Abstract
C-peptide, which contains the 13 NH2-terminal residues of RNase A, shows partial helix formation in water at low temperature (1 degree C, pH 5, 0.1 M NaCl), as judged by CD spectra; the helix is formed intramolecularly [Brown, J. E. & Klee, W. A. (1971) Biochemistry 10, 470-476]. We find that helix stability depends strongly on pH: both a protonated histidine (residue 12) and a deprotonated glutamate (residue 9 or 2 or both) are required for optimal stability. This information, together with model building, suggests that the salt bridge Glu-9- ... His-12+ stabilizes the helix. Formation of the helix is enthalpy driven [van't Hoff delta H, - 16Kcal/mol (1 cal = 4.18 J)] and the helix is not observed above 30 degrees C. Proton NMR data indicate that several side chains adopt specific conformations as the helix is formed. These results have two implications for the mechanism of protein folding. First, they indicate that short alpha-helices, stabilized by specific side-chain interactions within the helix, can be stable enough in water to function as folding intermediates. Second, they suggest that similar experiments with peptides of controlled amino acid sequence could be used to catalogue the intrahelix interactions that stabilize or destabilize alpha-helices in aqueous solution. These data might provide the code relating amino acid sequence to the locations of alpha-helices in proteins.
Full text
PDF
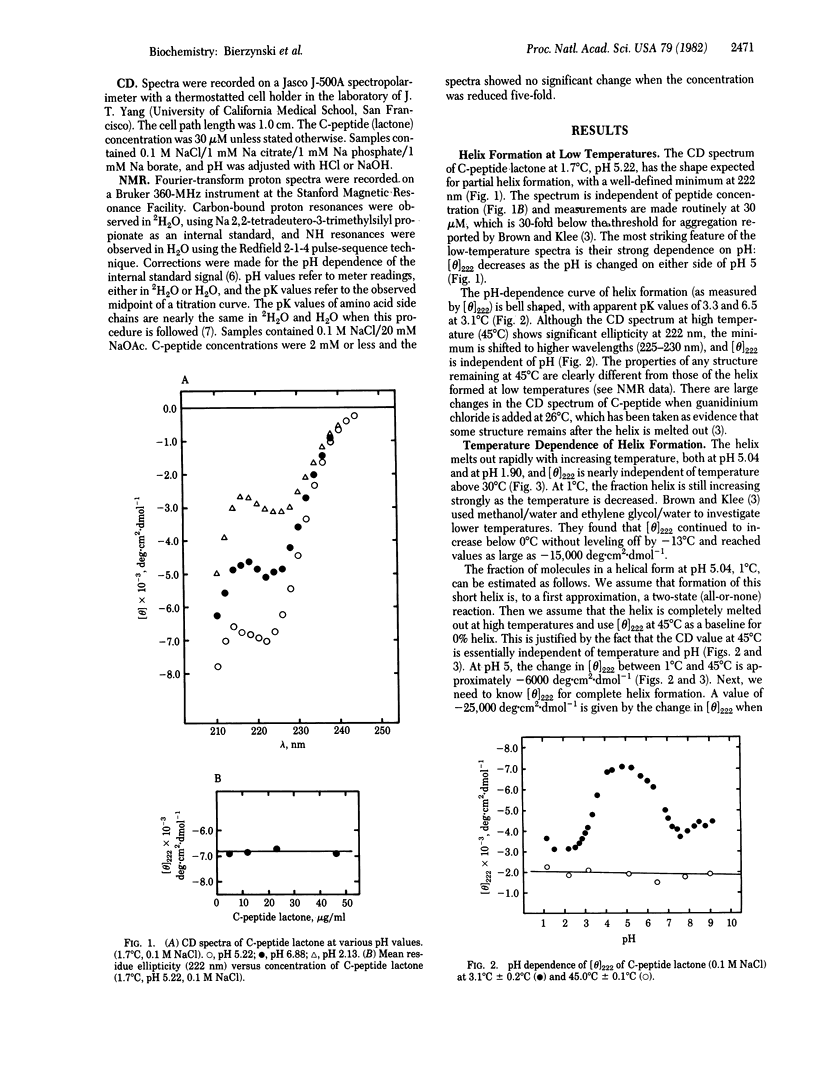

Selected References
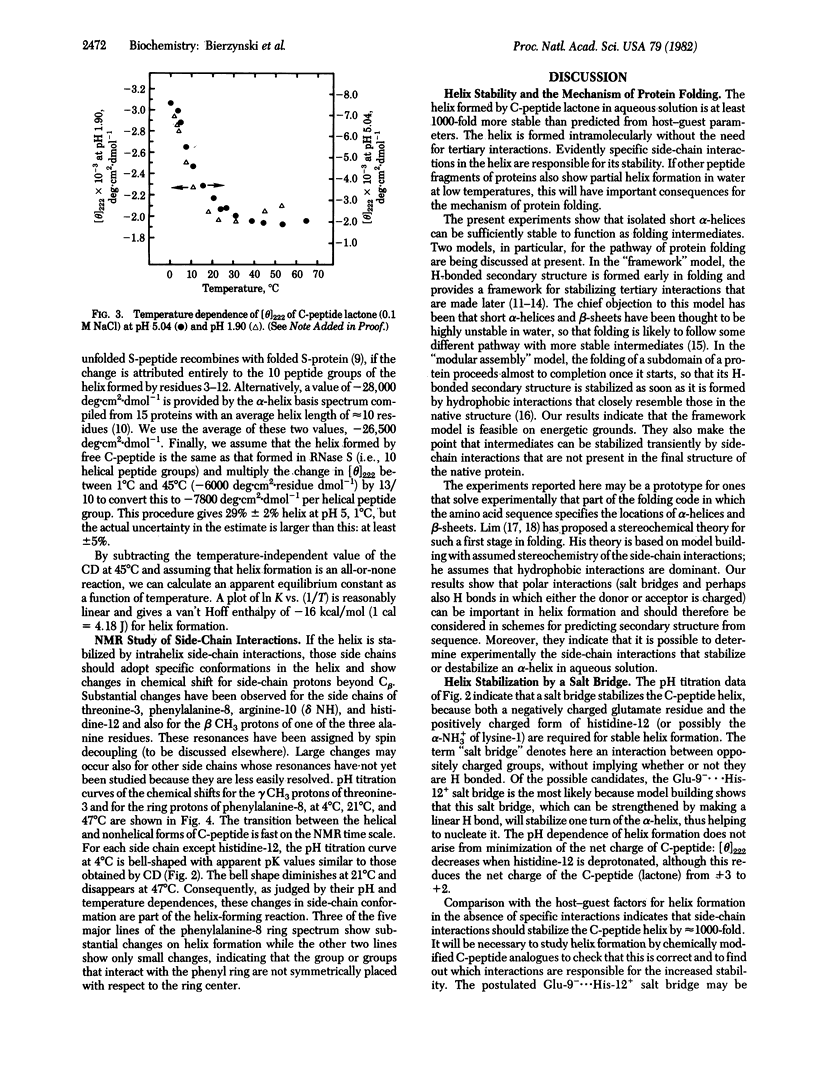
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Klee W. A. Helix-coil transition of the isolated amino terminus of ribonuclease. Biochemistry. 1971 Feb 2;10(3):470–476. doi: 10.1021/bi00779a019. [DOI] [PubMed] [Google Scholar]
- Brown L. R., De Marco A., Richarz R., Wagner G., Wüthrich K. The influence of a single salt bridge on static and dynamic features of the globular solution conformation of the basic pancreatic trypsin inhibitor. 1H and 13C nuclear-magnetic-resonance studies of the native and the transaminated inhibitor. Eur J Biochem. 1978 Jul 17;88(1):87–95. doi: 10.1111/j.1432-1033.1978.tb12425.x. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Scheraga H. A. The influence of long-range interactions on the structure of myoglobin. Biochemistry. 1968 Aug;7(8):2864–2872. doi: 10.1021/bi00848a024. [DOI] [PubMed] [Google Scholar]
- Hearn R. P., Richards F. M., Sturtevant J. M., Watt G. D. Thermodynamics of the binding of S-peptide to S-protein to form ribonuclease S.. Biochemistry. 1971 Mar 2;10(5):806–817. doi: 10.1021/bi00781a013. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Structural intermediates trapped during the folding of ribonuclease A by amide proton exchange. Biochemistry. 1980 Dec 23;19(26):6124–6129. doi: 10.1021/bi00567a027. [DOI] [PubMed] [Google Scholar]
- Lenstra J. A., Hofsteenge J., Beintema J. J. Invariant features of the structure of pancreatic ribonuclease. A test of different predictive models. J Mol Biol. 1977 Jan 15;109(2):185–193. doi: 10.1016/s0022-2836(77)80028-4. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Rose G. D. Folding units in globular proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4304–4308. doi: 10.1073/pnas.78.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Marchiori F., Borin G., Moroder L., Rocchi R., Scoffone E. Relation between structure and function in some partially synthetic ribonucleases S'. I. Kinetic determinations. Biochim Biophys Acta. 1972 Feb 29;257(2):210–221. doi: 10.1016/0005-2795(72)90272-3. [DOI] [PubMed] [Google Scholar]
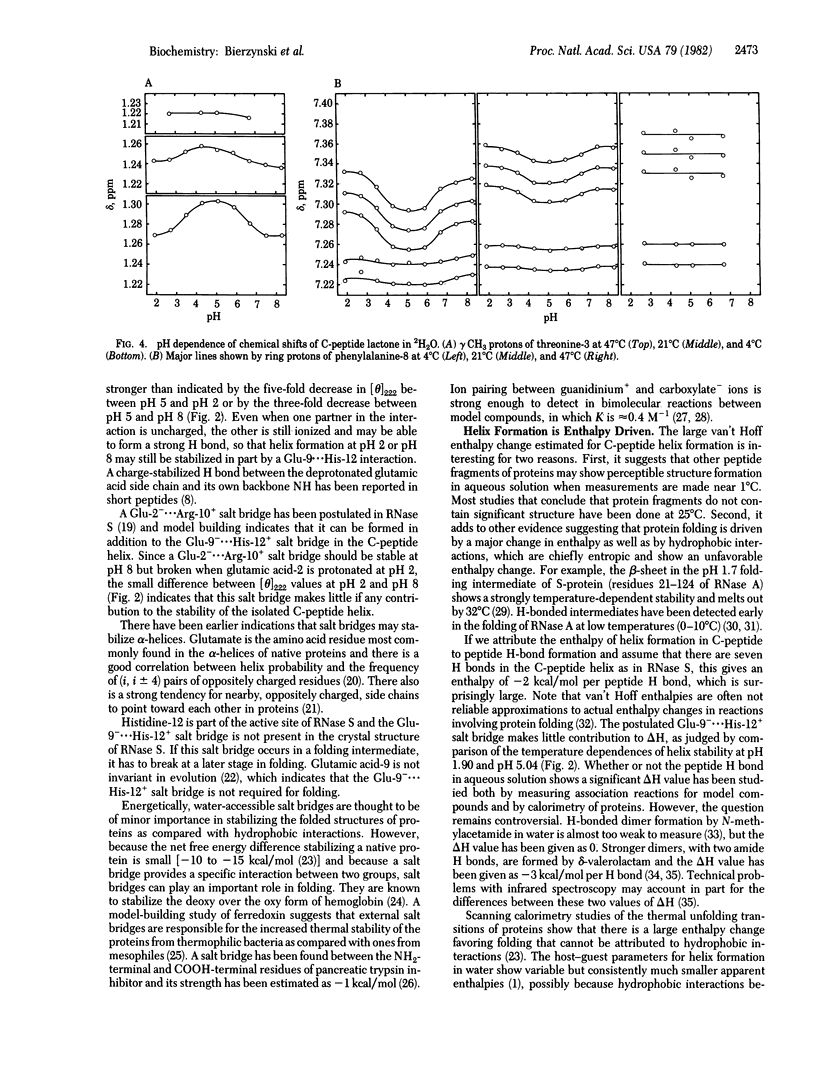
- Maxfield F. R., Scheraga H. A. The effect of neighboring charges on the helix forming ability of charged amino acids in proteins. Macromolecules. 1975 Jul-Aug;8(4):491–493. doi: 10.1021/ma60046a022. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Structure and mechanism of haemoglobin. Br Med Bull. 1976 Sep;32(3):195–208. doi: 10.1093/oxfordjournals.bmb.a071363. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O. B., Rashin A. A. A model of myoglobin self-organization. Biophys Chem. 1975 Feb;3(1):1–20. doi: 10.1016/0301-4622(75)80033-0. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- SUSI H., TIMASHEFF S. N., ARD J. S. NEAR INFRARED INVESTIGATION OF INTERAMIDE HYDROGEN BONDING IN AQUEOUS SOLUTION. J Biol Chem. 1964 Sep;239:3051–3054. [PubMed] [Google Scholar]
- Schmid F. X., Baldwin R. L. Detection of an early intermediate in the folding of ribonuclease A by protection of amide protons against exchange. J Mol Biol. 1979 Nov 25;135(1):199–215. doi: 10.1016/0022-2836(79)90347-4. [DOI] [PubMed] [Google Scholar]
- Warme P. K., Morgan R. S. A survey of amino acid side-chain interactions in 21 proteins. J Mol Biol. 1978 Jan 25;118(3):289–304. doi: 10.1016/0022-2836(78)90229-2. [DOI] [PubMed] [Google Scholar]


