Abstract
Herpesvirus capsids traverse the nuclear envelope by utilizing an unusual export pathway termed nuclear egress. In this process, the viral capsid is delivered into the perinuclear space, producing a vesicular intermediate after fission. After fusion with the outer nuclear membrane, the naked capsid is released into the cytosol. A recent study now suggests that this pathway might be an endogenous cellular pathway, co-opted by viruses, that serves to transport cellular cargo exceeding the size limit imposed by the nuclear pore complex. We propose that one function of this pathway is to transport nuclear protein aggregates to the cytosolic autophagy machinery. Our model has implications for our understanding of laminopathies and related diseases affecting proteins residing at the inner nuclear membrane and nuclear lamina.
Keywords: Torsin A, LINC complex, chaperones, nuclear envelope, egress of nuclear aggregates (EGNA), premature aging
Nuclear egress: A transport pathway for viral capsids
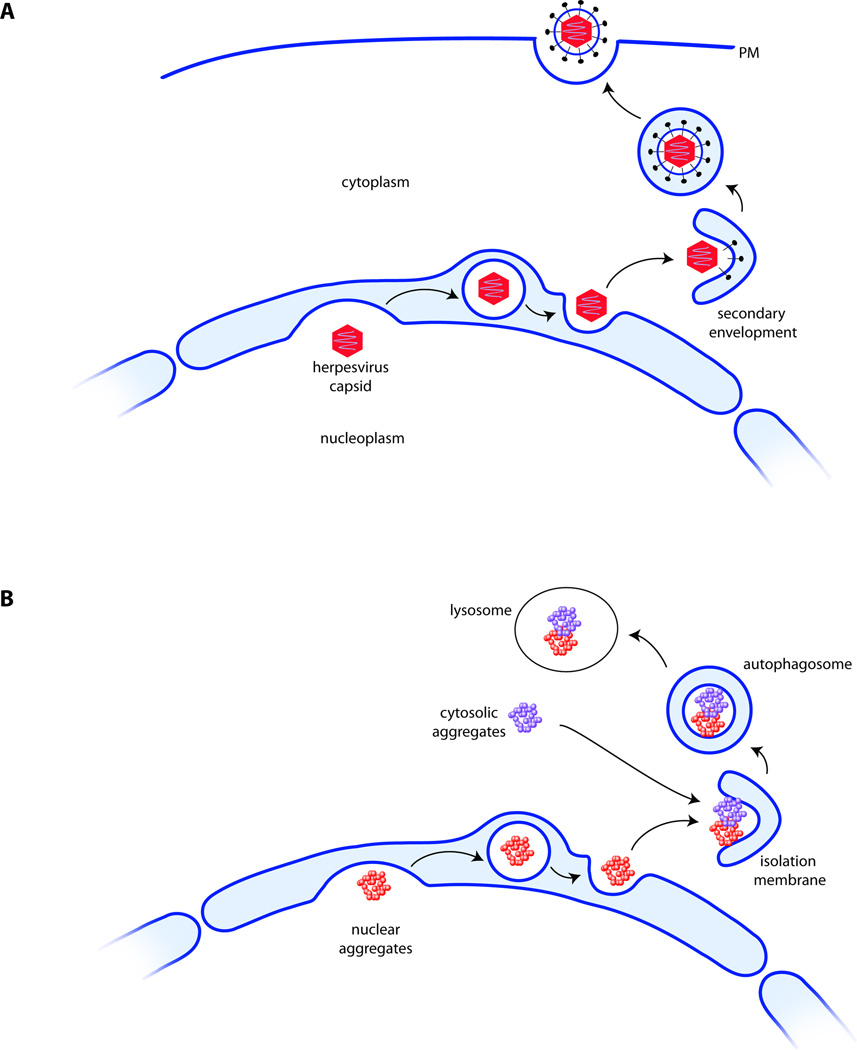
In the late phase of the lytic cycle of a productive herpesvirus infection, the DNA genomes of progeny viruses are packaged into viral capsids in the host cell nucleus. The completely assembled nucleocapsid has a diameter of approximately125 nm and although this number varies between different members of the herpesvirus family [1], the dimensions of the nucleocapsid greatly exceed the 39 nm size limit of the nuclear pore complex (NPC) [2]. For this reason, nuclear export of capsids cannot rely on the canonical machinery that utilizes the NPC as a conduit to the cytosol. Instead, most if not all herpesviruses follow a pathway termed nuclear egress (for a recent review, see [3]; Glossary). In the first step of nuclear egress, designated primary envelopment, the capsid apposes to the inner nuclear membrane (INM) of the nuclear envelope (NE) and buds into the perinuclear space (PNS) to produce a vesicular intermediate (Figure 1a). Upon fusion of this intermediate with the outer nuclear membrane (ONM), the naked capsid is released into the cytosol and has thus crossed the two membranes that define the borders of the nuclear envelope. What follows is a process termed secondary envelopment, in which the capsid is enveloped by a double membrane (Figure 1a). From a topological perspective, we consider this latter process highly reminiscent of isolation membrane formation [4] at the onset of autophagy. Finally, the double membrane closes. Following fusion of the outer membrane with the plasma membrane, the mature virion is then released from the cell.
Figure 1.
Egress of herpesvirus capsids from the nucleus: A paradigm for the delivery of nuclear protein aggregates to the cytosolic autophagy machinery? (a). Herpesvirus capsids are assembled in the nucleus and bud into the perinuclear space to form a membrane-enveloped intermediate, which then fuses with the outer nuclear membrane (ONM) to release the naked capsid into the cytosol, where the glycoprotein coat is acquired. (b). Using the same route, nuclear aggregates may be transported to the autophagic machinery that is confined to the cytosol (note that secondary envelopment, depicted in a, is topologically equivalent to isolation membrane formation at the onset of autophagy, depicted in b). The link to autophagy is at present hypothetical and awaits experimental validation (see main text and Box 1).
A large body of work ([3, 5], and references cited therein) has established the individual steps that underlie nuclear egress. Initially, the nuclear lamina is dissolved in a process that requires phosphorylation of lamins and other INMresident proteins, including Emerin, by recruitment of cellular kinases and via virus-encoded kinases [6–8]. The capsid is then recruited to the INM via the nuclear egress complex, conserved in all herpesvirus subfamilies. For human herpesvirus-1 (HSV-1), the egress complex consists of UL31 and UL34 (Figure 2). Remarkably, UL31/34 can induce NE budding upon transfection into mammalian cells in the complete absence of a viral infection [9]. Less is known about the fission events that produce the vesicular intermediate. The degree to which host cell factors are involved in fission and other egress reactions remains to be established. Once formed, HSV-1 glycoprotein B presumably acts as a fusion protein to execute the fusion of the vesicular intermediate with the ONM [10] (Figures 1a, 2). However, similar studies centered on Pseudorabiesvirus and human Cytomegalovirus do not support an essential function of glycoprotein B in nuclear egress [11, 12], suggesting that other, possibly cellular, factors mediate fusion.
Figure 2.
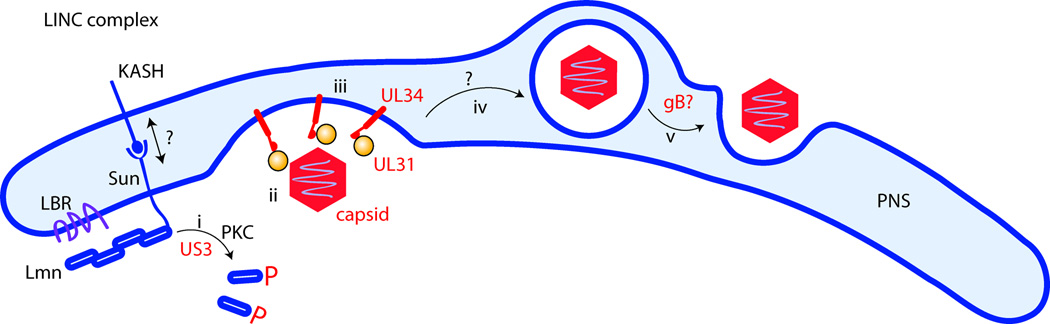
Manipulation of the nuclear envelope during viral egress. Dissolution of the nuclear lamina by local phosphorylation of nuclear lamins via kinases (i) enables the capsid to gain access to the inner nuclear membrane, and to dock onto the nuclear egress complex (ii). Budding (iii) and fission (iv) produce a vesicular intermediate, which fuses the outer nuclear membrane in a reaction that may involve gB (v). Lmn, Lamins; LBR, Lamin B receptor; PKC, protein kinase C; gB, Glycoprotein B. Cellular proteins are depicted in blue, viral proteins in red/orange. Note that the capsid is not drawn to scale: its diameter is approximately three times the width of the perinuclear space (PNS).
Long believed to be a virus-specific pathway, recent evidence now suggests that nuclear egress might be a cellular pathway that has been co-opted by herpesviruses [13]. As we shall explain below, we believe that this pathway may fill a major gap in our understanding of cellular protein quality control.
A pathway for nuclear protein aggregates
The proteome of every living organism is surveyed by an elaborate protein quality control system which serves to repair or eliminate misfolded proteins before they reach cytotoxic levels. The major routes for protein turnover in the cytosol of eukaryotes are the ubiquitin-proteasome system (UPS) and autophagy. While both systems cooperate on many levels, there is a key difference in selectivity with respect to the physical state of the substrate: soluble misfolded species are usually targeted to the UPS for degradation, while insoluble protein aggregates that cannot be handled by the proteasome are engulfed by an isolation membrane at the onset of autophagy [14]. Following the formation of a double membrane structure, the contents of the autophagosome are ultimately delivered to the lysosome via membrane fusion [4] (Figure 1b).
Because the autophagy machinery is confined to the cytosol in interphase cells (in a neuron, for example, no nuclear envelope breakdown occurs for decades), it is difficult to envision how the cytoplasmic machinery for autophagy can gain access to the nucleoplasm, separated from it by the nuclear envelope. How protein aggregates are handled in the nucleus – the largest organelle by far in many cell types – is therefore largely unknown.
We hypothesize that nuclear protein aggregates bud into the intermembrane space of the nuclear envelope, followed by fusion with the outer nuclear membrane for cytosolic release. Once inside the cytosol, aggregates are cleared by means of autophagy (Figure 1b).
This postulated mechanism, which we designate egress of nuclear aggregates (EGNA), derives support from the following considerations: (i) The autophagic machinery is confined to the cytosol and there is no known mechanism that accounts for removal of protein aggregates in the nucleus. Specifically, a potent nuclear disaggregating activity, which would be required to disassemble nuclear aggregates, has not been described in mammalian cells. (ii) The nuclear pore complex does not allow for the passage of structures greater than 39 nm in diameter [2], suggesting that aggregated protein structures must exit via alternative mechanisms. Nuclear inclusions that exceed the transport limit have been reported in trinucleotide repeat disorders ([15] and references cited therein), and in response to missense mutations [16]. (iii) Perinuclear autophagosomes and an upregulation of autophagy markers are present in cells that express pathogenic alleles encoding the INM/lamina proteins Emerin and Lamin A [17]. (iv) A profound thickening of the nuclear lamina is a hallmark of cells from patients with progeria syndromes [18, 19]. This phenomenon may be caused by aberrant protein deposition - visible as electron-dense material in electron micrographs of those cells - although for the moment this remains speculative. (v) In yeast, nucleus-vacuole (NV) junctions are assembled via membrane budding, fission and fusion events, allowing for the delivery of nuclear components to the vacuole in a process termed piecemeal microautophagy of the nucleus [20, 21]. One may therefore entertain the idea that herpesviruses did not establish this pathway de novo. We hypothesize that nuclear egress represents a conserved cellular pathway, subverted by herpesviruses to their own advantage. Indeed, blebbing-related nuclear transport pathways have been considered [13, 22, 23], but not as part of cellular quality control.
Export of RNA granules resembles nuclear egress: lessons from Drosophila
A recent discovery lends credence to the idea of nuclear aggregate egress: It was reported that ribonucleoprotein (RNP) granules in the neuromuscular junction of Drosophila exit the nucleus in a manner similar to the nuclear egress of HSV-1 capsids [13]. These specific RNP granules experience a size predicament similar to that of virus nucleocapsids, in that they exceed the size limit for exit via nuclear pores. In studying the Frizzled nuclear import pathway, the authors discovered that D-Frizzled2 (DFz2) foci in the nucleus colocalized with RNA and the Drosophila A-type LaminC (LamC). By electron microscopy these foci corresponded to membrane-enclosed granular intermediates within the perinuclear space. Some of these granules were present immediately outside of the nucleus, implying that they can be translocated across the membrane. LamC and an atypical protein kinase C (PKC) isoform are required for the formation of these foci. However, it remains to be established whether RNP export is entirely independent from NPC-dependent transport. Given that PKC is required in HSV-1 nuclear egress as well, and that both pathways rely on vesicles as transport intermediates in the perinuclear space, one may speculate that HSV-1 hijacks a cellular pathway to leave the nucleus [13].
We argue that aggregated nuclear proteins would likewise be too large to exit the nucleus via nuclear pores. Instead, utilization of a budding pathway through the nuclear envelope would allow access to the cytoplasm and the known mechanisms of autophagy. Given that cytosolic protein aggregates are actively transported to a perinuclear site [24, 25], the transport routes of cytosolic and nuclear protein aggregates might converge at such a site, followed by their sequestration into autophagosomes (see Figure 1b for a hypothetical model).
Cellular factors implicated in nuclear egress
A mechanistic understanding of this type of nuclear egress is in its infancy, and many crucial players await identification. The use of viruses as tools has illuminated many cellular transport pathways [26]. Herpesviruses may therefore prove helpful in dissecting both cellular and viral aspects of nuclear egress. By analogy to viral manipulation of the nuclear envelope, the cellular egress pathway may consist of the following steps (Figure 2): (i) local dissolution of nuclear lamina, (ii) recognition of cargo by a receptor at the INM, (iii) budding into the PNS, (iv) fission to yield a vesicular intermediate, and lastly (v) fusion with the ONM.
While cargo receptors remain elusive, the viral nuclear egress complex interacts with the Lamin B receptor indirectly leading to its relocalization [27] [28]. The functional implications of this observation remain to be established. More is known about the action of kinases, which function in a manner analogous to NE breakdown during mitosis [29]: PKC-type kinases are involved in lamin phosphorylation to facilitate egress [6] [13]. The mechanism that ensures that those kinases only act locally to prevent the bulk vesiculation of the NE are not known, but likely involve recruitment to egress sites defined by specific, INMresident receptors.
Which cellular components aid in membrane curvature, fission, and fusion, and how is the transport process energized? Topologically, the budding reaction is similar to formation of a multivesicular body (MVB) in endosomes (Glossary), responsible for the formation of intralumenal vesicles [30] [31]. CHMP1, a member of the Snf7 family required for MVB formation, was first identified as a nuclear protein [32]. Manipulation of the MVB machinery compromises HSV-1 production [33, 34], but whether MVB components affect only secondary envelopment in the cytosol or serve another function during nuclear egress is not known.
Many AAA+ (ATPases associated with a variety of cellular activities) ATPases [35] play important roles in membrane remodeling; examples include VPS4, a critical component of the MVB machinery [30] [31], and NSF, which disassembles SNARE proteins after membrane fusion [36]. There are several reasons why Torsin AAA+ ATPases are likely to be functionally important (four are encoded in the human genome: TorsinA, TorsinB, Torsin2A, Torsin3A). First, to our knowledge they are the only AAA+ ATPases found in the perinuclear space. Second, vesicular structures in the PNS resembling viral egress intermediates are visible in electron micrographs derived from mouse models of DYT1 dystonia, a disorder caused by a mutation in the TOR1A gene, as well as in primary cells of primary dystonia patients [37–41]. TorsinA - the product of the TOR1A gene - and possibly other Torsins may therefore play a major role in the formation or resolution of these vesicular structures [13, 42]. Consistent with this idea, expression of dominant negative alleles of Torsin A and B potently reduce HSV-1 production [43].
Apart from the nuclear lamina, another obstacle to nuclear egress that must be modulated is the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex. This complex serves as a molecular ruler to restrict the width of the perinuclear space to approximately 40 nm. It consists of a trimeric SUN (Sad1/UNC84) protein that spans the INM and binds the lamin network with its Nterminus, while binding Nesprin/KASH (Klarsicht, ANC-1, SYNE1 Homology) proteins in the perinuclear space [44–46]. These Nesprin proteins, which are the second component, span the outer nuclear membrane and attach to the cytoskeleton with their N-termini [45, 47]. While the precise number and density of LINC complexes in the nuclear membrane is unknown, it is likely that the LINC complex would need to be modulated (i.e. degraded, dissociated, or relocalized) to permit the formation of vesicular intermediates, the size of which exceed the typical width of the PNS by a factor of approximately three. Indeed, Sun2 is degraded during HCMV infection [48], indicating that herpesviruses are capable of manipulating these complexes. The LINC complex might otherwise play an active role in the nuclear egress pathway, for example by connecting exported cargo to cytoskeletal motor proteins for subsequent transport to their final destination.
The fact that TorsinA physically interacts with nesprin-3 and affects the localization of Sun2 suggests that Torsins may manipulate the LINC complex [49] [50]. The precise function of Torsins remains to be defined, but likely involves at least one of the following: disassembly of the LINC complex, disassembly/recycling of fusion proteins analogous to NSF [36], a role in membrane fission, or imposition of membrane curvature. Ultimately, an in vitro reconstitution of the egress reaction will be critical for distinguishing among these possibilities. A prerequisite to this approach is that the identities of most components must be known, which is a major limitation at this point. That many of these components are likely membrane proteins, which are often difficult to purify in their native state, will likely be another complication. Of note, multivesicular body formation, a similarly complicated and topologically related process, was successfully reconstituted in vitro [51], suggesting that a biochemical reconstitution lies within the realm of possibilities.
Implication for laminopathies and related diseases affecting nuclear envelope morphology
There are many examples of diseases caused by mutations in INM proteins or their interacting partners, collectively designated nuclear envelopathies or laminopathies [52, 53]. Among the most severe manifestations are progeria (premature aging) syndromes and muscular dystrophies. Autosomal dominant (AD) Emery-Dreifuss muscular dystrophy (EDMD) is caused by distinct lamin A mutations, whereas X-linked EDMD is caused by mutations in the emerin (EMD) gene, an integral protein of the nuclear envelope. AD-EDMD is more common than X-EDMD and is often more severe, with earlier onset, often in very early childhood. Both forms are similar in that they present with muscle weakness and wasting, early contractures of the elbow, the achilles tendons and the spine. Eventually, cardiac conduction defects can lead to complete heart block and death in EDMD patients [54].
Previous studies of laminopathies focused on alterations in gene expression, mechanical stability of the nucleus, or cell-cycle regulation. However, the underlying molecular mechanisms that give rise to pathology remain poorly understood [55]. Several pathogenic alleles that cause laminopathies act as dominant negatives to yield protein aggregates when overexpressed, and aberrant intranuclear foci have been observed in primary cells from patients [53, 55, 56]. Lamin A-deficient mouse models do not show obvious phenotypes [57], suggesting a gain-of-function mechanism, possibly through proteotoxicity. Proteinaceous deposits are a hallmark of many neurodegenerative diseases induced by proteotoxic - and often dominant - variants that create structures resistant to the cellular machinery responsible for repair and turnover of damaged proteins [58, 59]. There may be a link between these diseases and the LINC complex. Mice lacking nesprin-1, a KASH protein in the outer nuclear membrane, display an EDMD-like phenotype [60]. Mutants causing both forms of EDMD and progeria bind Sun1 and/or Sun2 less tightly [61, 62]. Sun1 is upregulated in mouse models of AD-EDMD and in primary cells of progeria patients, and genetic ablation of Sun1 from these animals reduces the toxicity of the disease alleles [63].
Thus, the aforementioned diseases deserve scrutiny from the perspective of nuclear egress. The recent seminal findings [13] provide compelling evidence for interference of RNP granule export caused by an aberrant Lamin C allele, and thus establish the idea that the toxicity exerted by laminopathy-associated alleles may be attributed at least in part to interference with the nuclear egress pathway [13]. Whether this also applies to the turnover of nuclear protein aggregates remains to be established. It is possible that the wide variety of symptoms manifested in different laminopathies is due to differential effects of the mutations in question on the nuclear egress of various types of cargo from the nucleus.
Concluding remarks
By analogy to nuclear transport via the nuclear pore complex, nuclear egress may be a versatile transport mechanism with the added advantage of an increased cargo size limit. An interdisciplinary effort will be required to identify the underlying machinery, and to determine if viral and cellular pathways indeed overlap substantially with respect to mechanistic underpinnings and shared components. It will also be interesting to explore additional biological functions of this unusual transport route. A complementary approach to classical genetics could entail scrutinizing the interactions of viral proteins with as yet unknown host cell proteins (Box 1). Determining the functional consequences of manipulating these interactions, for example by RNA interference, will aid in the identification and validation of key players in the nuclear egress machinery (Box 1). Suitable nuclear model substrates that can be tracked biochemically and via live cell imaging will have to be established to validate the existence of nuclear aggregate egress (Box 1). The breakdown of these substrates should be insensitive to nuclear export inhibitors, but sensitive to inhibitors of the autophagy pathway. Future work will reveal whether these criteria are matched, which would strongly support the idea of a functional link between the nucleus and autophagy machinery. The basic components of the egress machinery may constitute novel drug targets and present therapeutic opportunities for treatment of viral infections and laminopathies.
Box 1. Outstanding questions.
Is it possible to exploit herpesviruses to find the cellular components of the endogenous nuclear egress pathway and elucidate the molecular mechanism of transport?
Which proteins are responsible for generating membrane curvature, fission and fusion required for the nuclear egress pathway? Is ESCRTIII involved in creating the negative curvature required to vesiculate the INM?
What happens to the LINC complex when the nuclear egress pathway is activated? Is there a factor responsible for its disassembly, degradation, or relocalization?
Which function(s) are fulfilled by Torsin ATPases in the context of nuclear egress?
Are there other substrates that need to exit the nucleus but are too large for transport through nuclear pore complex? Photoconvertible model substrates suitable for live cell imaging will be required to provide conclusive evidence for nuclear egress.
Is transport unidirectional, or can large components located in the cytoplasm enter the nucleus via the same pathway?
Acknowledgments
We thank Mark Hochstrasser and members of the Schlieker lab for critical reading of the manuscript and Tom DiCesare for help with illustrations. C.S. is supported by the Ellison Medical Foundation (AG-NS-0662-10) and NIH (DP2 OD008624-01).
Glossary
- Autophagy
Cellular degradation pathway that degrades large structures such as aggregated proteins and organelles by engulfing the structure in an isolation membrane and targeting it to the lysosome for degradation.
- Isolation membrane
Double-membrane structure that elongates to engulf proteins or organelles destined to be degraded by the lysosome.
- Laminopathies
Designation for a large number of diseases such as Emery-Dreifuss Muscular Dystrophy and progeria caused by mutations in nuclear lamins and proteins associated with the lamina.
- Lysosome
Cellular organelle of low pH that is capable of degrading cellular components regardless of size.
- Multi-vesicular body (MVB)
Endosomes containing numerous vesicles formed by ESCRT (endosomal sorting complex required for transport) proteins containing proteins to be degraded. MVBs eventually fuse with the lysosome and the vesicles with their cargo are degraded.
- Nuclear egress
The process by which herpesvirus capsids are transported from the nucleus to the cytoplasm. This requires vesiculation of the inner nuclear membrane and formation of a vesicular intermediate in the perinuclear space that fuses with the outer nuclear membrane to allow naked capsids access to the cytoplasm.
- Primary envelopment
Acquisition by HSV1 of a membrane envelope derived from the inner nuclear membrane upon nuclear egress. This envelope is lost upon fusion with the outer nuclear membrane.
- RNP granule
Dense ribonucleoprotein structure composed of RNA and protein shown to traffic from the nucleus to the cytoplasm via a membrane budding pathway that takes it through the nuclear envelope.
- Secondary envelopment
Acquisition by HSV1 of a double-membrane envelope. The interior membrane is the final viral membrane envelope that includes glycoproteins required for subsequent host cell entry, while the exterior membrane of this structure will fuse with the plasma membrane to release the mature virion from the cell.
- Ubiquitin proteasome system (UPS)
Cellular degradation pathway that utilizes ubiquitin as a tag to signal degradation of small soluble and membrane-bound proteins. Proteins marked for degradation by ubiquitin are targeted to the proteasome, a large protein complex that proteolyzes the target protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fields BN, et al. Fields' virology. 5th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Pante N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 4.Nakatogawa H, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 5.Mettenleiter TC, et al. Herpesvirus assembly: a tale of two membranes. Curr Opin Microbiol. 2006;9:423–429. doi: 10.1016/j.mib.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Muranyi W, et al. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science. 2002;297:854–857. doi: 10.1126/science.1071506. [DOI] [PubMed] [Google Scholar]
- 7.Mou F, et al. Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane. J Virol. 2008;82:8094–8104. doi: 10.1128/JVI.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JB, et al. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J Virol. 2007;81:4429–4437. doi: 10.1128/JVI.02354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klupp BG, et al. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc Natl Acad Sci U S A. 2007;104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisner TW, et al. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J Virol. 2009;83:3115–3126. doi: 10.1128/JVI.01462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klupp B, et al. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J Virol. 2008;82:6299–6309. doi: 10.1128/JVI.00386-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacson MK, Compton T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J Virol. 2009;83:3891–3903. doi: 10.1128/JVI.01251-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speese SD, et al. Nuclear Envelope Budding Enables Large Ribonucleoprotein Particle Export during Synaptic Wnt Signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchberger A, et al. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2010;40:238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 16.Park R, et al. Efficient induction of nuclear aggresomes by specific single missense mutations in the DNA-binding domain of a viral AP-1 homolog. J Biol Chem. 2011;286:9748–9762. doi: 10.1074/jbc.M110.198325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park YE, et al. Autophagic degradation of nuclear components in mammalian cells. Autophagy. 2009;5:795–804. doi: 10.4161/auto.8901. [DOI] [PubMed] [Google Scholar]
- 18.Dechat T, et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci U S A. 2007;104:4955–4960. doi: 10.1073/pnas.0700854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman RD, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kvam E, Goldfarb DS. Nucleus-vacuole junctions and piecemeal microautophagy of the nucleus in S. cerevisiae. Autophagy. 2007;3:85–92. doi: 10.4161/auto.3586. [DOI] [PubMed] [Google Scholar]
- 21.Millen JI, et al. Measuring piecemeal microautophagy of the nucleus in Saccharomyces cerevisiae. Autophagy. 2009;5:75–81. doi: 10.4161/auto.5.1.7181. [DOI] [PubMed] [Google Scholar]
- 22.Gay H. Nucleocytoplasmic relations in Drosophila. Cold Spring Harb Symp Quant Biol. 1956;21:257–269. doi: 10.1101/sqb.1956.021.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Szollosi MS, Szollosi D. 'Blebbing' of the nuclear envelope of mouse zygotes, early embryos and hybrid cells. J Cell Sci. 1988;91(Pt 2):257–267. doi: 10.1242/jcs.91.2.257. [DOI] [PubMed] [Google Scholar]
- 24.Kaganovich D, et al. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 26.Mercer J, et al. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 27.Scott ES, O'Hare P. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J Virol. 2001;75:8818–8830. doi: 10.1128/JVI.75.18.8818-8830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milbradt J, et al. Cytomegaloviral proteins that associate with the nuclear lamina: components of a postulated nuclear egress complex. J Gen Virol. 2009;90:579–590. doi: 10.1099/vir.0.005231-0. [DOI] [PubMed] [Google Scholar]
- 29.Murray AW, Hunt T. The cell cycle : an introduction. Oxford University Press; 1993. [Google Scholar]
- 30.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henne WM, et al. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Stauffer DR, et al. CHMP1 is a novel nuclear matrix protein affecting chromatin structure and cell-cycle progression. J Cell Sci. 2001;114:2383–2393. doi: 10.1242/jcs.114.13.2383. [DOI] [PubMed] [Google Scholar]
- 33.Calistri A, et al. Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies. J Virol. 2007;81:11468–11478. doi: 10.1128/JVI.01364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawliczek T, Crump CM. Herpes simplex virus type 1 production requires a functional ESCRT-III complex but is independent of TSG101 and ALIX expression. J Virol. 2009;83:11254–11264. doi: 10.1128/JVI.00574-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuwald AF, et al. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 36.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hewett JW, et al. TorsinB--perinuclear location and association with torsinA. J Neurochem. 2004;89:1186–1194. doi: 10.1111/j.1471-4159.2004.02404.x. [DOI] [PubMed] [Google Scholar]
- 38.Gerace L. TorsinA and torsion dystonia: Unraveling the architecture of the nuclear envelope. Proc Natl Acad Sci U S A. 2004;101:8839–8840. doi: 10.1073/pnas.0402441101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim CE, et al. A molecular mechanism underlying the neural-specific defect in torsinA mutant mice. Proc Natl Acad Sci U S A. 2010;107:9861–9866. doi: 10.1073/pnas.0912877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodchild RE, et al. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Jungwirth M, et al. Relative tissue expression of homologous torsinB correlates with the neuronal specific importance of DYT1 dystonia-associated torsinA. Hum Mol Genet. 2010;19:888–900. doi: 10.1093/hmg/ddp557. [DOI] [PubMed] [Google Scholar]
- 42.Burns LT, Wente SR. Trafficking to uncharted territory of the nuclear envelope. Curr Opin Cell Biol. 2012 doi: 10.1016/j.ceb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maric M, et al. A functional role for TorsinA in herpes simplex virus 1 nuclear egress. J Virol. 2011;85:9667–9679. doi: 10.1128/JVI.05314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodzic DM, et al. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279:25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- 45.Crisp M, et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sosa BA, et al. LINC Complexes Form by Binding of Three KASH Peptides to Domain Interfaces of Trimeric SUN Proteins. Cell. 2012;149:1035–1047. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q, et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 48.Buchkovich NJ, et al. Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection. J Virol. 2010;84:7005–7017. doi: 10.1128/JVI.00719-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nery FC, et al. TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton. J Cell Sci. 2008;121:3476–3486. doi: 10.1242/jcs.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vander Heyden AB, et al. LULL1 retargets TorsinA to the nuclear envelope revealing an activity that is impaired by the DYT1 dystonia mutation. Mol Biol Cell. 2009;20:2661–2672. doi: 10.1091/mbc.E09-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somech R, et al. Nuclear envelopathies--raising the nuclear veil. Pediatr Res. 2005;57:8R–15R. doi: 10.1203/01.PDR.0000159566.54287.6C. [DOI] [PubMed] [Google Scholar]
- 53.Worman HJ, Bonne G. "Laminopathies": a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown SC, et al. Investigating the pathology of Emery-Dreifuss muscular dystrophy. Biochem Soc Trans. 2008;36:1335–1338. doi: 10.1042/BST0361335. [DOI] [PubMed] [Google Scholar]
- 55.Worman HJ, et al. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manju K, et al. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J Cell Sci. 2006;119:2704–2714. doi: 10.1242/jcs.03009. [DOI] [PubMed] [Google Scholar]
- 57.Fong LG, et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett EJ, et al. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- 59.Winklhofer KF, et al. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. Embo J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puckelwartz MJ, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ostlund C, et al. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122:4099–4108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haque F, et al. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CY, et al. Accumulation of the inner nuclear envelope protein sun1 is pathogenic in progeric and dystrophic laminopathies. Cell. 2012;149:565–577. doi: 10.1016/j.cell.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]