Abstract
Background
The high incidence of morbidity and mortality following major burn can in part be attributed to immune derangements and wound healing complications. Inflammation plays an important role in wound healing, of which inducible nitric oxide synthase (iNOS) derived nitric oxide is a central mediator. T-cells of the γδ TCR lineage have also been shown to be important in healing of the burn wound site. Nonetheless, the role of γδ T-cells in the regulation of the burn wound iNOS expression is unknown.
Methods
Wildtype (WT) and δ TCR−/− male C57BL/6 mice were subjected to burn (3rd degree, 12.5% TBSA) or sham treatment. Three days after injury, skin samples from non-injured and the burn wound were collected and analyzed for the expression of iNOS and cytokines and chemokine levels. In a second series of experiments, WT mice were subjected to burn and left untreated or treated with the iNOS inhibitor, L-Nil. Skin cytokine and chemokine levels were assessed 3 days thereafter.
Results
Burn induced an 18-fold increase in iNOS expression at the wound site as compared to the uninjured skin of WT sham mice. In δ TCR−/− mice iNOS expression at the wound site was significantly lower than that of the WT group. Burn also induced increased levels of IL-1β, IL-6, G-CSF, TNF-α, KC, MCP-1, MIP-1α and MIP-1β at the wound site in WT and δ TCR−/− mice, but G-CSF, TNF-α, and MIP-1β levels were greater in δ TCR−/− mice. Inhibition of iNOS activity in WT mice with L-Nil suppressed burn wound levels of IL-1β, G-CSF, and MIP-1α, whereas IL-6, TNF-α, KC, MCP-1 and MIP-1β were unaffected.
Conclusions
T-cells of the γδ TCR lineage significantly contribute to the up-regulation of iNOS expression which contributes to wound inflammation.
Keywords: iNOS, chemokines, cytokines, inflammation, trauma, wound healing
1. INTRODUCTION
In burn patients, the local inflammatory process plays a pivotal role controlling wound contamination and initiating wound healing; however, the inflammatory response can become a systemic response when the burn size exceeds 20% of the patient’s body surface, leading to a wide range of complications [1, 2]. With regard to inflammatory mediators, nitric oxide (NO) has been shown to play a major role in the development of complications after burn [2, 3]. Nitric oxide, a short-lived free radical, can act as a 2nd messenger molecule. It is produced from L-arginine by the NO synthase (NOS), enzyme that exists in three different isoforms; two constitutive isoforms [endothelial and neuronal (eNOS and nNOS respectively)] and one inducible isoform, (iNOS) [4, 5]. Numerous findings have implicated NO in the inflammatory response caused by burn [2, 6, 7]. Moreover, increases in NO synthesis has been associated with remote organ dysfunction [8–10] and immune dysfunction [11–14] after burn and iNOS has been detected in skin from burned rodents and humans [15–17]. Previous studies have also shown that iNOS is important in various aspects of wound healing including reepithelialization, wound closure and collagen deposition [18, 19]. Nonetheless, the critical factors responsible for iNOS induction after burn are unclear.
Several studies from our laboratory suggest an important role of γδ T-cells in the development of the immune-inflammatory response after burn [20–22]. In particular, findings suggest that this unique T-cell population is critical in the healing of the burn wound [20, 21]. Unique characteristics of these cells, such as their location in barrier tissues, fast expansion upon activation and their ability to independently recognize antigens make them ideal regulators of local injury and maintenance of skin homeostasis [23, 24]. Jones-Carson et al.[25] have shown that γδ T-cells enhance macrophage NO production and that γδ T-cell depletion abrogated iNOS expression in response to Candida albicans infection. The current study was undertaken to determine the relationship between the induction of burn wound iNOS expression and γδ T-cells.
2. MATERIALS AND METHODS
2.1 Animals
Male C57BL/6 (WT) and male mice lacking γδ T-cells (δ TCR−/−; C57BL/6J-Tcrdtm1Mom [22 to 25 g; 8 to 10 wk of age]) were obtained from Jackson Laboratories (Bar Harbor, ME). The mice were allowed to acclimatize in the animal facility for at least 1 week prior to experimentation. The experiments in this study were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and were performed in accordance with the National Institutes of Health guidelines for the care and handling of laboratory animals.
2.2 Burn procedure
Mice received a scald burn as described previously [26]. Briefly, the mice were anesthetized by intramuscular (i.m.) injection of ketamine/xylazine, and the dorsal surface was shaved. The anesthetized mouse was placed in a custom insulated mold exposing 12.5% of their total body surface area (TBSA) along the right dorsum. The mold was immersed in 70°C water for 10 sec to produce a 3rd degree burn. Previous studies have verified this injury to be a full thickness burn with damage to the epidermal, dermal and sub-dermal layers [26, 27]. The mice were then resuscitated with 1 ml of Ringer's lactate solution administered by intraperitoneal injection and returned to their cages. The cages were placed on a heating pad for 2 hrs until the mice were fully awake, at which time they were returned to the animal facility. Sham treatment consisted of anesthesia and resuscitation with Ringer's lactate solution only.
2.3 Administration of the iNOS inhibitor, L-Nil
L-Nil (Cayman Chemical, Ann Arbor, MI) was administered with s.c. implanted mini-osmotic pumps (ALZET model 2001 [mean pumping rate is 1 μl/hr], DURECT Corporation, Cupertino, CA). L-Nil was dissolved in PBS and the pumps were filled according to the manufacturer’s instructions. L-Nil dosage was 1.0 mg/day based on previously published findings [28].
2.4 Tissue collection and processing
Skin samples from the burn site and uninjured skin from the injured and sham mice was collected at 3 days after injury. Ventral skin was collected as the uninjured sample in the burn mice. Skin samples from the burn site included injured skin and the wound margin. The burn wound and uninjured skin were excised, down to the level of the musculofascia, including the submucosal layer by sharp dissection and immediately snap-frozen in liquid nitrogen. Skin samples were stored at −80°C until analysis for iNOS expression and cytokine/chemokine content.
2.5 Analysis of iNOS expression in skin lysates
For Western blot analysis the samples were homogenized in a protease inhibitor cocktail (Calbiochem®, San Diego, CA). The tissue extract was clarified by centrifugation at 14,000 rpm for 20 min. Samples (25 μg/lane) were run on a 12.5% NuPAGE® Novex® Bis-Tris Gel (Invitrogen Laboratories) (45 min at room temperature, voltage: 200 V) and transferred (2 hrs at 4°C, voltages: 100 V) to a nitrocellulose membrane (Immun-Blot PVDF Membrane, Bio-Rad Laboratories). The membranes were blocked with 5% non-fat milk for 1 hr, and incubated with a mouse antiserum against iNOS (1:500, Cell Signaling Technology) and anti-β-actin (1:2000, Sigma Lab) overnight at 4°C. The blots were then washed and incubated with secondary antibodies (goat anti-rabbit IgG for iNOS 1:10000 and goat anti-rabbit IgG for β-actin 1:20000, Santa Cruz, CA) for 1 hr. Antigens were visualized using a chemiluminescence kit (SuperSignal West Pico, Thermo Scientific, USA). Pictures of the membranes were scanned and analyzed using ImageJ software (NIH).
2.6 Analysis of cytokines/chemokines in skin lysates
Tissue samples were homogenized in protease inhibitor cocktail prior to analysis. The Bioplex (Bio-Rad) system was used for cytokine/chemokine levels analysis. Cytokines and chemokines’ levels in the skin samples were normalized to mgs of total protein as determined using a BCA (Sigma-Aldrich Laboratories, Saint Louis, MO).
2.7 Statistical analysis
Data are expressed as mean ± SE. Comparisons were analyzed using ANOVA. A p-value < 0.05 was considered to be statistically significant for all analyses.
3. RESULTS
3.1 Effect of burn on skin iNOS expression 3 days after injury
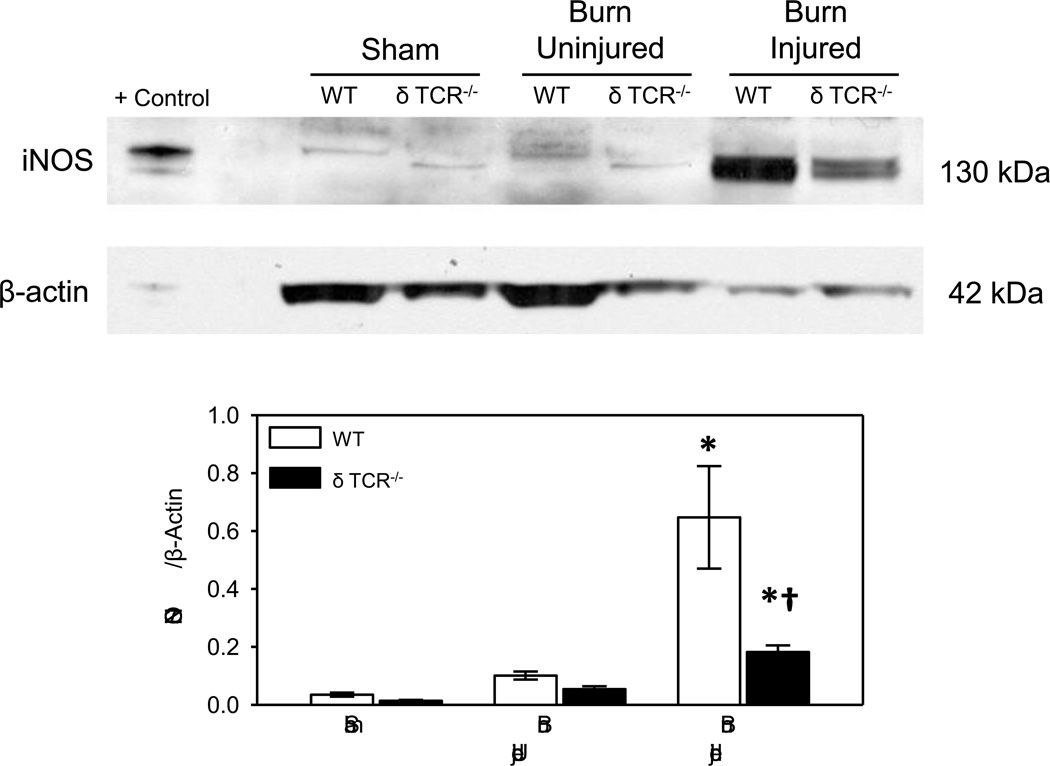
At 3 days after burn a profound increase in skin iNOS expression in WT mice was observed (Fig. 1). A significant increase in the iNOS expression was seen in the burn wound as compared with the uninjured skin of the burn mice or the sham mice. The most robust response was seen in the burn wounds with an 18-fold increase in iNOS expression as compared to the uninjured skin of WT sham mice. A 6-fold increase was observed as compared with uninjured skin from burn mice. In δ TCR−/− mice a slight, but significant increase in iNOS expression was observed at the wound site and in the uninjured skin of the burn mice, as compared to the uninjured skin of the sham δ TCR−/− mice. While, burn increased iNOS expression at the wound site of δ TCR−/− mice, it was significantly less (~20%) than that observed in WT mice.
Fig. 1. iNOS expression in uninjured and burn skin lysates.
Skin samples of WT and δ TCR−/− mice at 3 days after burn were assessed for iNOS expression by Western blot as described in Materials and Methods. Raw 264.7 whole cell lysates were used for the positive control (Santa Cruz Biotechnology). Data are mean ± SE for 6–8 mice/group. * p<0.05 vs. uninjured skin or sham skin. † p<0.05 vs. respective WT group.
The iNOS protein was expressed at 2 distinct molecular weights (mw) of approximately 135 and 130 kd. The 130 mw band predominated in the δ TCR−/− mice, whereas the 135 mw band was the predominant form in WT mice, irrespective of injury. In addition, β-actin and GAPDH levels were lower in wound samples from both WT and δ TCR−/− mice (Table 1).
Table 1.
β-actin and GAPDH expression in skin lysatesa
| Sham | Burn Uninjured | Burn Injured | ||
|---|---|---|---|---|
| β-Actin | WT | 2.51 ± 0.37 | 2.09 ± 0.38 | 0.96 ± 0.12b,c |
| δ TCR−/− | 3.01 ± 0.34 | 1.48 ± 0.17 | 1.30 ± 0.17 b,c | |
| GAPDH | WT | 7.26 ± 0.44 | n.d. | 0.42 ± 0.13b |
| δ TCR−/− | 3.88 ± 0.27 | n.d. | 0.93 ± 0.06b | |
Skin samples were collected at 3 days after injury or sham procedure and processed for β-actin content as described in the Materials and Methods. Data are the mean ± SEM for relative densitometric units; n=8 mice/group for β-actin and n=3 mice/group for GAPDH.
p<0.05 as compared with respective sham
p<0.05 as compared with respective burn uninjured
n.d. not determined
3.2 Skin cytokine content after burn
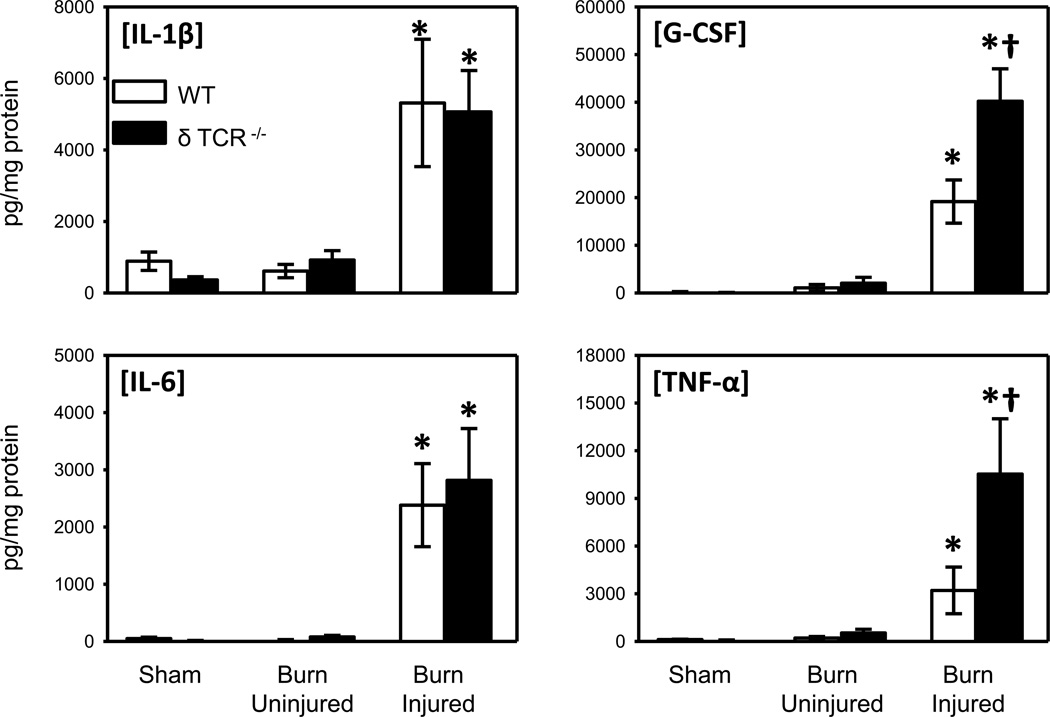
As markers of inflammation, we analyzed the expression of IL-1β, IL-6, G-CSF and TNF-α in skin lysates of the WT and the δ TCR−/− (Fig. 2). No significant differences in cytokine levels were observed between uninjured skin from sham and burn mice. Burn induced a significant increase in the expression of all cytokines at the wound sites of WT and δ TCR−/− mice as compared with the uninjured skin of the injured or sham mice skin. G-CSF and TNF-α levels were approximately 2-fold greater in the wound skin of δ TCR−/− mice as compared with that of WT mice. In contrast, IL-1β and IL-6 levels at the wound site were comparable in WT and δ TCR−/− mice.
Fig. 2. Cytokine content in uninjured and burn skin lysates.
Skin samples of wild type and δ TCR−/− mice at 3 days after burn were assessed for IL-1β, IL-6, G-CSF and TNF-α expression by Bioplex as described in Materials and Methods. Data are expressed as the mean ± SEM for 8 mice/group. * p<0.05 vs. uninjured skin or sham skin. † p<0.05 vs. respective WT group.
3.3 Skin chemokine content after burn
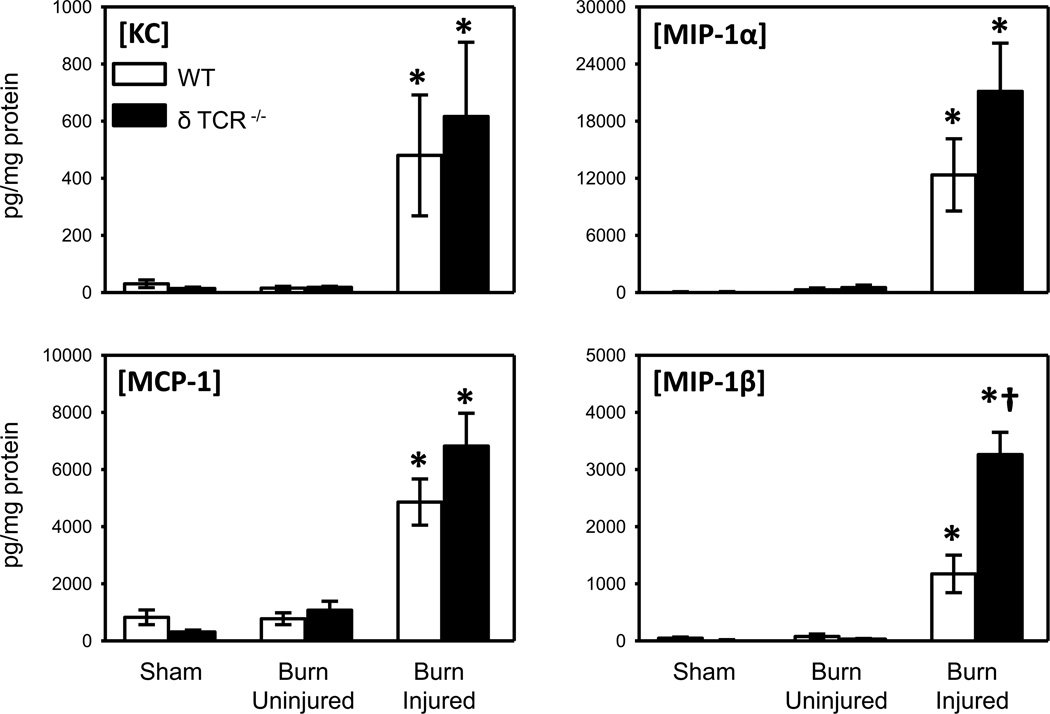
The expression of the chemokines KC, MCP-1, MIP-1α and MIP-1β was measured in the skin lysates of the WT and the δ TCR−/− mice (Fig. 3). Similar to our findings with inflammatory cytokine content, no significant differences in chemokine levels were observed between uninjured skin from sham and burn mice. A significant increase in the expression of all chemokines at the burn site of the WT and δ TCR−/− mice, as compared with the uninjured skin of the burn or sham mice was observed. KC, MCP-1, MIP-1α levels in the injured skin of the δ TCR−/− mice did not differ from those observed in the WT mice; however, MIP-1β levels were elevated 3-fold in the δ TCR−/− mice.
Fig. 3. Chemokine content in uninjured and burn skin lysates.
Skin samples of wild type and δ TCR−/− mice at 3 days after burn were assessed for KC, MCP-1, MIP-1α and MIP-1β content by Bioplex as described in Materials and Methods. Data are expressed as the mean ± SEM for 8 mice/group. * p<0.05 vs. uninjured skin or sham skin. † p<0.05 vs. respective WT group.
3.4 Effect of iNOS inhibition on skin cytokine and chemokine content
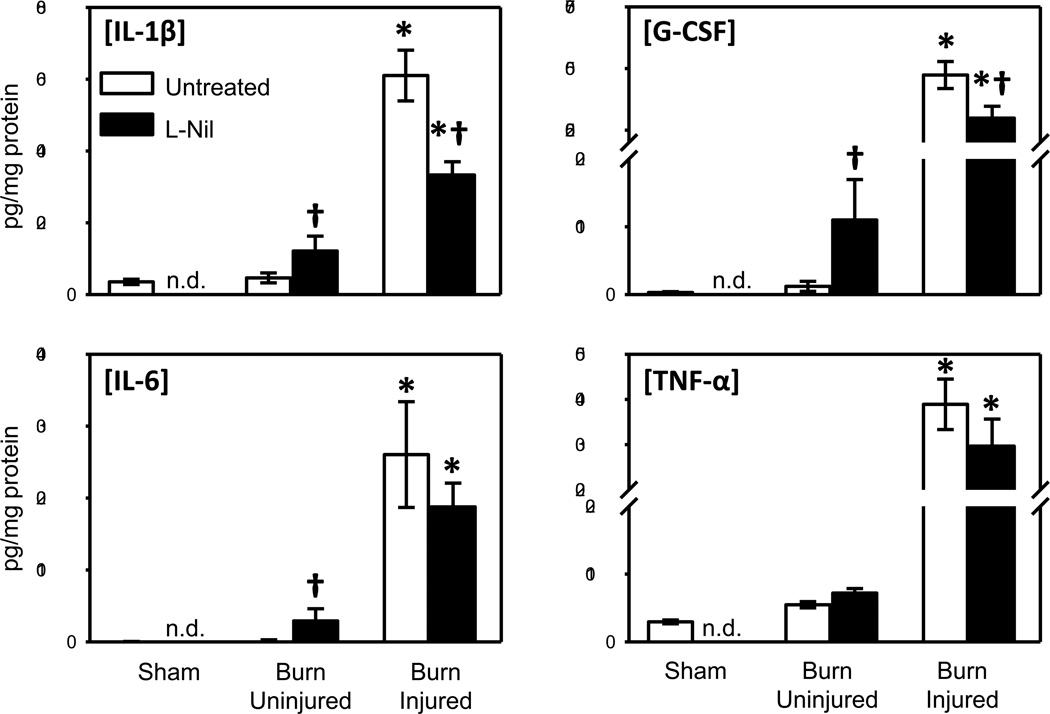
As with the first set of experiments (see Fig. 2), IL-1β, IL-6, G-CSF and TNF-α levels in burn skin were significantly elevated as compared with uninjured skin from burn mice or sham mice. Treatment of mice with L-Nil after burn resulted in a significant elevation in IL-1β, IL-6 and G-CSF in uninjured skin from burn mice (Fig. 4). In contrast, burn skin from L-Nil treated mice had significantly lower levels of IL-1β and G-CSF as compared with untreated mice. IL-6 and TNF-α level in burn skin did not differ between the untreated and L-Nil treated groups.
Fig. 4. Effect of iNOS inhibition on skin cytokine content.
Skin samples of untreated and L-Nil treated mice at 3 days after burn were assessed for IL-1β, IL-6, G-CSF and TNF-α expression by Bioplex as described in Materials and Methods. Data are expressed as the mean ± SEM for 7–12 mice/group. * p<0.05 vs. uninjured skin or sham skin. † p<0.05 vs. respective untreated group. n.d. = not determined.
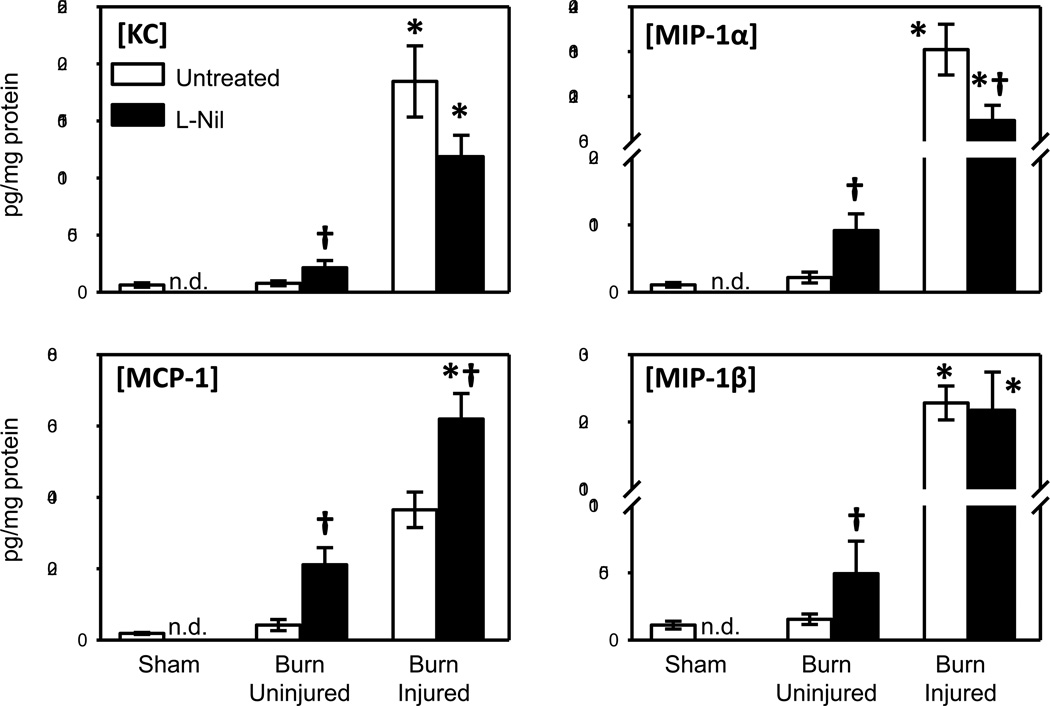
Chemokine levels were elevated in burn skin, as initially observed (see Fig. 3), and also affected by L-Nil treatment (Fig. 5). L-Nil treatment increased the levels of KC, MCP-1, MIP-1α and MIP-1β in uninjured skin from burn mice. In burn skin, L-Nil treatment resulted in a 50% increase in MCP-1 levels as compared with untreated mice. In contrast, burn mice treated with L-Nil displayed a 35–50% drop in the levels of KC and MIP-1α at the injury site. MIP-1β levels at the injury site were unaffected by L-Nil treatment.
Fig. 5. Effect of iNOS inhibition on skin chemokine content.
Skin samples of untreated and L-Nil treated mice at 3 days after burn were assessed for KC, MCP-1, MIP-1α and MIP-1β expression by Bioplex as described in Materials and Methods. Data are expressed as the mean ± SEM for 7–12 mice/group. * p<0.05 vs. uninjured skin or sham skin. † p<0.05 vs. respective untreated group. n.d. = not determined.
4. DISCUSSION
Given the important role of iNOS-derived NO in the wound healing process, the current study was conducted to assess the role of epidermal γδ T-cells in the expression of iNOS at the burn wound site. Our findings here, for the first time, demonstrate that γδ T-cells are critical in the up-regulation of iNOS at the burn injury site, since iNOS expression was markedly reduced in burn δ TCR−/− mice as compared to WT mice. This γδ T-cell-dependent iNOS expression appears to be in part independent of effects on other aspects of the wound inflammatory response, since the expression of only selected inflammatory cytokines and chemokines at the burn site were elevated in δ TCR−/− mice.
Nitric oxide (NO), a multifunctional biological messenger, has been shown to be involved in several pathways of the inflammatory process, including granulation tissue formation, epithelial proliferation, collagen synthesis and angiogenesis [29]. The high expression of iNOS in the acute inflammatory phase after burn by the WT mice, as compared with the δ TCR−/− mice, suggests a pivotal role for γδ T-cells in the acute host response, NO synthesis regulation and subsequent wound healing. Studies by Isenberg et al, have shown the role of NO in multiple steps of the wound healing process and how disruption of its delicate balance can compromise adequate healing [30].
In the current study we observed two distinct bands for the iNOS protein, in which the lower molecular weight band predominated in the δ TCR−/− mice. The reason for the double band is unclear, but may be related to post-translational modifications, such as phosphorylation or ubiquination [31]. Other investigators have also shown modification of iNOS molecular weight in non-burn models [32, 33]. The reasons and mechanisms responsible for these γδ T-cell-dependent changes in iNOS expression remain to be elucidated.
Previous studies have shown that γδ T-cells display properties of innate immune cells, highlighting the role of γδ T cells in the early immune response after injury [34, 35]. Gamma delta T-cells are involved in all stages of the wound healing process, from the early inflammatory infiltration at the injury site to modulation of macrophage [36], keratinocytes and fibroblast responses [37]. Gamma delta (γδ) T-cells are an important source of chemokines [23] that attract monocytes and macrophages early post burn, and respond rapidly and efficiently to invading pathogens through their ability to recognize specialized patterns of antigen without antigen-presenting cells or prior antigen degradation. Studies by Alexander et al, suggest that γδ T-cells are critical to burn wound healing as evidenced by decreased levels of growth factors and immune cell infiltration in the burn wounds of γδ T-cell deficient mice [21]. Additional studies by Daniel et al in a murine wound sponge model have shown that wound infiltration is lacking in γδ T-cell deficient mice [20]. In contrast, to the current study, Daniel et al showed a profound attenuation in cytokine/chemokine levels at the wound site in δ TCR−/− mice. This suggests that γδ T-cell-mediated regulation of resident immune cells (in the current study) and infiltrating cells (Daniel et al.) is markedly different.
Once the burn disrupts the skin equilibrium, an inflammatory cascade is initiated involving the production and release of multiple inflammatory mediators. In the present study we found a significant inflammatory response at the burn site at 3 days after burn. This inflammatory response was associated with significantly higher levels of the pro-inflammatory cytokines and chemokines. Somewhat surprisingly, a number of these inflammatory mediators were elevated further in δ TCR−/− mice. These findings suggest that, unlike growth factors [21], the burn inflammatory response appears to be differentially regulated γδ T-cells at 3 days post-injury and γδ T-cells appear to play either directly or indirectly a somewhat anti-inflammatory role. The current study focused on 3 days after injury, as this has been shown to be the time most critical for activation of macrophages, the likely wound cell expressing iNOS [38–41]. The effect of γδ T-cells at other times post-injury remains to be determined.
Our findings showed a differential effect of iNOS inhibition on cytokine and chemokine expression after burn. A number of inflammatory mediators (IL-1β, G-CSF and MIP-1α) were suppressed by iNOS inhibition with L-Nil, whereas MCP-1 was elevated under such conditions. This effect of iNOS inhibition may be in part related to decreased recruitment of mononuclear cells to the site that produce IL-1β, G-CSF and MIP-1α and elevated production of MCP-1 by resident cells to recruit the inflammatory mononuclear cells. In this regard, iNOS has been shown to be important in cellular recruitment in models of pulmonary fibrosis and hepatic ischemia/reperfusion [42, 43]. These effects of iNOS inhibition or depletion may be related to angiogenesis, as angiogenesis has been shown to be stimulated by NO in a rat burn model [29]. Studies by Yamasaki et al have shown that iNOS knockout mice exhibit delayed angiogenesis [19], probably related to an iNOS-dependent depletion of VEGF levels [41].
In conclusion, T-cells of the γδ TCR lineage have a significant role in the burn wound healing process through the regulation of iNOS expression at the injury site. Wound healing is a relevant clinical problem, and clearer understanding of potential targets of therapeutic intervention (such as γδ T-cells) may provide burn care improvement leading to decreased morbidity and mortality.
HIGHLIGHTS.
Burn is associated with profound inflammation and innate immune system activation
Burn wounds had a massive increase in iNOS expression and inflammatory cytokines
iNOS expression in the burn wound of γδ T-cell deficient mice was markedly reduced
Gamma delta T-cells regulate wound iNOS expression independent of inflammation
ACKNOWLEDGEMENTS
Support was provided by NIH grant GM079122. Dr. Oppeltz is a General Surgery Resident who was enrolled in the Master of Science in Clinical Investigation of UTHSCSA. RFO, QZ and MR were responsible for the animal experiments, Western blot and Bioplex analysis. RFO was responsible for the data analysis, scientific interpretation and drafted the manuscript. MGS was responsible for scientific conception, design and interpretation and assisted in the drafting of the manuscript. All authors read and approved the final version of the manuscript. The authors declare that they have no competing interest.
Grant information: NIH grant GM079122
ABBREVIATIONS
- γδ
gamma-delta
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- δ TCR−/−
gamma-delta T-cell deficient animal
- WT
wild type
- TBSA
total body surface area
- IL
interleukin
- TNF-α
tumor necrosis factor alpha
- MCP-1
Monocyte chemotactic protein-1
- MIP-1α
Macrophage Inflammatory Protein 1 alpha
- MIP-1β
Macrophage Inflammatory Protein 1 beta
- KC
keratinocyte chemoattractant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Richard F. Oppeltz, Email: oppeltz@uthscsa.edu.
Meenakshi Rani, Email: rani@uthscsa.edu.
Qiong Zhang, Email: zhangq0@uthscsa.edu.
Martin G. Schwacha, Email: schwacha@uthscsa.edu.
REFERENCES
- 1.Schwacha MG, Nickel E, Daniel T. Burn injury-induced alterations in wound inflammation and healing are associated with suppressed hypoxia inducible factor-1alpha expression. Mol Med. 2008;14:628–633. doi: 10.2119/2008-00069.Schwacha. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira GV, Shimoda K, Enkhbaatar P, Jodoin J, Burke AS, Chinkes DL, et al. Skin nitric oxide and its metabolites are increased in nonburned skin after thermal injuries. Shock. 2004;22:278–282. doi: 10.1097/01.shk.0000135259.90311.33. [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Wei X, Bian K, Murad F. Effects of nitric oxide on skin burn wound healing. J Burn Care Res. 2008;29:804–814. doi: 10.1097/BCR.0b013e3181848119. [DOI] [PubMed] [Google Scholar]
- 4.Curran JN, Winter DC, Bouchier-Hayes D. Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Repair Regen. 2006;14:376–386. doi: 10.1111/j.1743-6109.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Palmer RM, Higgs EA. Nitric oxide; physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 6.Gamelli RL, George M, Sharp-Pucci M, Dries DJ, Radisazljevic Z. Burn-induced nitric oxide release in humans. J Trauma. 1995;39:869–878. doi: 10.1097/00005373-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Daniel T, Alexander M, Hubbard WJ, Chaudry IH, Choudhry MA, Schwacha MG. Nitric oxide contributes to the development of a post-injury Th2 T-cell phenotype and immune dysfunction. J Cell Physiol. 2006;208:418–427. doi: 10.1002/jcp.20677. [DOI] [PubMed] [Google Scholar]
- 8.Soejima K, Traber LD, Schmalstieg FC, Hawkins H, Jodoin JM, Szabo C, et al. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 2001;163:745–752. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 9.Chen LW, Hsu CM, Cha MC, Chen JS, Chen SC. Changes in gut mucosal nitric oxide synthase (NOS) activity after thermal injury and its relation with barrier failure. Shock. 1999;11:104–110. doi: 10.1097/00024382-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Chen LW, Hsu CM, Wang JS, Chen HL, Chen JS. Inhibition of inducible nitric oxide synthase (iNOS) prevents lung neutrophil deposition and damage in burned rats. Shock. 2001;15:151–156. doi: 10.1097/00024382-200115020-00012. [DOI] [PubMed] [Google Scholar]
- 11.Schwacha MG, Somers SD. Thermal injury induced immunosuppression in mice: the role of macrophage derived reactive nitrogen intermediates. J Leuko Biol. 1998;63:51–58. doi: 10.1002/jlb.63.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Schwacha MG, Samy TSA, Cantania RA, Chaudry IH. Thermal injury alters macrophage responses to prostaglandin E2: contribution to the enhancement of inducible nitric oxide synthase activity. J Leuko Biol. 1998;64:740–746. doi: 10.1002/jlb.64.6.740. [DOI] [PubMed] [Google Scholar]
- 13.Bamberger T, Masson I, Mathieu J, Chauvelot-Moachon L, Giroud JP. Nitric oxide mediates the depression of lymphoproliferative responses following burn injury in rats. Biomed Pharmacother. 1992;46:445–450. doi: 10.1016/0753-3322(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 14.Masson I, Mathieu J, Nolland XB, Sousa MD, Chanaud B, Stralko S, et al. Role of nitric oxide in depressed lymphproliferative responses and altered cytokine production following thermal injury in rats. Cell Immunol. 1998;186:121–132. doi: 10.1006/cimm.1998.1296. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Ando K, Wakisaka N, Matsuzaki K, Aihara M, Kumagai N. Effects of nitric oxide synthase inhibitors on vascular hyperpermeability with thermal injury in mice. Nitric Oxide. 2001;5:334–342. doi: 10.1006/niox.2001.0350. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen SM, Wurster SH, Nanney LB. Expression of inducible nitric oxide synthase in human burn wounds. Wound Repair Regen. 1998;6:142–148. doi: 10.1046/j.1524-475x.1998.60208.x. [DOI] [PubMed] [Google Scholar]
- 17.Rawlingson A, Shendi K, Greenacre SA, England TG, Jenner AM, Poston RN, et al. Functional significance of inducible nitric oxide synthase induction and protein nitration in the thermally injured cutaneous microvasculature. Am J Pathol. 2003;162:1373–1380. doi: 10.1016/S0002-9440(10)63933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stallmeyer B, Kampfer H, Kolb N, Pfeilschifter J, Frank S. The function of nitric oxide in wound repair: inhibition of inducible nitric oxide-synthase severely impairs wound reepithelialization. J Invest Dermatol. 1999;113:1090–1098. doi: 10.1046/j.1523-1747.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki K, Edington HD, McClosky C, Tzeng E, Lizonova A, Kovesdi I, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest. 1998;101:967–971. doi: 10.1172/JCI2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel T, Thobe BM, Chaudry IH, Choudhry MA, Hubbard WJ, Schwacha MG. Regulation of the postburn wound inflammatory response by gammadelta T-cells. Shock. 2007;28:278–283. doi: 10.1097/shk.0b013e318034264c. [DOI] [PubMed] [Google Scholar]
- 21.Alexander M, Daniel T, Chaudry IH, Choudhry M, Schwacha MG. T-cells of the T-cell receptor lineage play an important role in the postburn wound healing process. Journal of Burn Care and Research. 2006;27:18–25. doi: 10.1097/01.bcr.0000188325.71515.19. [DOI] [PubMed] [Google Scholar]
- 22.Schneider DF, Glenn CH, Faunce DE. Innate lymphocyte subsets and their immunoregulatory roles in burn injury and sepsis. J Burn Care Res. 2007;28:365–379. doi: 10.1097/BCR.0B013E318053D40B. [DOI] [PubMed] [Google Scholar]
- 23.Schwacha MG. Gammadelta T-cells: potential regulators of the post-burn inflammatory response. Burns. 2009;35:318–326. doi: 10.1016/j.burns.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones-Carson J, Vazquez-Torres A, van der Heyde HC, Warner T, Wagner RD, Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–557. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki JR, Zhang Q, Schwacha MG. Burn induces a Th-17 inflammatory response at the injury site. Burns. 2011;37:646–651. doi: 10.1016/j.burns.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14:623–628. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 28.Badn W, Hegardt P, Fellert MA, Darabi A, Esbjornsson M, Smith KE, et al. Inhibition of inducible nitric oxide synthase enhances anti-tumour immune responses in rats immunized with IFN-gamma-secreting glioma cells. Scand J Immunol. 2007;65:289–297. doi: 10.1111/j.1365-3083.2007.01901.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu H, Ka B, Murad F. Nitric oxide accelerates the recovery from burn wounds. World J Surg. 2007;31:624–631. doi: 10.1007/s00268-007-0727-3. [DOI] [PubMed] [Google Scholar]
- 30.Isenberg JS, Ridnour LA, Espey MG, Wink DA, Roberts DD. Nitric oxide in wound-healing. Microsurgery. 2005;25:442–451. doi: 10.1002/micr.20168. [DOI] [PubMed] [Google Scholar]
- 31.Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. J Cell Sci. 2004;117:2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 32.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Kibbe M, Billiar T, Tzeng E. Inducible nitric oxide synthase and vascular injury. Cardiovasc Res. 1999;43:650–657. doi: 10.1016/s0008-6363(99)00130-3. [DOI] [PubMed] [Google Scholar]
- 34.Augustin A, Kubo RT, Sim GK. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989;340:239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 35.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 36.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 37.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406–412. doi: 10.1016/s0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- 39.Lee RH, Efron D, Tantry U, Barbul A. Nitric oxide in the healing wound: a time-course study. J Surg Res. 2001;101:104–108. doi: 10.1006/jsre.2001.6261. [DOI] [PubMed] [Google Scholar]
- 40.Schwacha MG, Thobe BM, Daniel T, Hubbard WJ. Impact of thermal injury on wound infiltration and the dermal inflammatory response. J Surg Res. 2010;158:112–120. doi: 10.1016/j.jss.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kane AJ, Barker JE, Mitchell GM, Theile DR, Romero R, Messina A, et al. Inducible nitric oxide synthase (iNOS) activity promotes ischaemic skin flap survival. Br J Pharmacol. 2001;132:1631–1638. doi: 10.1038/sj.bjp.0703944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naura AS, Zerfaoui M, Kim H, Abd Elmageed ZY, Rodriguez PC, Hans CP, et al. Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. J Immunol. 2010;185:3076–3085. doi: 10.4049/jimmunol.0904214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamada T, Duarte S, Tsuchihashi S, Busuttil RW, Coito AJ. Inducible nitric oxide synthase deficiency impairs matrix metalloproteinase-9 activity and disrupts leukocyte migration in hepatic ischemia/reperfusion injury. Am J Pathol. 2009;174:2265–2277. doi: 10.2353/ajpath.2009.080872. [DOI] [PMC free article] [PubMed] [Google Scholar]