Abstract
The neuropeptide vasopressin has traditionally been associated with vasoconstriction and water reabsorption by the kidneys. However, data from experimental animal studies also implicate vasopressin in social bonding processes. Preliminary work suggests that vasopressin also plays a role in social behaviors in humans. The goal of this cross-sectional study was to evaluate associations among plasma vasopressin and self-reported interpersonal functioning in a sample of married couples. During a 24-hour admission to a hospital-based research unit, 37 couples completed measures of interpersonal functioning and provided blood samples for neuropeptide analyses. Results showed that vasopressin was associated with markers of interpersonal functioning, but not with general psychological distress. Specifically, greater plasma vasopressin levels were related to a larger social network, fewer negative marital interactions, less attachment avoidance, more attachment security, and marginally greater spousal social support. These results indicate that vasopressin is likely implicated in different relationship maintenance processes in humans.
Keywords: vasopressin, social functioning, couples, social support, marital quality
Arginine vasopressin (AVP) is a neuropeptide that has traditionally been associated with the regulation of vasoconstriction and water reabsorption by the kidneys (Vincent and Su, 2008). However, mounting evidence from animal studies also implicates AVP in social behaviors (Caldwell et al., 2008). To date, only a handful of studies have examined the role of AVP in social bonding processes in humans.
Manipulation of central AVP levels via intra-nasal peptide administration facilitated perceptions of friendliness in females, but diminished perception of friendliness and recognition of negative facial expressions in males (Thompson et al., 2006; Uzefovsky et al., 2011). Furthermore, intra-nasal AVP administration increased the likelihood of reciprocated cooperation (i.e., they gave money back to their partner when the partner had initially entrusted them with some money), but it did not impact initial trust behavior in a Prisoner’s Dilemma game (Rilling, et al., 2011). Pharmacological AVP manipulation thus impacts cognitive and behavioral responses during interaction with strangers.
Different markers of vasopressinergic activity have been related to social bonding and marital quality. Gene polymorphisms of the AVP V1a receptor have been associated with autism, a disorder characterized by social behaviors deficits and verbal and non-verbal communication difficulties (Kim, et al., 2002; Wassink, et al., 2004; Yirmiya et al., 2006). Among healthy individuals, AVP V1a receptor polymorphisms have also been related to a greater likelihood of being married, decreased frequency of marital conflict, and perception of better marital quality in males and in their female partners (Walum, et al., 2008). Moreover, higher plasma AVP levels have been related to greater self-reported marital distress in men, but not in women (Taylor et al., 2010). In contrast, higher levels of plasma AVP were associated with lower frequencies of negative communication behaviors during a marital interaction task among both males and females (Gouin, et al., 2010).
Results of experimental animal studies parallel human data. Pharmacological manipulation of AVP improved social memory and recognition (Engelmann and Landgraf, 1994), facilitated social bonding (Cho et al., 1999; Winslow et al., 1993), promoted paternal care (Parker and Lee, 2001), and increased inter-male and maternal aggression (Bester-Meredit et al., 1999; Wersinger et al. 2007). Collectively, these data implicate AVP in a range of processes involved in interpersonal relationships initiation and maintenance.
Given the paucity of data linking AVP with interpersonal processes in humans, the purpose of this cross-sectional study was to examine the associations of plasma AVP with a range of indicators of interpersonal functioning. We predicted that in a sample of non-depressed, married individuals, plasma AVP would be more strongly associated with indices of interpersonal functioning than with markers of psychological distress.
Methods
Participants
As part of a larger study on marital stress and wound healing, 37 heterosexual, married couples (74 participants) were recruited through newspaper and radio ads, notices posted on campus and in the community, and referrals from other participants (Kiecolt-Glaser, et al., 2005). Exclusion criteria included 1) health problems or related medications that had an obvious immunological or endocrinological component or consequence for wound healing (e.g., cancer, recent surgeries, strokes, diabetes mellitus, peripheral vascular disease, conditions such as asthma or arthritis that required regular use of anti-inflammatory medications), 2) blood pressure medication, smoking, or using excessive alcohol or caffeine, and 3) pregnancy, current involvement in breastfeeding, or anti-diuretic medication use. All women were scheduled during the follicular phase of their menstrual cycle. The Institutional Biomedical Research Review Committee approved the project; all subjects gave written informed consent prior to participation.
Protocol
Individuals participated in a 24-hour visit at a hospital-based research unit. Upon admission at 7:00 AM, a heparin well was inserted in each participant’s arm to facilitate blood sample collection throughout the day. Couples subsequently underwent a blistering procedure for the wound healing study and participated in two marital interaction tasks, as described elsewhere (Kiecolt-Glaser, et al., 2005). The psychological distress and marital quality questionnaires were completed during the adaptation period in the morning. The social network, social support, and attachment style questionnaires were administered after the experimental tasks in the afternoon. Throughout the admission, couples were encouraged to drink water regularly to facilitate blood draws and prevent AVP fluctuations due to changes in plasma osmolarity. Blood samples for AVP were taken at 7:45 AM (baseline) and 12:00 PM (after the second interaction task).
Self-report measures
Psychological distress
The Beck Depression Inventory (BDI) assessed cognitive and vegetative depressive symptoms in the past two weeks (Beck et al., 1988). The Beck Anxiety Inventory (BAI) assessed anxious symptoms in the past two weeks (Beck et al., 1988). Higher scores on these measures indicated greater psychological distress.
Interpersonal functioning
The Social Network Inventory (SNI) provided an index of social integration by evaluating regular participation in different types of social roles (Cohen et al., 1997). The types of relationships and social roles evaluated included spouse, parents, parents-in-law, children, other close family member, close neighbors, friends, coworkers, fellow volunteers, member of religious and non-religious groups. Individuals with larger social network had higher scores on this measure.
The Social Support Questionnaire (SSQ) quantified the perceived availability of social support (Sarason et al., 1983). Participants were asked to list who they could turn to or rely on when they were faced with a given sets of circumstances. Examples of items include: “who can console you when you are very upset?”, and “who can you really count to help out in a crisis situation?” Two indexes were derived from the scales: 1) number of times the spouse was mentioned as a source of support and 2) the total number of people (excluding the spouse) listed as available to provide support when needed. Because 73% of the sample listed their spouse as a support provider for all the situations listed, a dichotomous variable distinguishing whether the participants listed their spouse as being supportive in all vs. some of the situations described was created. Higher scores indicated greater perceived social support.
The Marital Adjustment Test (MAT) assessed marital adjustment and satisfaction (Locke and Wallace, 1959). Participants were asked to rate their current level of happiness in their marriage, and the degree agreement with their spouse on several topics such as finance, in-laws, or sexual relations. Higher scores were associated with greater relationship satisfaction.
The Test of Negative Social Exchanges (TENSE) is an 18-item questionnaire assessing the frequency of occurrence of negative social interactions in the past month (Ruehlman and Karoly, 1991). Participants completed two versions of the questionnaire: one referring to marital interactions and one referring to non-marital interactions. The total score was computed by summing the average of each subscale. Higher scores were indicative of more frequent negative social interactions.
Adult attachment style was measured using both categorical and continuous measures of attachment. With the Hazan and Shaver’s categorical attachment style measures, participants indicated their degree of agreement to one-sentence descriptions of secure, anxious, and avoidance attachment style using a 6-point Likert scale (Hazan and Shaver, 1987). Higher scores represented greater endorsement of the specific attachment style. The Experiences in Close Relationships-Revised (ECRr) is a 36-item scale assessing attachment style at a dimensional level (Brennan et al., 1998). It comprised two subscales evaluating attachment anxiety and avoidance. Higher scores were associated with greater attachment anxiety and avoidance, respectively.
Plasma Vasopressin Assay
Plasma was kept chilled and immediately frozen at −80° prior to thawing for assays. The plasma was diluted in assay buffer (1:4) to give results reliably within the linear portion of the standard curve. Plasma AVP was assayed in duplicate by enzyme immunoassay (Assay Designs, Ann Arbor, MI), per kit instructions. This EIAs are highly sensitive (minimal detection levels = 4 pg/ml vasopressin) with very little antibody cross-reactivity for other neuropeptides. For the vasopressin EIA kits, the cross-reactivity between oxytocin and vasopressin was <0.04%. All samples were run in duplicates. The inter- and intra-assay coefficients of variation were less than 8% for the assay. The raw vasopressin values obtained (m = 75.75 pg/ml, SD = 62.19) were similar to those reported in the Taylor et al., (2010) study using the same assay (women mean = 71.73 pg/ml, SD = 47.50; men mean = 67.09 pg/ml, SD = 33.38). Due to technical difficulties, peptide analyses were not performed for one participant.
Statistical analysis
To account for the dependency between the husband and the wife data, hierarchical linear models treating each individual as nested within a couple were fitted with interpersonal functioning variables as dependent variables and AVP as independent variable (Kenny & Kashy, 1991). Intercepts were estimated as random effects, but all the slopes of the level-1 predictors were constrained to be equal because estimating a random effect for these variables may lead to non-convergence problems with dyadic data (Newsom and Nishishiba, 2002). Restricted maximum likelihood was used as the estimation method given the study sample size. A grand mean centering of the independent variables was performed to facilitate the data interpretation.
As reported elsewhere, levels of AVP in the two blood samples were highly correlated (r =.87, p < .001) and the marital interaction task did not lead to significant changes in AVP, F (1,73) = 0.01, p = .93 (Gouin, et al., 2010). The two peptide measurements were therefore averaged to provide a better estimate of the basal AVP values. Spearman’s rho correlations were used for the bivariate analyses because of the non-normal distributions of some of the study variables. For the multivariate analyses, a log10 logarithmic transformation normalized the positively skewed distributions of AVP and the Experiences in Close Relationship-Revised’s continuous attachment variables. Given the skewed response distributions with the Hazan & Shaver’s categorical attachment measure, 3-level categorical variables were created for the attachment avoidance and anxiety variables and a dichotomous variable was computed for the attachment security variable. Gender was effect coded (−1: men and 1: women) to facilitate interpretation. The covariates included in the models were age and sex. The α significance level was set at .05. Statistical analyses were performed using SAS version 9.2.
Results
Most participants (n = 68) were Caucasian (91.3%), 3 were African-American, 2 Hispanic and 1 Asian. About half (56.5%) of the sample had a college education. Participants’ ages ranged from 22 to 73 years with a mean of 38.47 (SD = 11.99). Couples had been married from 2 to 39 years, with a mean of 11 years (SD = 9.63). Most participants (78%) had only been married once. Couples had two children on average (SD=1.63) with a range of 0 to 5. Table 1 provides the descriptive statistics of the participants’ psychological and social characteristics.
Table 1.
Study participants’ psychological and social characteristics.
| M (SD) | Range | |
|---|---|---|
| Depression (BDI) | 2.33 (2.38) | 0–12 |
| Anxiety (BAI) | 5.11 (4.33) | 0–21 |
| Social Network Size (SNI) | 7.29 (1.77) | 3–12 |
| Spousal social support (SSQ) | 5.4 (1.34) | 0–6 |
| Non-spousal social support (SSQ) | 17.42 (11.42) | 0–48 |
| Marital Satisfaction (MAT) | 113 (25.50) | 39–155 |
| Negative Marital Interactions (TENSE) | 1.95 (2.32) | 0–11.65 |
| Negative Non-Marital Interactions (TENSE) | 1.56 (1.67) | 0–7.20 |
| Hazan & Shaver Attachment anxiety | 2.03 (1.41) | 1–6 |
| Hazan & Shaver Attachment avoidance | 2.37 (1.62) | 1–6 |
| Hazan & Shaver Attachment security | 4.23 (1.55) | 1–6 |
| ECR-R Attachment Avoidance | 2.23 (.95) | 1–5.94 |
| ECR-R Attachment Anxiety | 2.39 (.92) | 1–5.39 |
Age was not associated with AVP, β (SE) = −.0004 (.003), p = .91. There was no significant gender difference in AVP levels, β (SE) = −.06 (.07), p = .42. Length of marriage was not significantly correlated with the peptide, β (SE) = .11 (.10), p = .27. Household income was not related to AVP, β (SE) = .008(.02), p =.58. The within-couple intra-class AVP correlations between the husband and the wife was ρ = .08. Table 2 includes the Spearman’s rho correlations among the study variables.
Table 2.
Spearman’s rho correlations among the study variables.
| AVP | BDI | BAI | SNI | SSQ-S | SSQ-NS | MAT | TENSE-S | TENSE-NS | ATT-Av | ATT-Ax | ATT-S | ECR-Av | ECR-Ax | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVP | 1 | −.09 | 0.3 | .24* | .29* | .01 | .13 | −.30 | −.18 | −.24* | .−.5 | .19 | .02 | .08 |
| BDI | 1 | .26* | −.07 | −.14 | −.01 | −.32* | .23* | .33* | .37* | .25* | −.24* | .39* | .36* | |
| BAI | 1 | .05 | −.19 | −.11 | −.20 | .13 | .13 | .14 | .09 | −.12 | .02 | .30* | ||
| SNI | 1 | .04 | .21 | −.02 | −.01 | .02 | −.22 | −.12 | .40* | −.13 | −.13 | |||
| SSQ-S | 1 | .24* | .41* | −.33* | −.17 | −.15 | −.11 | .26* | −.34 | −.04 | ||||
| SSQ-NS | 1 | −.14 | −.07 | .20 | −.20 | −.12 | .32* | .04 | .03 | |||||
| MAT | 1 | −.41* | −.35* | −.19 | −.22 | .22 | −.40* | −.27* | ||||||
| TENSE-S | 1 | .62* | .25* | .17 | −.23* | −.20 | .15 | |||||||
| TENSE-NS | 1 | .37* | .21 | −.22 | .26* | .12 | ||||||||
| ATT-Av | 1 | .30 | −.61 | .31 | .07 | |||||||||
| ATT-Ax | 1 | −.41* | .27* | .33* | ||||||||||
| ATT-S | 1 | −.29* | −.12 | |||||||||||
| ECR-Av | 1 | .32* | ||||||||||||
| ECR-Ax | 1 |
AVP: Vasopressin; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; SNI: Social Network Index; SSQ-S: Social Support Questionnaire- Spousal version; SSQ-NS: Social Support Questionnaire-Non-Spousal version; MAT: Marital Adjustment Test; TENSE-S: Test of Negative Social Exchange-Spousal version; TENSE-NS: Test of Negative Social Exchange-Non-Spousal Version; ATT-Av: Hazan & Shaver Attachment avoidance; ATT-Ax: Hazan & Shaver Attachment anxiety; ATT-S: Hazan & Shaver Attachment security; ECR-Av: Experiences in Close Relationships-Avoidance Scale; Experiences in Close Relationships-Anxiety Scale.
indicates p < .05
Table 3 presents the associations between measures of psychological and interpersonal functioning and plasma AVP. The psychological distress indices of depression and anxiety were not significantly related to AVP. Among the interpersonal functioning variable, greater plasma AVP was associated with a larger social network (Figure 1), less negative marital interactions (Figure 2), lower attachment-related avoidance (Figure 3), greater attachment-related security (Figure 4), and marginally with greater spousal social support. Notably, for the self-reported negative social interaction (TENSE) and social support (SSQ) measures, the spousal versions, but not the general interpersonal relationships versions of the measures, were related to AVP levels. No significant gender differences were observed in the relationships between interpersonal functioning and AVP, all ps’ > .19.
Table 3.
Social functioning and plasma AVP levels.
| β (SE) | p-value | |
|---|---|---|
| Depression (BDI) | −.14 (12) | .24 |
| Anxiety (BAI) | .05 (.14) | .71 |
| Social Network Size (SNI) | 1.37 (.69) | .05** |
| Spousal social support (SSQ) | .33 (.17) | .06* |
| Non-spousal social support (SSQ) | −.88 (4.29) | .83 |
| Marital Satisfaction (MAT) | 7.92 (9.84) | .42 |
| Negative Marital Interactions (TENSE) | −.25 (.11) | .03** |
| Negative Non-Marital Interactions (TENSE) | −.14 (10) | .18 |
| Hazan & Shaver Attachment avoidance | −.73 (.32) | .03** |
| Hazan & Shaver Attachment anxiety | −.25 (.33) | .45 |
| Hazan & Shaver Attachment security | 1.71 (.83) | .04** |
| ECR-R Attachment Avoidance | .02 (.07) | .78 |
| ECR-R Attachment Anxiety | .01 (.06) | .88 |
p < .10;
p ≤ .05
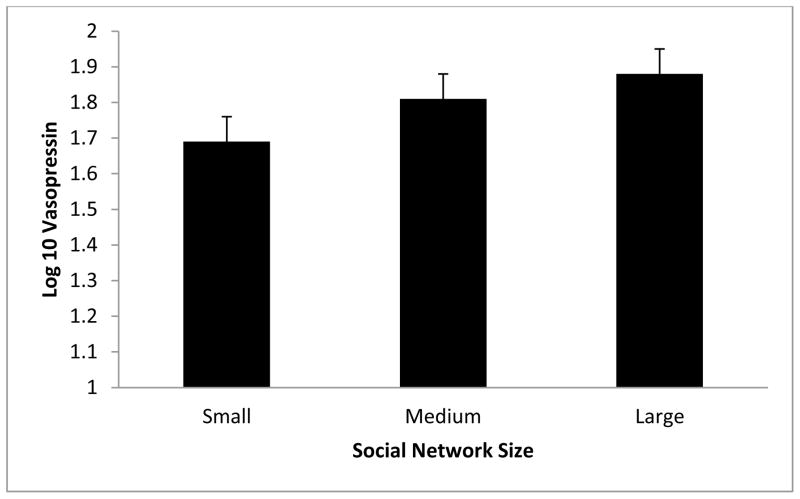
Figure 1.
Relationship between plasma vasopressin and social integration. Results revealed that vasopressin was significantly associated with the number of regular social roles, p = .05. For illustration purposes, the social integration variable (SNI) was divided into tertiles. Error bars represent standard errors of the mean.
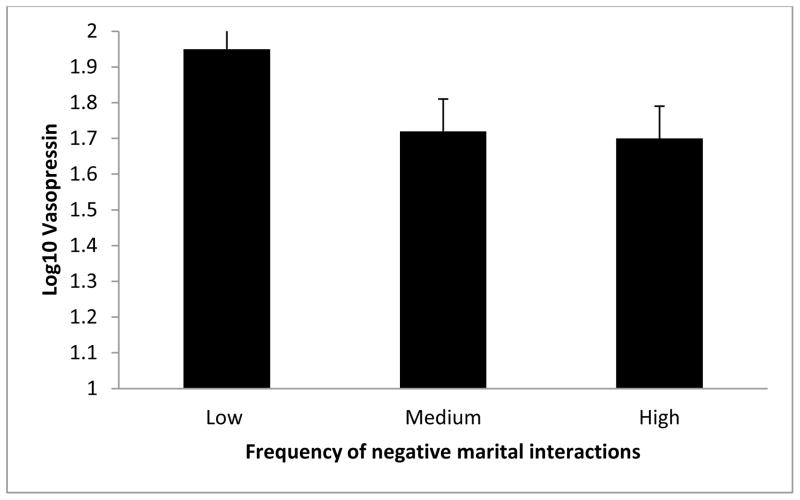
Figure 2.
Relationship between plasma vasopressin and the frequency of negative marital interactions. Results revealed that vasopressin was significantly associated with the frequency of negative marital interactions, p = .03. For illustration purposes, the negative marital interaction variable (TENSE) was divided into tertiles. Error bars represent standard errors of the mean.
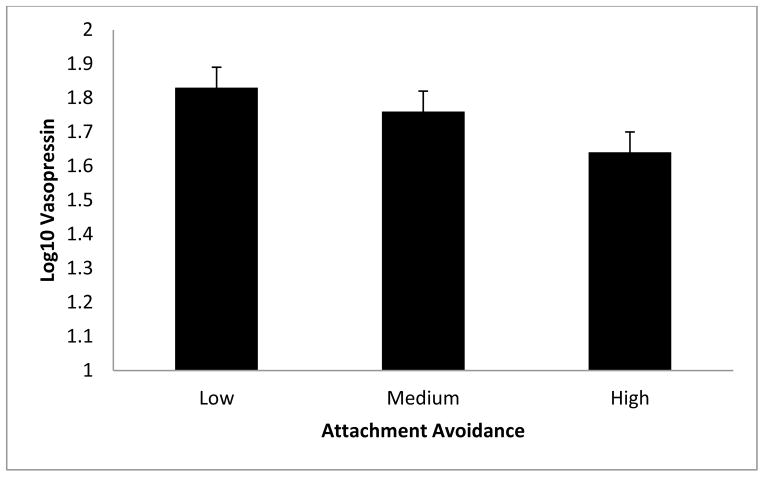
Figure 3.
Relationship between plasma vasopressin and Hazan and Shaver’s attachment avoidance. Results revealed that vasopressin was significantly associated with attachment avoidance, p = .03. Error bars represent standard errors of the mean.
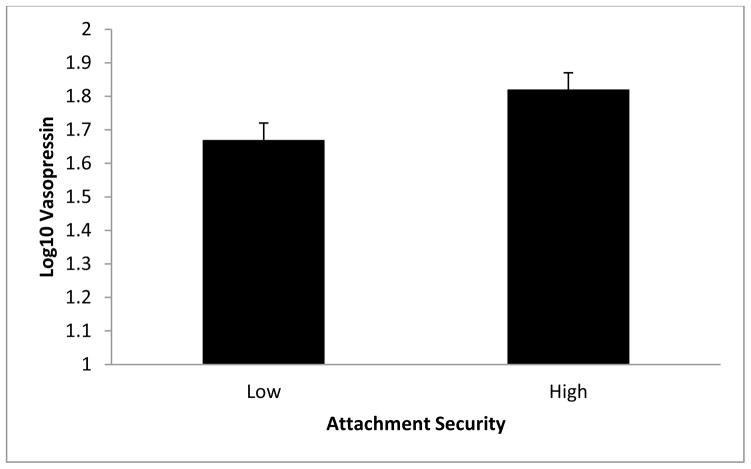
Figure 4.
Relationship between plasma vasopressin and Hazan and Shaver’s attachment security. Results revealed that vasopressin was significantly associated with attachment security, p = .04. Error bars represent standard errors of the mean.
Discussion
This study evaluated the relationships among plasma AVP and interpersonal functioning. Among these married couples, peripheral AVP was associated with a range of indicators of positive interpersonal functioning such as a larger social network, fewer negative marital interactions, less attachment avoidance, more attachment security, and, marginally, more spousal social support. Given that AVP was associated with different facets of interpersonal functioning, these data support the hypothesis that AVP may be involved in a range of interpersonal relationships initiation and maintenance processes in humans.
Consistent with research in animals implicating AVP in the formation of social bonds in both males and females (Cho, et al., 1999; Winslow, et al., 1993), circulating AVP levels were significantly related to facets of interpersonal functioning, but not to psychological distress. Furthermore, on two measures distinguishing general interpersonal functioning from marital functioning, AVP was only associated with aspects of marital quality. Interestingly, in non0-human primates, AVP administration increased social contact with a partner, but not with a stranger (Jarcho et al., 2011). In humans, intra-nasal AVP manipulation increased reciprocated cooperation, but not initial trust behavior in a Prisoner’s Dilemma game (Rilling, et al., 2011). These data suggest that AVP may be specifically implicated in relationship maintenance processes.
In the current study, plasma AVP was associated with indices of positive interpersonal functioning. In contrast, among individuals with personality disorders, greater cerebro-spinal fluid AVP levels were associated with a history of aggression (Coccaro et al., 1998). Furthermore, among partnered undergraduate students, higher plasma AVP levels were related to greater marital distress in men, but not in women (Taylor et al., 2010). Moreover, AVP administration decreased perception of friendliness to neutral faces in men (Thompson et al., 2006). While these data support the role of AVP in social behaviors in humans, the directionality of the association between AVP levels and interpersonal functioning varies across studies. Interestingly, similar results have been found for the closely-related nonapeptide oxytocin (Grewen et al., 2005; Taylor, et al., 2006). These findings raise the possibility that individual and contextual differences might influence the association between the peptide and social behaviors (Bartz et al., 2011).
A large body of literature implicates AVP in the activity of the hypothalamic-pituitary-adrenal axis (Aguilera and Rabadan-Diehl, 2000). Furthermore, plasma AVP levels were elevated in a subgroup of patients with major depression in some (van Londen, et al. 1996; Goekoop, et al., 2006) but not all studies (Parker, et al., 2011). In the current study, plasma AVP was not associated with markers of psychological distress. This is likely due to the fact that participants in the current sample reported very low levels of psychological distress (mean BDI score = 2.33; BDI clinical cut-off score = 14; Beck, et al. 1988). It is possible that a different pattern of associations might emerge in samples taken in a more stressful context or among clinically depressed individuals.
A limitation of the study is the fact that AVP was measured from plasma. Although the AVP that is detected in plasma is likely made in the brain, it is unclear to what extent peripheral AVP is related to levels of the peptide in specific brain regions (Landgraf and Neumann, 2004). Furthermore, because of the cross-sectional design of the study, the current data do not speak to the causality or direction of the relationships among peptide levels and social functioning. Thus, high levels of AVP as well as the patterns of behavior measured here might both be secondary to other central processes. Moreover, a large number of statistical tests were conducted given the study’s sample size, inflating the possibility of type I errors. However, it is important to note that AVP was significantly related to several measures of interpersonal functioning, but not to measures of psychological distress.
The present results extend our understanding of the role of AVP in social behaviors in humans. Among married couples, higher levels of AVP were associated with a range of indicators of positive interpersonal functioning. Future work should focus on identifying the specific relationship maintenance processes modulated by the vasopressinergic system.
Highlights.
Healthy, married couples provided blood samples and completed questionnaires.
Greater plasma vasopressin levels were related to better interpersonal functioning.
Vasopressin related to social integration, attachment style, and marital quality.
Acknowledgments
This research was supported by National Institutes of Health (NIH) grants DE13749, CA16058, and UL1RR025755 AG16321 and CA158868, NCRR Grant UL1RR025755 which funds the Clinical Research Center, and by Ohio State Comprehensive Cancer Center Core Grant CA16058, and grant MH 072935 to University of Illinois at Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic- pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Clark CL, Shaver PR. Self-report measures of adult romantic attachment. An integrative overview. In: Simpson JA, Rholes WS, editors. Attachment Theory and Close Relationships. New York: Guilford; 1998. pp. 46–76. [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS. Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Goekoop JG, de Winter RP, de Rijk R, Zwinderman KH, Frankhuijzen-Sierevogel A, Wiegant VM. Depression with above-normal plasma vasopressin: validation by relations with family history of depression and mixed anxiety and retardation. Psychiatry Res. 2006;141:201–211. doi: 10.1016/j.psychres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-Vander J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA. Analyzing interdependence in dyads. In: Montgomery BM, Duck S, editors. Studying interpersonal interaction. New York: Guilford; 1991. pp. 275–285. [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Locke HJ, Wallace KM. Short marital adjustment and prediction tests: Their reliability and validity. Marriage and Family Living. 1959;21:251–255. [Google Scholar]
- Newsom JT, Nishishiba M. Nonconvergence and sample bias in hierarchical linear modeling of dyadic data. 2002 http://www.upa.pdx.edu/IOA/newsom/mlrdyad4.doc.
- Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in microtus pennsylvanicus (meadow voles) Horm Behav. 2001;39:285–294. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010;178:359–362. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnogni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2011;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehlman LS, Karoly P. With a little flak from my friends: Development and preliminary validation of the Test of Negative Social Exchange (TENSE) Journal of Consulting and Clinical Psychology. 1991;3:97–104. [Google Scholar]
- Sarason IG, Levine HM, Basham RB, Sarason BR. Assessing social support: The Social Support Questionnaire. Journal of Personality and Social Psychology. 1983;44:127–139. [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci USA. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzefovsky F, Shalev I, Israel S, Knafo A, Ebstein RP. Vasopressin selectively impairs emotion recognition in men. Psychoneuroendocrinology. 2011;37:576–580. doi: 10.1016/j.psyneuen.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Su F. Physiology and pathophysiology of the vasopressinergic system. Best Pract Res Clin Anaesthesiol. 2008;22:243–252. doi: 10.1016/j.bpa.2008.03.004. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacol. 1996;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Christiansen M, Young WS. Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 2007;6:653–660. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]