A Unique Reproductive Tissue
The mammary gland is a unique reproductive organ, being one of the few tissues that undergo the majority of their development after birth. Because it is in an immature state at and around the time of birth, the rapidly developing mammary gland may be particularly sensitive to the effects of transplacental or lactationally-transferred exposures. The branching epithelium of the gland arborizes the fat pad quickly after birth, with terminal end buds (TEB; highly mitotic, cap-cell layer) leading the outgrowth. These TEB cells form the branch points in the gland and are known to be susceptible to the effects of chemical carcinogens (first established by Russo and Russo, 1978). The number of TEB and the timing of their presence in the rodent mammary gland play an important role in the susceptibility and the sensitivity of the gland to exogenous (environmental) or endogenous (changes in hormones/receptors of the dam or offspring) perturbations (Fenton, 2006). The human breast and rodent mammary gland undergo very similar development, with few exceptions (as reviewed in Fenton, 2006). The timing of the formation of the mammary bud is probably the most important difference; the rodent bud appears and begins to grow rapidly in the final days of fetal life, whereas human mammary bud outgrowth occurs toward the end of the first trimester. However, the periods of life during which TEB are present in the gland are similar across species – before, during and just after puberty.
Translation of Research Findings in Animals to Human Health
According to the most recent statistics of the American Cancer Society (ACS, 2008), it is estimated that 1 in 8 women in America will be diagnosed with breast cancer in their lifetime, and the incidence rate has grown at a rate of 2.0% per year from 1988–2000 for tumors ≤2.0 cm, and 1.7% from 1992 to present for larger tumors (>5.0 cm). This common health affliction has many known risk factors (Table 1), but most are controlled by genetics. It has been increasingly evident over the last 20 years that a woman’s gene pool explains only about 25–30% of the increased rate of breast cancer and the rate of risk change is faster than any documented change in the gene pool of humans. Many of the known endogenous factors that influence the risk for breast cancer in women (Table 1) are completely out of the control of the woman (inherited), and a few can be manipulated to some extent (i.e., timing of the first child, obesity, high-dose radiation exposure to the chest). One breast cancer risk factor, pubertal timing, has a hereditary component (specifically for menarche), but may also be controlled by other factors such as body mass index and environmental factors (Euling et al., 2008). A panel of experts in the puberty field reviewed years of published literature (1940–1994) on the timing of puberty and concluded that there was strong evidence for precocious breast development and menarche in girls, but that pubertal timing of boys was unchanged. The expert panel also suggested a role of the environment in early pubertal progression in girls. The environment is thought to contribute a majority of the risk for breast cancer, also. However, this theory has been difficult to test.
Table 1.
Factors That Increase the Relative Risk for Breast Cancer in Women
| Relative Risk | Factor |
|---|---|
| >4.0 |
|
|
| |
| 2.1–4.0 |
|
|
| |
|
1.1–2.0 Factors that affect circulating hormones |
|
| Other factors |
|
Source: American Cancer Society, 2008.
There have been numerous epidemiological studies that have selected populations with higher than average breast cancer risk, in which environmental factors have been the specific study targets (i.e., MWS, 2006; LIBCSP, 2002). In these studies, women diagnosed with breast cancer were evaluated for current environmental stressors in their body (circulating), home and surroundings. Few direct correlations were made with environmental chemical levels, per se, and no single result fully explained the increased breast cancer risk in those communities. Some clues as to why there are few correlations between cancer risk and environmental factors in those studies, as conducted, may be garnered from laboratory animal studies.
Endocrine disrupting compounds (EDCs), defined by the US EPA (ORD, 1997) as “an exogenous agent that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development, and/or behavior”, have been shown in several laboratories to disrupt the development, function, and tumor susceptibility of the mammary gland in rodent models (reviewed in Fenton, 2006; Birnbaum and Fenton, 2003). One of the most important lessons learned from these collective studies is that early life vs. adult exposure to EDCs causes a more severe, permanent, or sensitive response in the rodent mammary gland.
Dioxin is one of the best examples of a transplacental and lactational-transfer of environmental EDC causing early life perturbation of the mammary gland, which translates to increased risk of mammary tumor formation in the adult rat. Several studies (Brown et al., 1998; Lewis et al., 2001; Fenton et al., 2002) reported a single exposure to dioxin during late fetal life caused irreversible abnormalities in mammary gland development, resulting in longer TEB presence in the gland than normal. When exposed as a virgin adult, rat mammary tissues were without phenotypic response to this long-lasting EDC (Fenton et al., 2002; Vorderstrasse et al., 2004). However, when challenged with a chemical carcinogen, the animals with numerous TEB present, at an inappropriate time of development when control rat TEB had differentiated, developed more mammary tumors (Brown et al., 1998). Not only did dioxin stunt mammary gland development of the rat, but in a polluted area of Belgium, girls (N=200; aged 15.8 to 19.6 years old) exposed to polychlorinated aromatic hydrocarbons (including dioxin) via pollution, demonstrated a significant delay in breast development that was associated with a doubling of serum dioxin concentrations. However, the young women did not exhibit delayed menarche (den Hond et al., 2002).
Several other EDCs have been reported to alter the timing of mammary epithelial outgrowth, branching, and inappropriate timing of TEB presence and result in long-lasting effects (many reviewed in Fenton, 2006). Among these are phenols found in plastics, such as bisphenol A and nonylphenol (Moon et al, 2007); the pharmaceutical estrogen agonist, diesthylstilbesterol; the xenoestrogens genestein, diadzein, and resveratrol; environmental metals; the perfluorinated surfactant, PFOA (White et al., 2007, 2009); and a few pesticides (Enoch et al., 2007). Although DDT, the first synthetic pesticide used to control mosquito populations, is likely the most studied pesticide, there have been no consistent reports of deleterious effects on the mammary gland of rodents. A pesticide that has repeatedly demonstrated an effect on the mammary gland of the rat is the chlorotriazine herbicide, atrazine. Studies using this proven EDC (EPA, 2000) confirmed the identification of a “critical window” of sensitivity of the neonatal mammary gland (Rayner et al., 2004, 2005). Another important outcome of these studies was that this short half-life compound caused mammary growth delays persistent/severe enough to affect the growth of offspring from the prenatally exposed females, apparently from lack of functional differentiation of the gland (Rayner et al., 2005). A similar lactational effect has been reported for dioxin (Vorderstrasse et al., 2004) and PFOA (White et al., 2007, 2009). Although the early atrazine studies on the mammary gland used high exposures (100 mg/kg), later studies used a mixture of the metabolites to the chlorotriazine herbicides (as they might be found in the environment) at ≥10X lower levels, via the same route of exposure, and found identical delays in mammary development in early life as those seen with the parent compound (Enoch et al., 2007).
Figure 1 demonstrates the importance of the “critical windows” of EDC exposure and the adverse consequences (compiled from many studies) that may follow in later life. These consequences of exposure during sensitive or susceptible periods of development, largely identified using animal models, have similar human health correlates. Therefore, the fact that epidemiologic studies in the breast cancer field have not uncovered major environmental exposures associated with risk for breast cancer at the time that breast cancer is diagnosed is not surprising. Early life exposures may be more relevant to the risk, and explain the outcome better. A positive indicator for this theory is the recent evaluation of serum samples collected between 1959 and 1967 as part of a prospective study from women whose California health records indicated that they had died from or were diagnosed with breast cancer before the age of 50. Women exposed to relatively high levels of DDT prior to 14 years old had a 5 times greater risk of developing breast cancer later in life than women with lower exposures. But, exposure after adolescence did not increase risk (Cohn et al., 2007). The latter outcome is consistent with DDT findings in the previously mentioned studies, and others, that have “looked for the key under the lightpost.”
FIG. 1.
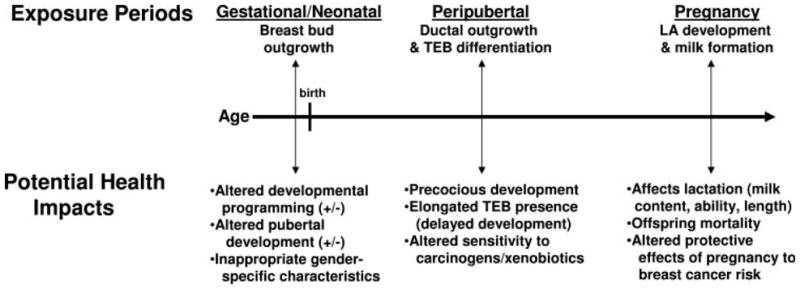
Adverse Consequences of Mammary Gland Perturbation. Timeline highlighting the critical periods of mammary gland development most likely to be altered by EDCs and the correlating potential health effects of these exposures. Three time periods are suggested from rodent model literature. LA, lobuloalveolar; TEB, terminal end bud. Source: Fenton, 2006.
What can be done to increase awareness of chemical effects on the mammary gland?
It has become apparent that an evaluation of early life exposures (pollutants, radiation, pharmaceutical use, personal hygiene items, dietary intake) may lead to better correlations with breast cancer risk, but at the present we have few clues as to which exposures to look for. Because the mammary gland is not a homogeneous tissue (consists of epithelial, fibroblast, and adipose cells) and relies on cell-cell communication for outgrowth, branching and differentiation, the use of clonal cell lines will likely fail to identify environmental exposures of concern. Improved use of animal models for identification of these potential risk factors is needed.
The Food Quality Protection Act and the Safe Drinking Water Act, both of 1996, mandated the EPA to evaluate pesticides and toxic chemicals for endocrine disrupting activities. EPA’s Endocrine Disruptor Screening Program (EDSP) is a two-tier system in which Tier 1 studies are screening assays, identifying potential EDCs, and Tier 2 assays are designed to be more definitive and include dose response and mode of action information. Tier 1 tests include assays that will identify EDCs altering the pubertal end points of vaginal opening in females and preputial separation in males, as well as other reproductive development indices and some serum hormone concentrations. The Tier 2 level includes an expanded one-generation study, which is similar to a standard 2-generation reproductive study conducted by the NTP and most industrial laboratories.
The assays are currently undergoing validation, but do not include in them the mammary gland as a required or optional tissue of study. The primary reason for this is the lack of standardized assays to evaluate mammary effects across species. It is very important to evaluate mammary gland response to all of the potential EDCs that will be run through these vigorous screening assays, which could assess all three of the “critical windows” of mammary gland development defined in Figure 1. This information would provide invaluable information on the EDCs with the potential to alter human breast development, pubertal timing, or the ability of a new mother to breast feed her infant.
In regulatory decision making on chemical safety, a weight-of-evidence (WOE) approach has great value for understanding the relationship between the affected endpoints and the dose or time response relationships. Some effects may be considered “adverse” by current testing or risk assessment guidelines, whereas the adversity for other endpoints may be more complicated to assess. In the rodent model, the adversity of a change in timing of external, visible reproductive indices are not clear, but the adversity of persistent mammary gland developmental delays has been established in the above mentioned studies. The inability to sustain the body weight of offspring, increased mammary tumor risk, and increased mortality of offspring are adverse consequences reported after early life exposure to EDCs. These outcomes in rodents translate in women to the inability to breast feed a child because sufficient milk is not produced (and the consequences on the immune status of the child), or precocious beginnings of breast development (≤ 8 years of age) that takes longer than normal to reach full maturity, thus increasing the chance that the susceptible TEB structures present during this life stage to be exposed to a secondary affector (radiation, another chemical, dietary constituent, etc), potentially increasing life long breast cancer risk.
To decrease the risk of breast cancer in women caused by environmental factors, prevention of exposure is the key, but before that can be accomplished the primary exposure factors must be ascertained. Published methods for the evaluation of mammary gland effects in animal models need to be validated to facilitate their use in all types of chemical screening assays. This could result in earlier identification of chemicals and pharmaceuticals likely to affect lactational ability or breast cancer risk.
Another major change that would greatly benefit the breast cancer field is the focus of epidemiological studies on early life exposures. A handful of excellent studies have incorporated this concept and are studying exposures in children/adolescents to understand pubertal timing and other exposure-related illness. The NIEHS-sponsored Breast Cancer and the Environment Research Centers (BCERC, 2006) recruited 6–8 year old girls in New York City, San Francisco, and Cincinnati to evaluate the environmental factors affecting pubertal timing. The Agricultural Health Study (AHS, 2004), finding few health links to adult pesticide exposure in highly exposed farm workers, is now evaluating the exposures and health outcomes in the farming children. Last, but not least, the National Children’s Study (NCS, 2000) will evaluate the environmental exposures of 100,000 children from conception to at least age 21. This prospective, longitudinal study will ascertain associations of early life environmental factors to puberty and obesity, two major endpoints known to affect breast cancer risk. Continued federal funding of these types of integrated, longitudinal studies is imperative in the fight for breast cancer prevention.
References
- Agricultural Health Study (AHS) [Accessed 24 Sept, 2008];National Institute for Environmental Health Sciences. 2004 http://www.niehs.nih.gov/research/atniehs/labs/epi/studies/ahs/index.cfm.
- American Cancer Society. Breast Cancer Fact & Figures. Atlanta: ACS, Inc; 2007–2008. [Accessed 24 Sept, 2008.]. http://www.cancer.org/downloads/STT/BCFF-Final.pdf. [Google Scholar]
- Breast Cancer and the Environment Research Centers (BCERC) [Accessed 24 Sept, 2008];National Institute for Environmental Health Sciences. 2006 http://www.bcerc.org/home.htm.
- Brown NM, Manzolillo PA, Zhang JX, Wang J, Lamartiniere CA. Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis. 1998;19:1623–1629. doi: 10.1093/carcin/19.9.1623. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. Environmental Health Perspectives. 2007. DDT and breast cancer in young women: New data on the significance of age at exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hond ED, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, Vandermeulen C, Winneke G, Vanderschueren D, Staessen JA. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisted. Environ Health Perspect. 2002;110:771–776. doi: 10.1289/ehp.02110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDSP, US EPA. Endocrine Disruptor Screening Program (EDSP) 2008 http://www.epa.gov/endo/index.htm.
- EPA’s FIFRA Scientific Advisory Panel Report No. 2000–05. Atrazine:Hazard and dose-response assessment and characterization. 2000 Jun 27; http://www.epa.gov/scipoly/sap/2000/june27/finalatrazine.pdf.
- Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008;121(Suppl 3):S167–71. doi: 10.1542/peds.2007-1813C. [DOI] [PubMed] [Google Scholar]
- Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- Lewis BC, Hudgins S, Lewis A, Schorr K, Sommer R, Peterson RE, Flaws JA, Furth PA. In utero and lactational treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs mammary gland differentiation but does not block the response to exogenous estrogen in the postpubertal female rat. Toxicol Sci. 2001;62:46–53. doi: 10.1093/toxsci/62.1.46. [DOI] [PubMed] [Google Scholar]
- Long Island Breast Cancer Study Project (LIBCSP) National Cancer Institute (NCI); 2004. [Accessed 01 Oct, 2008.]. http://www.cancer.gov/cancertopics/libcsp. [Google Scholar]
- Marin Women’s Study (MWS), Breast Cancer Resaerch. [Accessed 01 Oct, 2008];County of Marin Department of Health and Human Services. 2006 http://www.marinwomensstudy.org/
- Moon HJ, Han SY, Shin J-H, Kang IH, Kim TS, Hong JH, Kim S-H, Fenton SE. Gestational Exposure to Nonylphenol Causes Precocious Mammary Gland Development in Female Rat Offspring. J Reprod Dev. 2007;53:333–344. doi: 10.1262/jrd.18055. [DOI] [PubMed] [Google Scholar]
- National Children’s Study Research Plan (NCS) [Accessed 24 Sept, 2008 ];Demonstration of numerous methods and study tools from ORD researchers. 2000 http://www.nationalchildrensstudy.gov/research/studydesign/researchplan/Pages/default.aspx.
- ORD, US EPA. Special Report on Environmental Endocrine Disruption: An Effects Assessment and Analysis. Washington, D.C: 1997. [Accessed 01 Oct, 2008]. International Workshop on Endocrine Disruptors. http://www.epa.gov/edrlupvx/Pubs/smithrep.html. [Google Scholar]
- Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci. 2005;87:255–266. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol Appl Pharmacol. 2004;195:23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenzanthracene. J Natl Cancer Inst. 1978;61:1439–1449. [PubMed] [Google Scholar]
- Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci. 2004;78:248–257. doi: 10.1093/toxsci/kfh062. [DOI] [PubMed] [Google Scholar]
- White SS, Calafat AM, Kuklenyik Z, Thibodeaux J, Wood C, Fenton SE. Gestational PFOA Exposure of Mice is Associated with Altered Mammary Gland Development in Dams and Female Offspring. Toxicol Sci. 2007;96:133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, Stanko JP, Wolf CJ, Abbott BD, Fenton SE. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol. 2008 doi: 10.1016/j.reprotox.2008.11.054. special issue on PFOA (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


