | |||||||||||
| HB | 28 | 1.14 (1.04–1.25) | 0.200 | 1.75 (1.41–2.16) | <0.001 | 1.33 (1.16–1.54) | <0.001 | 1.53 (1.30–1.79) | <0.001 | 1.34 (1.20–1.50) | <0.001 |
| PB | 22 | 1.17 (1.01–1.35) | 0.009 | 1.34 (1.11–1.62) | <0.001 | 1.24 (1.08–1.44) | 0.001 | 1.21 (1.04–1.41) | <0.001 | 1.19 (1.07–1.32) | <0.001 |
| HWE | |||||||||||
| Yes | 42 | 1.16 (1.06–1.28) | 0.014 | 1.55 (1.32–1.80) | <0.001 | 1.29 (1.16–1.43) | <0.001 | 1.37 (1.22–1.54) | <0.001 | 1.26 (1.16–1.36) | <0.001 |
| No | 8 | 1.13 (0.91–1.40) | 0.246 | 1.57 (0.99–2.48) | 0.005 | 1.34 (0.95–1.90) | 0.011 | 1.42 (0.97–2.10) | 0.003 | 1.36 (1.02–1.84) | <0.001 |
| Subtype | |||||||||||
| SVD | 17 | 1.16 (1.00–1.33) | 0.128 | 1.45 (1.13–1.87) | 0.011 | 1.25 (1.04–1.50) | 0.045 | 1.30 (1.06–1.59) | 0.006 | 1.20 (1.05–1.36) | 0.003 |
| LVD | 17 | 1.09 (0.94–1.26) | 0.141 | 1.47 (1.07–2.04) | <0.001 | 1.24 (0.99–1.53) | 0.014 | 1.36 (1.04–1.76) | <0.001 | 1.23 (1.04–1.46) | <0.001 |
| Gender | |||||||||||
| Male | 5 | 1.16 (0.76–1.79) | 0.035 | 1.11 (0.83–1.49) | 0.078 | 1.20 (0.78–1.85) | 0.018 | 1.04 (0.82–1.32) | 0.476 | 1.13 (0.87–1.45) | 0.041 |
| Female | 5 | 1.26 (0.92–1.73) | 0.090 | 2.16 (0.86–5.41) | 0.003 | 1.61 (0.87–2.99) | 0.014 | 1.46 (0.87–2.46) | 0.050 | 1.37 (0.93–2.02) | 0.008 |
P value of Q-test for heterogeneity test.
HB: Hospital-based; PB: Population-based; SVD: small vessel disease; LVD: large vessel disease.
In the stratified analysis by ethnicity, significantly higher risks were found in Asians (ID vs II: OR = 1.30, 95% CI = 1.12–1.50; DD vs II: OR = 2.20, 95% CI = 1.70–2.84; dominant model: OR = 1.54, 95% CI = 1.30–1.82; recessive model: OR = 1.87, 95% CI = 1.51–2.31; allele model: OR = 1.52, 95% CI = 1.33–1.75) but with borderline statistical significance in Europeans (DD vs II: OR = 1.12, 95% CI = 1.01–1.23; recessive model: OR = 1.10, 95% CI = 1.02–1.18; allele model: OR = 1.06, 95% CI = 1.01–1.12; Fig. 2). Moreover, when stratified by source of control, statistically significantly elevated risk was also observed, and this elevated risk was more pronounced among hospital-based studies (Table 2).
Figure 2. Forest plot of stroke risk associated with the ACE I/D polymorphism (DD vs ID/II).
In the present meta-analysis, seventeen studies provided detailed genotype information according to stroke subtype. As shown in Table 2, significantly higher stroke risk was found in small vessel disease (SVD) but with borderline statistical significance in large vessel disease (LVD). However, in the subgroup analysis by gender, we did not find significant associations in any genetic models.
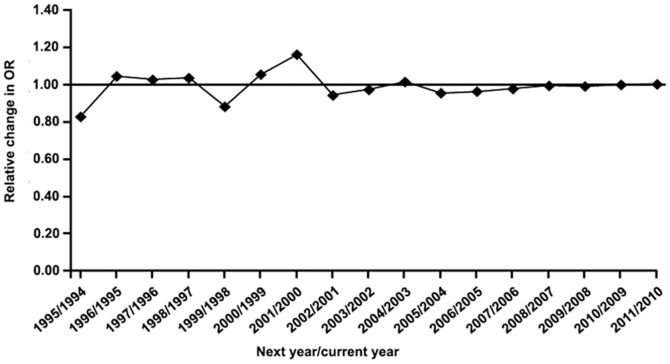
The cumulative meta-analysis for the recessive model showed a trend of association as published data information accumulated (Fig. 3). In recursive cumulative meta-analysis, the relative change in the random effects ORs fluctuated around 1.00 until 2008 and then stabilized, indicating that there is sufficient evidence for investigating the association (Fig. 4).
Figure 3. Results of the cumulative meta-analysis.
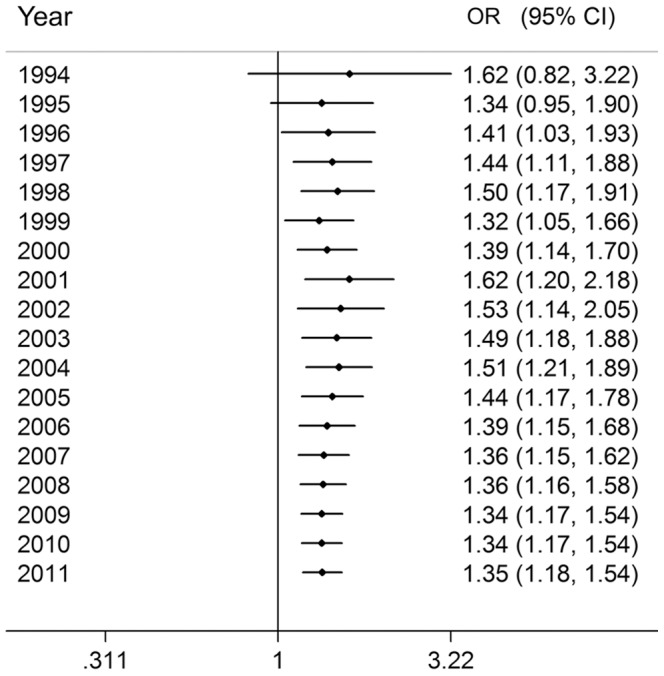
The random effects pooled OR with 95% CI at the end of each information step is shown.
Figure 4. Results of the recursive cumulative meta-analysis.
The relative change in random effects pooled OR in each information step (OR in the next year/OR in the current year) for the recessive model is shown.
Test of Heterogeneity
Obvious heterogeneity between studies was observed in overall and subgroup analyses (P<0.05, I 2>30%, Table 2). Then, we assessed the source of heterogeneity for recessive model comparison by ethnicity, sample size (subjects >800), and source of controls. As a result, ethnicity (χ2 = 33.13, df = 2, P<0.001) and source of controls (χ2 = 13.99, df = 1, P<0.001), but not the sample size (χ2 = 3.13, df = 1, P = 0.077), was found to contribute to substantial heterogeneity.
Sensitivity Analyses
Sensitivity analyses indicated that four independent studies [10], [12], [39], [46] were the main origin of the heterogeneity in Asians. The heterogeneity was effectively lowered or removed after exclusion of these four studies (DD vs ID/II: P heterogeneity = 0.081). Although the genotype distributions in eight of the included studies did not follow HWE, the corresponding pooled ORs were not materially altered with or without including these studies (Table 2). In further sensitivity analysis in which 1 study at a time was removed and the rest analyzed, the pooled ORs ranged from 1.32 to 1.39, indicating that the pooled estimate was robust and not influenced by a single study.
Publication Bias
Visual inspection of the Begg's funnel plot showed some asymmetry. Then, the Egger's test was used to provide statistical evidence of funnel plot asymmetry. As expected, the results indicated an obvious evidence of publication bias (t = 3.70, P = 0.001 for DD vs ID/II). Random-effects OR corrected for publication bias using the trim and fill method was 1.19 (95% CI, 1.05–1.35) for all studies combined. Correction for potential publication bias therefore did not materially alter the combined risk estimate.
Discussion
The present meta-analysis, including 10 070 cases and 22 103 controls from 50 published studies, explored the association between the ACE I/D polymorphism and stroke risk. We found that the variant genotypes of this polymorphism were associated with significant increase in overall stroke risk.
ACE activates angiotensin I and inactivates bradykinin, resulting in decreased tissue perfusion, vascular smooth muscle cell growth [70], and stimulation of plasminogen-activator inhibitor type I [71]. Moreover, plasma ACE concentration is an important factor in cardiovascular and cerebrovascular risk profiling, since chronic exposure to high levels of plasma ACE may result in vascular wall thickness and stiffness [72]. Thus, the ACE gene is a good candidate gene for ischemic stroke. Early studies demonstrated a strong correlation between the D allele and levels of circulating, intracellular, and tissue activity of ACE [8], [73]. Since both alleles have codominant effects on ACE levels, individuals who are homozygous for the D allele have the highest levels of the enzyme, those homozygous for the I allele have the lowest, and heterozygous individuals have an intermediate level. Given the important roles of ACE in the pathogenesis of cerebrovascular disease, it is biologically plausible that ACE polymorphism may modulate the risk of ischemic stroke. In present meta-analysis, we found that variant genotypes of ACE I/D polymorphism were associated with higher stroke risk, which was consistent with experimental findings.
Significant associations were found in Asians but with borderline statistical significance in Europeans, suggesting a possible role of ethnic differences in genetic backgrounds and the environment they lived in. The impact of this polymorphism may be masked by the presence of other as-yet unidentified causal genes involved in stroke development in Europeans. Other factors such as selection bias, different matching criteria may also play a role. The above differences may lead to the inconsistent results. In addition, there is only one reported study using African population for this polymorphism. So it is also likely that the observed ethnic differences may be due to chance because studies with small sample size may have low statistical power to detect a slight effect. Thus, additional studies are warranted to further validate ethnic difference in the effect of this polymorphism on stroke risk, especially in Africans.
In the subgroup analysis by subtype, significantly higher stroke risk was observed in SVD but with borderline statistical significance in LVD. Stroke is a heterogenous disease and it is possible that ACE I/D polymorphism may play different roles in the differing subtypes of strokes. Our findings indicate that genetic risk factors for different subtypes are likely different, supporting the view that they are pathologically distinct entities, with SVD having a greater genetic liability compared to LVD.
No significant association between variant genotypes and stroke risk was observed when the included studies were stratified by gender. The null result may be due to limited number of studies with available data, which had insufficient statistical power to detect a slight effect or may have generated a fluctuated risk estimate.
Our findings confirmed results from previous meta-analyses. With the accumulative evidence, we were able to enhance the precision of the risk estimates and perform subgroup analyses to explore sources of heterogeneity, thereby increasing the clinical relevance of our findings. However, some limitations of this meta-analysis should be addressed. First, lacking of the original data of the reviewed studies limited our further evaluation of potential interactions, because the gene-gene interaction and gene-environment interaction may modulate stroke risk. Second, a potential publication bias may exist, as shown by the funnel plot and the Egger's test. Nevertheless, correction for this bias using the trim and fill method did not materially alter the combined risk estimate.
In conclusion, this meta-analysis provided evidence of the association between the ACE I/D polymorphism and stroke risk, supporting the hypothesis that the ACE I/D polymorphism may be a low-penetrance susceptibility marker of stroke. However, additional large studies are warranted to validate our findings. Future studies should use standardized genotyping methods and homogeneous patients and well-matched controls and include multi-ethnic populations. Furthermore, detailed gene-gene interaction and gene-environment interaction should also be considered in future studies, which should lead to better understanding of the association between the ACE I/D polymorphism and stroke risk.
Funding Statement
This study was supported by National Natural Science Foundation of China (31200938, 31171016, 81070922, 81171099), Natural Science Foundation of Jiangsu Province (BK2011021), and Natural Science Foundation of Jinling Hospital (2012009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, et al. (2004) The global stroke initiative. Lancet Neurol 3: 391–393. [DOI] [PubMed] [Google Scholar]
- 2. Sharma P (1996) Genes for ischaemic stroke: strategies for their detection. J Hypertens 14: 277–285. [DOI] [PubMed] [Google Scholar]
- 3. Jiang X, Sheng H, Li J, Xun P, Cheng Y, et al. (2009) Association between renin-angiotensin system gene polymorphism and essential hypertension: a community-based study. J Hum Hypertens 23: 176–181. [DOI] [PubMed] [Google Scholar]
- 4. Morishita R, Gibbons GH, Ellison KE, Lee W, Zhang L, et al. (1994) Evidence for direct local effect of angiotensin in vascular hypertrophy. In vivo gene transfer of angiotensin converting enzyme. J Clin Invest 94: 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pitt B (1994) Angiotensin-converting enzyme inhibitors in patients with coronary atherosclerosis. Am Heart J 128: 1328–1332. [DOI] [PubMed] [Google Scholar]
- 6. Raynolds MV, Bristow MR, Bush EW, Abraham WT, Lowes BD, et al. (1993) Angiotensin-converting enzyme DD genotype in patients with ischaemic or idiopathic dilated cardiomyopathy. Lancet 342: 1073–1075. [DOI] [PubMed] [Google Scholar]
- 7. Kim S, Iwao H (2000) Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 52: 11–34. [PubMed] [Google Scholar]
- 8. Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, et al. (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86: 1343–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma P, Carter ND, Barley J, Brown MM (1994) Molecular approach to assessing the genetic risk of cerebral infarction: deletion polymorphism in the gene encoding angiotensin 1-converting enzyme. J Hum Hypertens 8: 645–648. [PubMed] [Google Scholar]
- 10. Notsu Y, Nabika T, Park HY, Masuda J, Kobayashi S (1999) Evaluation of Genetic Risk Factors for Silent Brain Infarction. Stroke 30: 1881–1886. [DOI] [PubMed] [Google Scholar]
- 11. Zee RY, Ridker PM, Stampfer MJ, Hennekens CH, Lindpaintner K (1999) Prospective evaluation of the angiotensin-converting enzyme insertion/deletion polymorphism and the risk of stroke. Circulation 99: 340–343. [DOI] [PubMed] [Google Scholar]
- 12. Kalita J, Somarajan BI, Kumar B, Mittal B, Misra UK (2011) A study of ACE and ADD1 polymorphism in ischemic and hemorrhagic stroke. Clin Chim Acta 412: 642–646. [DOI] [PubMed] [Google Scholar]
- 13. Sharma P (1998) Meta-analysis of the ACE gene in ischaemic stroke. J Neurol Neurosurg Psychiatry 64: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ariyaratnam R, Casas JP, Whittaker J, Smeeth L, Hingorani AD, et al. (2007) Genetics of ischaemic stroke among persons of non-European descent: a meta-analysis of eight genes involving approximately 32,500 individuals. PLoS Med 4: e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banerjee I, Gupta V, Ganesh S (2007) Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: a meta-analysis. J Hum Genet 52: 205–219. [DOI] [PubMed] [Google Scholar]
- 16. Rao R, Tah V, Casas JP, Hingorani A, Whittaker J, et al. (2009) Ischaemic stroke subtypes and their genetic basis: a comprehensive meta-analysis of small and large vessel stroke. Eur Neurol 61: 76–86. [DOI] [PubMed] [Google Scholar]
- 17. Bentley P, Peck G, Smeeth L, Whittaker J, Sharma P (2010) Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants. PLoS One 5: e9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casas JP, Hingorani AD, Bautista LE, Sharma P (2004) Meta-analysis of genetic studies in ischemic stroke: thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol 61: 1652–1661. [DOI] [PubMed] [Google Scholar]
- 19. Xu X, Li J, Sheng W, Liu L (2008) Meta-analysis of genetic studies from journals published in China of ischemic stroke in the Han Chinese population. Cerebrovasc Dis 26: 48–62. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, et al. (1992) Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 327: 248–254. [DOI] [PubMed] [Google Scholar]
- 22. Ioannidis J, Lau J (2001) Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses. Proc Natl Acad Sci U S A 98: 831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 24. Ueda S, Weir CJ, Inglis GC, Murray GD, Muir KW, et al. (1995) Lack of association between angiotensin converting enzyme gene insertion/deletion polymorphism and stroke. J Hypertens 13: 1597–1601. [PubMed] [Google Scholar]
- 25. Catto A, Carter AM, Barrett JH, Stickland M, Bamford J, et al. (1996) Angiotensin-converting enzyme insertion/deletion polymorphism and cerebrovascular disease. Stroke 27: 435–440. [PubMed] [Google Scholar]
- 26. Kario K, Kanai N, Saito K, Nago N, Matsuo T, et al. (1996) Ischemic stroke and the gene for angiotensin-converting enzyme in Japanese hypertensives. Circulation 93: 1630–1633. [DOI] [PubMed] [Google Scholar]
- 27. Margaglione M, Celentano E, Grandone E, Vecchione G, Cappucci G, et al. (1996) Deletion polymorphism in the angiotensin-converting enzyme gene in patients with a history of ischemic stroke. Arterioscler Thromb Vasc Biol 16: 304–309. [DOI] [PubMed] [Google Scholar]
- 28. Agerholm-Larsen B, Tybjaerg-Hansen A, Frikke-Schmidt R, Gronholdt ML, Jensen G, et al. (1997) ACE gene polymorphism as a risk factor for ischemic cerebrovascular disease. Ann Intern Med 127: 346–355. [DOI] [PubMed] [Google Scholar]
- 29. Doi Y, Yoshinari M, Yoshizumi H, Ibayashi S, Wakisaka M, et al. (1997) Polymorphism of the angiotensin-converting enzyme (ACE) gene in patients with thrombotic brain infarction. Atherosclerosis 132: 145–150. [DOI] [PubMed] [Google Scholar]
- 30. Nakata Y, Katsuya T, Rakugi H, Takami S, Sato N, et al. (1997) Polymorphism of angiotensin converting enzyme, angiotensinogen, and apolipoprotein E genes in a Japanese population with cerebrovascular disease. Am J Hypertens 10: 1391–1395. [DOI] [PubMed] [Google Scholar]
- 31. Pfohl M, Fetter M, Koch M, Barth CM, Rudiger W, et al. (1998) Association between angiotensin I-converting enzyme genotypes, extracranial artery stenosis, and stroke. Atherosclerosis 140: 161–166. [DOI] [PubMed] [Google Scholar]
- 32. Seino Y, Ikeda U, Maeda Y, Haga Y, Yashima H, et al. (1998) Angiotensin-Converting Enzyme Gene Polymorphism and Plasminogen Activator Inhibitor 1 Levels in Subjects with Cerebral Infarction. J Thromb Thrombolysis 5: 263–267. [DOI] [PubMed] [Google Scholar]
- 33. Shen D, Ha D (1998) The relationship between angiotensin-converting enzyme gene polymorphism and brain infarction in Chinese hypertensives. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 15: 136–138. [PubMed] [Google Scholar]
- 34. Xu Y, Wang X, Zhu J, Wang Y, Dai L (1998) Angiotensin converting enzyme gene polymorphism and cerebrovascular disease. Chin J Neurol 31: 152–155. [Google Scholar]
- 35. Kostulas K, Huang WX, Crisby M, Jin YP, He B, et al. (1999) An angiotensin-converting enzyme gene polymorphism suggests a genetic distinction between ischaemic stroke and carotid stenosis. Eur J Clin Invest 29: 478–483. [DOI] [PubMed] [Google Scholar]
- 36. Lin JJ, Yueh KC, Lin GY, Chang DC, Chang CY, et al. (2000) Lack of association between angiotensin I-converting enzyme gene deletion polymorphism and cerebrovascular disease in Taiwanese. J Formos Med Assoc 99: 895–901. [PubMed] [Google Scholar]
- 37. Wei X, Wang G, Jiang C, Li D, Zhao G (2000) Association between hypertensive cerebrovascular stroke and renin-angiotensin system gene polymorphism from Chinese cohort in Shanghai. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 17: 256–258. [PubMed] [Google Scholar]
- 38. Zhang X, Wang D, Xu L, Ma Y, Zhang S (2001) Association between renin-angiotensin system gene polymorphism and type 2 diabetics with stroke in China. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 18: 462–466. [PubMed] [Google Scholar]
- 39. Zhang X, Xia J, Jin D (2001) The relationship between angiotensin converting enzyme gene polymorphism and risk factors for cerebral infarct. Zhonghua Liu Xing Bing Xue Za Zhi 22: 435–438. [PubMed] [Google Scholar]
- 40. Ohkubo R, Nakagawa M, Ikeda K, Kodama T, Arimura K, et al. (2002) Cerebrovascular disorders and genetic polymorphisms: mitochondrial DNA5178C is predominant in cerebrovascular disorders. J Neurol Sci 198: 31–35. [DOI] [PubMed] [Google Scholar]
- 41. Szolnoki Z, Somogyvari F, Kondacs A, Szabo M, Fodor L, et al. (2003) Evaluation of the modifying effects of unfavourable genotypes on classical clinical risk factors for ischaemic stroke. J Neurol Neurosurg Psychiatry 74: 1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Um JY, Joo JC, Kim KY, An NH, Lee KM, et al. (2003) Angiotensin converting enzyme gene polymorphism and traditional Sasang classification in Koreans with cerebral infarction. Hereditas 138: 166–171. [DOI] [PubMed] [Google Scholar]
- 43. Yuan XD, Hou QX, Wu SL, Pei HZ, Li HF (2003) A cross-sectional study on angiotensin-converting enzyme and angiotensin II type I receptor gene polymorphism and cerebral infarction. Zhonghua Liu Xing Bing Xue Za Zhi 24: 822–826. [PubMed] [Google Scholar]
- 44. Karagiannis A, Balaska K, Tziomalos K, Tokalaki-Nikolaidou L, Papayeoryiou A, et al. (2004) Lack of an association between angiotensin-converting enzyme gene insertion/deletion polymorphism and ischaemic stroke. Eur Neurol 51: 148–152. [DOI] [PubMed] [Google Scholar]
- 45. Rubattu S, Di Angelantonio E, Stanzione R, Zanda B, Evangelista A, et al. (2004) Gene polymorphisms of the renin-angiotensin-aldosterone system and the risk of ischemic stroke: a role of the A1166C/AT1 gene variant. J Hypertens 22: 2129–2134. [DOI] [PubMed] [Google Scholar]
- 46. Wang YM, Liu XD, Dong WW, Yang ZC (2004) The relationship between angiotensin-converting enzyme gene polymorphism and heart rate variability in cerebral stroke. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 21: 156–160. [PubMed] [Google Scholar]
- 47. Zhang JH, Kohara K, Yamamoto Y, Nakura J, Tabara Y, et al. (2004) Genetic predisposition to neurological symptoms in lacunar infarction. Cerebrovasc Dis 17: 273–279. [DOI] [PubMed] [Google Scholar]
- 48. Brenner D, Labreuche J, Poirier O, Cambien F, Amarenco P (2005) Renin-angiotensin-aldosterone system in brain infarction and vascular death. Ann Neurol 58: 131–138. [DOI] [PubMed] [Google Scholar]
- 49. Dikmen M, Gunes HV, Degirmenci I, Ozdemir G, Basaran A (2006) Are the angiotensin-converting enzyme gene and activity risk factors for stroke? Arq Neuropsiquiatr 64: 211–216. [DOI] [PubMed] [Google Scholar]
- 50. Gao X, Yang H, ZhiPing T (2006) Association studies of genetic polymorphism, environmental factors and their interaction in ischemic stroke. Neurosci Lett 398: 172–177. [DOI] [PubMed] [Google Scholar]
- 51. Pera J, Slowik A, Dziedzic T, Wloch D, Szczudlik A (2006) ACE I/D polymorphism in different etiologies of ischemic stroke. Acta Neurol Scand 114: 320–322. [DOI] [PubMed] [Google Scholar]
- 52. Tuncer N, Tuglular S, Kilic G, Sazci A, Us O, et al. (2006) Evaluation of the angiotensin-converting enzyme insertion/deletion polymorphism and the risk of ischaemic stroke. J Clin Neurosci 13: 224–227. [DOI] [PubMed] [Google Scholar]
- 53. Gawel B, Wajgt A, Gałka S, Głogowska-Ligus J, Mazurek U, et al. (2007) Polymorphism of the angiotensin-converting enzyme encoding gene in stroke patients. Udar Mozgu - Problemy Interdyscyplinarne 9: 8–13. [Google Scholar]
- 54. Gormley K, Bevan S, Markus HS (2007) Polymorphisms in genes of the renin-angiotensin system and cerebral small vessel disease. Cerebrovasc Dis 23: 148–155. [DOI] [PubMed] [Google Scholar]
- 55. Lalouschek W, Endler G, Schillinger M, Hsieh K, Lang W, et al. (2007) Candidate genetic risk factors of stroke: results of a multilocus genotyping assay. Clin Chem 53: 600–605. [DOI] [PubMed] [Google Scholar]
- 56. Li CM, Zhang C, Lu XL, Feng HY, Zeng Y, et al. (2007) Association between angiotensin-converting enzyme and polymorphisms of N5, N10-methylenetetrahydrofolic acid reductase gene in patients with ischemic stroke. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 29: 359–363. [PubMed] [Google Scholar]
- 57. Polupanov A, Halmatov A, Pak O, Romanova T, Kim E, et al. (2007) The I/D polymorphism of the angiotensin converting enzyme gene as a risk factor for ischemic stroke in patients with essential hypertension in Kyrgyz population. Turk Kardiyoloji Dernegi Arsivi 35: 347–353. [Google Scholar]
- 58. Tseng CH, Tseng CP, Chong CK, Sheu JJ, Cheng JC (2007) Angiotensin-converting enzyme gene polymorphism and stroke in type 2 diabetic patients in Taiwan. Eur J Clin Invest 37: 483–491. [DOI] [PubMed] [Google Scholar]
- 59. Hong SH, Park HM, Ahn JY, Kim OJ, Hwang TS, et al. (2008) ACE I/D polymorphism in Korean patients with ischemic stroke and silent brain infarction. Acta Neurol Scand 117: 244–249. [DOI] [PubMed] [Google Scholar]
- 60. Mollsten A, Stegmayr B, Wiklund PG (2008) Genetic polymorphisms in the renin-angiotensin system confer increased risk of stroke independently of blood pressure: a nested case-control study. J Hypertens 26: 1367–1372. [DOI] [PubMed] [Google Scholar]
- 61. Munshi A, Sultana S, Kaul S, Reddy BP, Alladi S, et al. (2008) Angiotensin-converting enzyme insertion/deletion polymorphism and the risk of ischemic stroke in a South Indian population. J Neurol Sci 272: 132–135. [DOI] [PubMed] [Google Scholar]
- 62. Celiker G, Can U, Verdi H, Yazici AC, Ozbek N, et al. (2009) Prevalence of thrombophilic mutations and ACE I/D polymorphism in Turkish ischemic stroke patients. Clin Appl Thromb Hemost 15: 415–420. [DOI] [PubMed] [Google Scholar]
- 63. Saidi S, Zammiti W, Slamia LB, Ammou SB, Almawi WY, et al. (2009) Interaction of angiotensin-converting enzyme and apolipoprotein E gene polymorphisms in ischemic stroke involving large-vessel disease. J Thromb Thrombolysis 27: 68–74. [DOI] [PubMed] [Google Scholar]
- 64. Tascilar N, Dursun A, Ankarali H, Mungan G, Ekem S, et al. (2009) Angiotensin-converting enzyme insertion/deletion polymorphism has no effect on the risk of atherosclerotic stroke or hypertension. J Neurol Sci 285: 137–141. [DOI] [PubMed] [Google Scholar]
- 65. Domingues-Montanari S, Fernandez-Cadenas I, del Rio-Espinola A, Mendioroz M, Ribo M, et al. (2010) The I/D polymorphism of the ACE1 gene is not associated with ischaemic stroke in Spanish individuals. Eur J Neurol 17: 1390–1392. [DOI] [PubMed] [Google Scholar]
- 66. Li Y, Chen F, Zhou L, Coulter D, Chen C, et al. (2010) COC use, ACE/AGT gene polymorphisms, and risk of stroke. Pharmacogenet Genomics 20: 298–306. [DOI] [PubMed] [Google Scholar]
- 67. Chutinet A, Suwanwela NC, Snabboon T, Chaisinanunkul N, Furie KL, et al. (2011) Association between Genetic Polymorphisms and Sites of Cervicocerebral Artery Atherosclerosis. J Stroke Cerebrovasc Dis [DOI] [PubMed] [Google Scholar]
- 68. Indrajaya T (2011) The role of ACE gene polymorphism on pathogenesis of ischemic stroke. Acta Med Indones 43: 152–157. [PubMed] [Google Scholar]
- 69. Markoula S, Giannopoulos S, Kostoulas C, Tatsioni A, Bouba I, et al. (2011) Gender association of the angiotensin-converting enzyme gene with ischaemic stroke. J Renin Angiotensin Aldosterone Syst 12: 510–515. [DOI] [PubMed] [Google Scholar]
- 70. Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM (1991) Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 68: 450–456. [DOI] [PubMed] [Google Scholar]
- 71. Ridker PM, Gaboury CL, Conlin PR, Seely EW, Williams GH, et al. (1993) Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin II. Evidence of a potential interaction between the renin-angiotensin system and fibrinolytic function. Circulation 87: 1969–1973. [DOI] [PubMed] [Google Scholar]
- 72. Juo SH (2009) Genetics of carotid atherosclerosis. Front Biosci 14: 4525–4534. [DOI] [PubMed] [Google Scholar]
- 73. Danser AH, Schalekamp MA, Bax WA, van den Brink AM, Saxena PR, et al. (1995) Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 92: 1387–1388. [DOI] [PubMed] [Google Scholar]