Abstract
A potential topical psoriasis therapy has been developed consisting of tyrosine-derived nanospheres (TyroSpheres) with encapsulated anti-proliferative paclitaxel. TyroSpheres provide enhancement of paclitaxel solubility (almost 4,000 times greater than PBS) by effective encapsulation and enable sustained, dose-controlled release over 72 hours under conditions mimicking skin permeation. TyroSpheres offer potential in the treatment of psoriasis, a disease resulting from over-proliferation of keratinocytes in the basal layer of the epidermis, by (a) enabling delivery of paclitaxel into the epidermis at concentrations >100 ng/cm2 of skin surface area and (b) enhancing the cytotoxicity of loaded paclitaxel to human keratinocytes (IC50 of paclitaxel-TyroSpheres was approximately 45% lower than that of free paclitaxel). TyroSpheres were incorporated into a gel-like viscous formulation to improve their flow characteristics with no impact on homogeneity, release or skin distribution of the payload. The findings reported here confirm that the TyroSpheres provide a platform for paclitaxel topical administration allowing skin drug localization and minimal systemic escape.
Keywords: psoriasis, topical drug delivery, tyrosine-derived nanospheres, cytotoxicity, skin distribution, viscous formulation
1. Introduction
Psoriasis is a chronic inflammatory disease of the skin that, according to the National Psoriasis Foundation, affects approximately 125 million people worldwide. The most common form of psoriasis is characterized by pink colored plaques and white flakes appearing on top of the skin. The pathogenesis of psoriasis has still not been completely elucidated. It is generally accepted that initial stimulation of dermal dendritic cells results in a cascade of events that leads to an interaction between epidermal keratinocytes and the immune system [1, 2]. The immune system upregulates the production of cytokines, which in-turn leads to over-proliferation of keratinocytes at the basal layer of the epidermis and the overall inflammation associated with psoriasis lesion formation [2].
Treatment options for psoriasis are based on the severity of the disease. Patients with moderate to severe cases of psoriasis typically receive systemic treatments (e.g. anticancer agents such as methotrexate, immune system suppressants such as cyclosporine, or biological agents) or phototherapy, while those with more mild cases are generally prescribed topical agents including vitamin analogs, corticosteroids, and retinoids [3]. Unfortunately, each treatment option is associated with side effects such as toxicity for systemic options, carcinogenicity associated with phototherapy, and skin thinning and irritation for the topical options. Based on the understanding of the disease pathology and outcomes of the current therapies, the next generation of psoriasis treatments should, most likely, combine the benefits of (a) topical application, which permits significant drug concentration in the skin strata as well as limiting or eliminating side effects associated with systemic exposure, with (b) therapeutics that control and reduce the over involved in the origination and progression of psoriasis.
Paclitaxel (PTX), a mitotic inhibitor that promotes the assembly and stabilization of microtubules, resulting in eventual cell death [4], is used commonly in cancer chemotherapy to help regulate rapidly proliferating cells. This ability to inhibit cell division should also enable PTX to address the hyperproliferative pathophysiologic process in psoriasis. However, the low aqueous solubility of PTX [5] limits its wide clinical use. In order to address this issue, a number of formulations and delivery systems for PTX including but not limited to Cremophor [6], nanoparticles [4, 7, 8], liposomes [9], emulsions [10], and foams [11] have been investigated. To the best of our knowledge only one instance has been reported using a PTX delivery system for the treatment of psoriasis. In this case, PTX-nanoparticles composed of poly (d,l-lactide) and methoxypolyethylene co-polymers were administered systemically, resulting in the reduction of disease severity and reduced epidermal thickness [12]. Although data showed promise, adverse events were reported including cases of fatigue, infusion reactions, and alopecia [12].
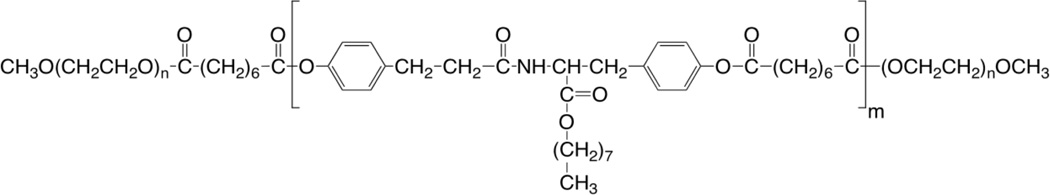
In order to develop a topical, skin-targeted PTX formulation with reduced side effects, we propose the use of the previously reported tyrosine-derived nanospheres for the delivery of PTX. These nanospheres (now referred to as “TyroSpheres” and in previous publications as “NSP”), are composed of the tyrosine-derived block copolymer poly(ethylene glycol)-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-poly(ethylene glycol), shown in Scheme 1. Several recent studies using TyroSpheres have demonstrated their ability to (a) efficiently encapsulate PTX [13], (b) retain the activity of the encapsulated drug [13, 14], (c) efficiently deliver a wide range of hydrophobic compounds into the skin [15, 16], and (d) cause no detrimental effects to skin morphology [15].
Scheme 1.
Structure of the tyrosine-derived, ABA-type poly(ethylene glycol)-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-poly(ethylene glycol) triblock copolymer (DTO-SA/5K) used in the preparation of TyroSpheres.
Since the TyroSphere preparation is an aqueous suspension, the flow characteristics are very similar to that of water and are therefore not ideal for topical administration. This has been addressed by increasing the viscosity of the TyroSphere formulation with hydroxypropyl methylcellulose, a pharmaceutically acceptable thickening agent [15].
Based on the potential effectiveness of PTX in the treatment of psoriasis and the safety and effectiveness of TyroSpheres in topical delivery, the aim of this study is to evaluate the applicability of PTX-TyroSpheres for psoriasis treatment. These studies include formulation development and optimization, investigation of cytotoxicity of PTX-TyroSpheres using a keratinocyte cell-line, and quantifying the extent of PTX delivery into the epidermis and dermis via an in vitro method utilizing mass spectrometry.
2. Materials and methods
2.1. Materials
Suberic acid (SA), poly(ethylene glycol) monomethyl ether MW 5000 (PEG5K), Tween-80, Dimethyl sulfoxide (DMSO), and dulbecco’s phosphate buffered saline (PBS) were obtained from Sigma Aldrich (St. Louis, MO). Acetonitrile, methanol, and HPLC grade water were obtained from Fisher Scientific (Pittsburgh, PA). Paclitaxel was obtained from LC Laboratories (Woburn, MA). Hydroxypropyl methylcellulose (Methocel K15M, ~500kDa) was obtained from Colorcon (West Point, PA). Desaminotyrosyl-tyrosine octyl ester (DTO) and 4-dimethylaminopridinium-p-toluene sulfate (DPTs) were synthesized according to previously published and established procedures in the New Jersey Center for Biomaterials and their chemical structure and purity were confirmed by 1H NMR. Dulbecco’s modified eagle medium, high glucose (DMEM), Penicillin (10,000 units/mL) and Streptomycin (10,000 µg/mL) (pen/strep), Trypsin (0.25% Trypsin-EDTA), and Dulbecco’s phosphate buffered saline without calcium chloride and magnesium chloride (DPBS) were purchased from Invitrogen (Grand Island, NY). 10% heat-inactivated Fetal Calf Serum (FCS) was obtained from Atlanta Biologicals (Lawrenceville, GA). Hyclone Trypan Blue (0.2 µM) was ordered from Thermo Scientific (South Logan, UT). AlamarBlue® Reagent was obtained from AbD Serotec (Raleigh, NC). All reagents were used as received.
2.2. Polymer synthesis and preparation of PTX-TyroSpheres and drug free TyroSpheres
ABA triblock copolymer PEG5K-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-PEG5K (DTO-SA/5K, Scheme 1) was synthesized and characterized as previously reported [13, 17]. PTX-TyroSpheres and drug free TyroSpheres were prepared and purified as previously reported unless specified below [13, 14].
2.3. TyroSpheres and PTX-TyroSpheres characterization
2.3.1. Size and size distribution
Particle size and polydispersity index (PDI) of drug-free TyroSpheres and PTX-TyroSpheres were established using dynamic light scattering (Beckman Coulter Delsa™ Nano). Samples were measured at 25°C at concentrations of approximately 10 mg/mL polymer. The TyroSphere suspensions were examined by normalized intensity distribution by the CONTIN method for cumulants, size distribution, and polydispersity. All measurements were performed in triplicates with average of 50 measurements recorded per replicate.
2.3.2. PTX HPLC method
A Hewlett-Packard 1100 series HPLC with diode array detector and HP Chemstation software was used for PTX method validation. Optimized chromatographic conditions include: column – Agilent Eclipse XDB-C18, 4.6 × 150 mm, 5 µm particle size; column temperature at 25°C; 20 µL injection volume; detection wavelength set at 230 nm; mobile phase consisted of acetonitrile : HPLC water (65:35 v/v, isocratic); flow rate of 0.75 mL/min; and run time of 8 minutes. Standard calibration curve was prepared at concentrations ranging from 0.4 to 100 µg/mL. Within- and between-day precision and accuracy determination of quality control samples were better than an RSD of 2% across the range of the calibration curve.
2.3.3. PTX encapsulation efficiency
PTX concentration in the final purified PTX-TyroSpheres was measured using previously established extraction [13] and HPLC techniques (described above). PTX entrapment by the TyroSpheres was characterized by two calculated ratios:
| (Equation 1) |
| (Equation 2) |
2.4. PTX solubility in different matrices
The solubility of PTX in aqueous media was measured to allow comparison with the amount of PTX "solubilized" in TyroSpheres. Excess PTX was added to PBS (pH 7.4) containing surfactant Tween 80 (0–1% w/v). Samples were vortexed and placed in a shaking water bath (120 rpm) at 37 °C. After 48 hours, samples were filtered through 0.22 µm filters (PTFE, Puradisc Syringe Filters, Fisher Scientific) to remove non-solubilized drug, lyophilized, and re-dissolved in methanol. HPLC was used to establish PTX concentration in these different media. All tests and measurements were performed in duplicate.
2.5. In vitro release of PTX from PTX-TyroSpheres
Release of PTX from PTX-TyroSpheres was measured using two independent techniques: dialysis cassettes and vertical glass Franz diffusion cells (Logan Instruments, Somerset, NJ). All experiments utilized 10K MWCO cellulose dialysis membranes (Spectrum Laboratories, Rancho Dominguez, CA) separating the dialysis outer solution / receptor and dialysis cassette / donor compartments. In both methods, PTX-TyroSpheres were diluted with PBS (to obtain PTX concentrations reported below) and added to the dialysis cassette or donor compartment. At pre-determined time-points (up to 72 hours), the contents of the dialysis outer solution or receptor compartments were collected and replaced with equivalent amounts of temperature equilibrated PBS. PTX content in the withdrawn samples was analyzed by HPLC after lyophilization and extraction. All release studies were conducted in triplicate. For the dialysis cassette method, 1 mL of 5% (w/w) loaded PTX-TyroSpheres was diluted to a concentration of 250 µg/mL PTX and added to the dialysis cassette. Cassettes were dialyzed against 200 mL of PBS and stored in a shaking water bath (120 rpm) at 37 °C. To confirm that there was no delaying effect of PTX release from PTX-TyroSpheres due to the membranes, permeation of free PTX across the dialysis membrane (MWCO=10000; 3mL) was also performed. For the Franz diffusion cell method, 1 mL of 5% (w/w) loaded PTX-TyroSpheres was diluted to a concentration of 20 µg/mL PTX. 0.5 mL of diluted 5% PTX-TyroSpheres was added to the donor compartment which was subsequently occluded. Receptor media contained 5 mL PBS which was stirred continuously at 600 rpm and maintained at 37 °C.
2.6. Cytotoxicity of free PTX and PTX-TyroSpheres to HaCaT
HaCaT (a cell line of human keratinocytes) was cultured in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (37°C, 10% CO2, RH 95%). When 90% confluency was reached, cells were rinsed with DPBS, trypsinized, and passaged at a 1:4 ratio. HaCaTs with passage number below 50 were applied in the study. 100 µL of HaCaT cell suspension was added to each well of a 96-well cell culture plate; the seeding density was 2,000 cells/well (cell count measured with a Cellometer Auto A4, Nexcelom Bioscience, MA). The cells were incubated for 4 hours to ensure sufficient attachment and then the media was changed in all wells to the experimental conditions. Below is the design of the experimental conditions:
(a) 0.5 nM to 25 nM PTX in PTX-TyroSpheres (in triplicate, prepared and characterized as described in Section 2.2 with initial 5% wt/wt PTX to polymer ratio); for each PTX concentration, the cell culture medium containing equivalent amount of drug-free TyroSpheres was applied as 100% viability.
(b) 0.5 nM to 25 nM free PTX dissolved in the culture medium using 0.1% v/v DMSO (in hexaplicates). Cell culture medium containing 0.1% v/v DMSO was used as 100% viability.
Cell culture medium, cell culture medium with 10% DMSO, and 10% AlamarBlue® containing medium were applied as negative control, positive control, and blank, respectively (in hexaplicates). Cells subjected to these experimental conditions were allowed to culture for 3 days at which point cell viability was measured using the AlamarBlue® metabolic assay. Cell culture medium containing 10% AlamarBlue® reagent was added and the fluorescent intensity was measured (560nm/590nm; manual gain 97%) when the negative controls started to turn pink. The optimal post AlamarBlue® treatment time was 2–4 hours. The cytotoxicity data and the half maximum inhibition concentration (IC50) were determined following the protocol provided together with the AlamarBlue® assay reagent (AbD Serotec).
2.7. High viscosity formulation of TyroSpheres (gel-like formulation)
2.7.1. Preparation of gel-like TyroSpheres formulation
The gel-like formulation was prepared by the addition of HPMC to PBS (1.5% w/v) under constant stirring for 18 hours. Thereafter, PTX-TyroSpheres or drug-free TyroSpheres, prepared by the above described methods, were added at a ratio of 1:2 (v/v) of 5% (w/w) loaded PTX-TyroSpheres : HPMC phase and allowed to stir over an additional 24 hours resulting in a gel-like formulation with the final HPMC concentration of 1% w/v.
2.7.2. Homogeneity Analysis
To ensure homogeneity of PTX-TyroSpheres in the sample, ten samples were analyzed for PTX concentration (w/w). Samples were weighed to allow calculation of PTX concentration (w/w) in relation to total mass of formulation and then lyophilized. The dried samples were re-suspended in methanol and sonicated to allow complete extraction of PTX. Samples were filtered and HPLC was performed to measure the PTX distribution within the gel-like formulation.
2.7.3. In vitro release of PTX from gel-like TyroSphere formulation
PTX release from the gel-like TyroSphere formulation was studied using the Franz cell diffusion method described above with slight modification. Due to the high viscosity of gel-like PTX-TyroSpheres, weights of formulation applied were recorded for determination of the exact amount of drug applied. An amount of formulation resulting in 10 µg PTX in the donor compartment was applied to allow for a direct comparison with the release profile of aqueous 5% (w/w) loaded PTX-TyroSpheres. Samples were taken from the receptor compartment every 6 hours over 48 hours. At the conclusion of the experiment, excess formulation was removed and donor compartments were flushed with fresh PBS three times to capture the remaining gel-like formulation and PTX. Next, the compartment itself, membrane, and all donor fluids were lyophilized and extracted in methanol. The validated HPLC method was used to determine PTX release. All release studies were conducted in triplicate.
2.7.4. PTX stability in gel-like TyroSphere formulation
PTX stability in the gel-like TyroSphere formulation was evaluated for the formulation containing 1% w/v HPMC. At time-zero, ten replicate samples of gel-like PTX-TyroSpheres were weighed and lyophilized. These samples were extracted in methanol for HPLC analysis and used to establish baseline drug content for stability studies. Concentration of PTX in the gel-like TyroSphere formulation stored at 4 °C was tested at pre-determined time-points over 56 days as an indication of formulation stability.
2.8. In vitro skin distribution studies of PTX-TyroSpheres and gel-like PTX-TyroSphere formulation
Human cadaver skin samples obtained from the New York Fire Fighters Skin Bank (New York City, NY) were used to evaluate the in vitro skin permeation of PTX delivered via PTX-TyroSpheres and the gel-like PTX-TyroSphere formulation. The permeation studies were performed using the Franz diffusion cells described above according to previously described methods [15]. Treated skin samples (6 hours with each test formulation) were processed according to the following procedure: at the conclusion of the experiment, the skins were washed and removed from the Franz cells. Epidermal and dermal layers were manually separated using tweezers. PTX was extracted in 5 mL methanol with homogenization (Polytron® PT10/35 - Kinematica, Switzerland) of each skin layer and extracted samples were assayed for PTX concentration by mass spectroscopy described below.
2.8.1. Liquid chromatography and mass spectroscopy (LC-MS)
LC-MS experiments were performed using a U3000 (Dionex) on line with linear trap quadrapole (ThermoFisher). In general, sample was injected in full loop mode and separated by a reverse phase column (Discovery BIO Wide Pore C18, 5cm × 2.1mm, Supelco analytical) with 50% acetonitrile and 0.1% formic acid as mobile phase at a flow rate of 250 µL/min for 3 minutes. Mass spectroscopy data was acquired with full MS from 300 to 1000 (positive mode) followed by MSMS of 854 (mono-protonated ion of PTX). MSMS detailed parameters include: isolation width - 7 Da; normalized collision energy - 35%; activation Q - 0.15, activation time - 16 milliseconds. Full MS and MSMS data were both acquired in centroid mode. Quantification of PTX was by peak integration and peak area calculation of the mono-protonated ion of PTX (m/z 854) or of the MSMS fragments 569 or 561. Linearity for molecular weight 854 Da (corresponding to mono-protonated PTX) was established in test matrix over the range of 5.0 to 500 ng/mL.
2.9 Statistics
For binding and loading, solubility, and release testing, results are reported as mean with its standard error indicated (mean ± SE) and p-values < 0.05 were considered significant. For cytotoxicity analysis, three independent experiments were performed with the mean and standard error (mean ± SE) calculated according to the general approach in determining the error bars in experimental biology [18]. Statistical differences for skin distribution studies were determined using a one-way ANOVA followed by Tukey’s post hoc test for comparison of PTX concentration in different layers of skin (epidermis vs dermis) for TyroSpheres and gel-like TyroSpheres. All analyses were carried out with KaleidaGraph version 4.02 graphing and data analysis software (Synergy Software, Reading, PA). p-values < 0.0001 (*) considered to be statistically significant
3. Results and Discussion
3.1. PTX-Tyrospheres: design, fabrication and characterization
The rationale for choosing the tyrosine-based triblock copolymer system (Scheme 1) used to fabricate TyroSpheres in this study was based on the ability of this amphiphilic copolymer to self-assemble in aqueous media. The middle block oligo(DTO-SA) is composed of naturally occurring metabolites [19] which renders the final degradation products benign, while mPEG end blocks provide a stable dispersion in an aqueous environment. Additionally, this particular formulation has been previously reported to have a strong affinity in binding hydrophobic drugs including PTX [17].
3.1.1. PTX binding and loading efficiency by TyroSpheres
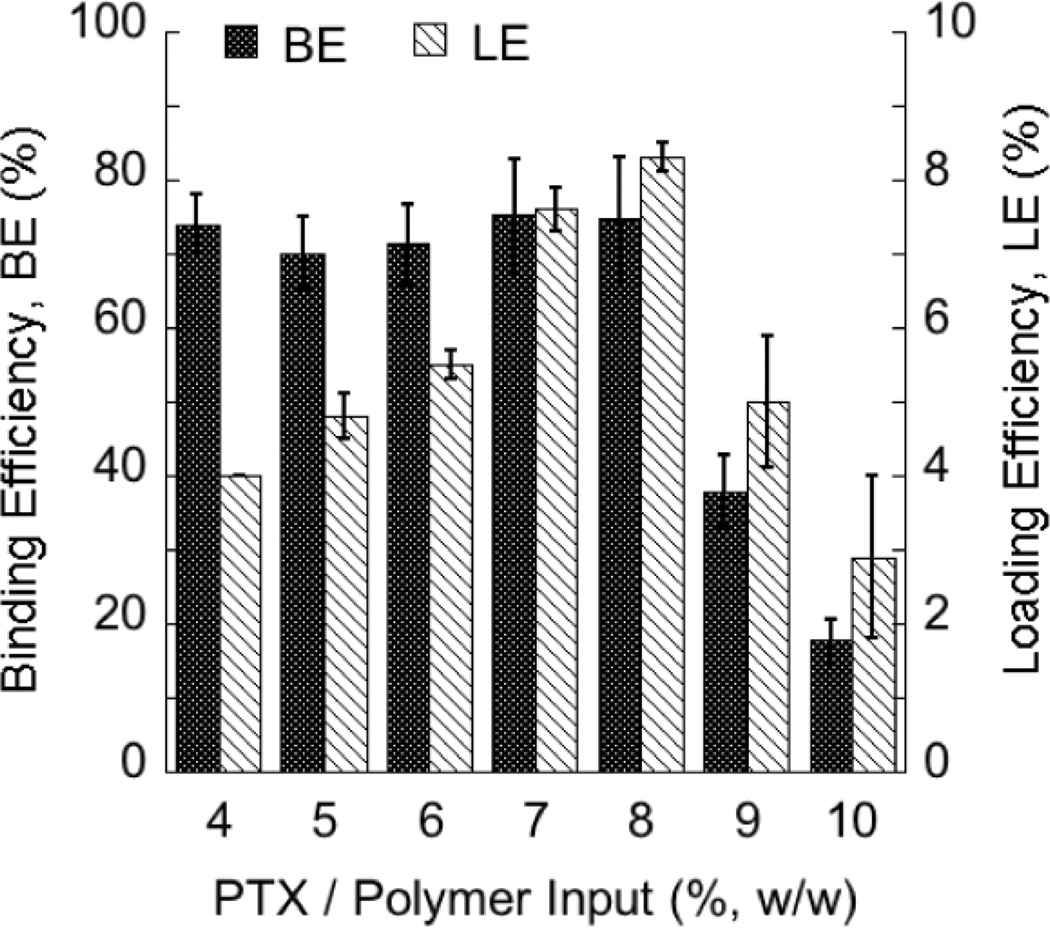
To evaluate the maximum loading of PTX into TyroSpheres, the amount of PTX input (drug in feed) was gradually increased while keeping the amount of DTO-SA/5K polymer constant. As shown in Figure 1, PTX is effectively encapsulated by TyroSpheres up to an initial drug input of 8% (w/w) to polymer. Up to this level, the loading efficiency (LE) is proportional to the initial drug input. Beyond this level, LE drops off markedly. The binding efficiency (BE), which was greater than 70% for all inputs less than or equal to 8% (w/w) input, also decreased corresponding with the LE. The limit of PTX loading can potentially be explained by the previously proposed mechanism of drug binding by the TyroSphere core [20]. PTX-TyroSphere interactions and binding rely on several aspects including drug-polymer structural compatibility and availability of putative binding sites (“hot spots”) for PTX in the oligo(DTO-SA) core. When the ”hot spots” become fully occupied, no more PTX can be dissolved (bound) within the core and the rest of unbound or loosely bound molecules eventually precipitate out in the aqueous environment surrounding the PTX-TyroSpheres.
Figure 1.
Binding and loading efficiencies of PTX by TyroSpheres.
The results are presented as mean ± SE. n-values for each tested condition were (w/w): 4% (n=16), 5% (n=6), 6% (n=8), 7% (n=12), 8% (n=16), 9% (n=12), 10% (n=7).
3.1.2. Size and size distribution
The size and size distribution of PTX-TyroSpheres with PTX input up to 8% (w/w) remain approximately 70 nm (PDI ≤ 0.22) regardless of the amount of PTX relative to polymer. The relatively small size and size distribution are important characteristics in the design of topical drug delivery formulations as smaller nanoparticles may increase the amount of surface contact with the skin and preferentially accumulate in hair follicles and skin furrows [21, 22].
3.1.3. PTX solubility
As shown in Table 1, PTX (Log P of 3.9) has a very limited aqueous solubility. With the addition of the pharmaceutically acceptable nonionic surfactant Tween 80, the solubility of PTX increased. However, even at 1% (w/v) of Tween 80, the solubility of PTX was still approximately 100-fold lower than that achieved by encapsulation (solubilization) of PTX in the TyroSpheres. The high solubility of PTX in TyroSpheres allows for an increased concentration gradient which is a key driving force in topical delivery [23].
Table 1.
Solubility of PTX in PBS ± surfactant and TyroSpheres.
| Sample | Concentration of PTX (µg/mL) |
|---|---|
| PTX in PBS | 0.3 * |
| PTX in 0.1% (w/v) Tween 80 | 2.7 |
| PTX in 1.0% (w/v) Tween 80 | 13.8 |
| PTX-TyroSpheres (LE=8.4% w/w) | 1160 |
3.2. In Vitro PTX Release from PTX-TyroSpheres
Equally as important as the ability to encapsulate the drug into the TyroSpheres, is the ability to release the drug from the delivery system. Typically, nanoparticle-based delivery systems exhibit release of their payload by one or a combination of three mechanisms: 1) diffusion, 2) polymer degradation and 3) particle dissociation [27]. The stability of TyroSpheres over a time-course relevant for release experiments was confirmed as no change was measured in particle size or polymer molecular weight compared to the initial formulation (data not shown). Therefore, dissociation and degradation are assumed to play negligible roles over the time-course of the release experiments. Additionally, control experiments with free PTX confirmed that the membrane had no delaying effect on PTX release as greater than 90% of the free PTX was measured in the release medium after 1 minute (data not shown). In vitro release of PTX from PTX-TyroSpheres across a semi-permeable membrane under physiological conditions (PBS; 37 °C) was performed using two methods: 1) jar and dialysis cassettes and 2) Franz diffusion cells (the amount released is determined as the amount of drug permeated to the receptor compartment of the diffusion cell).
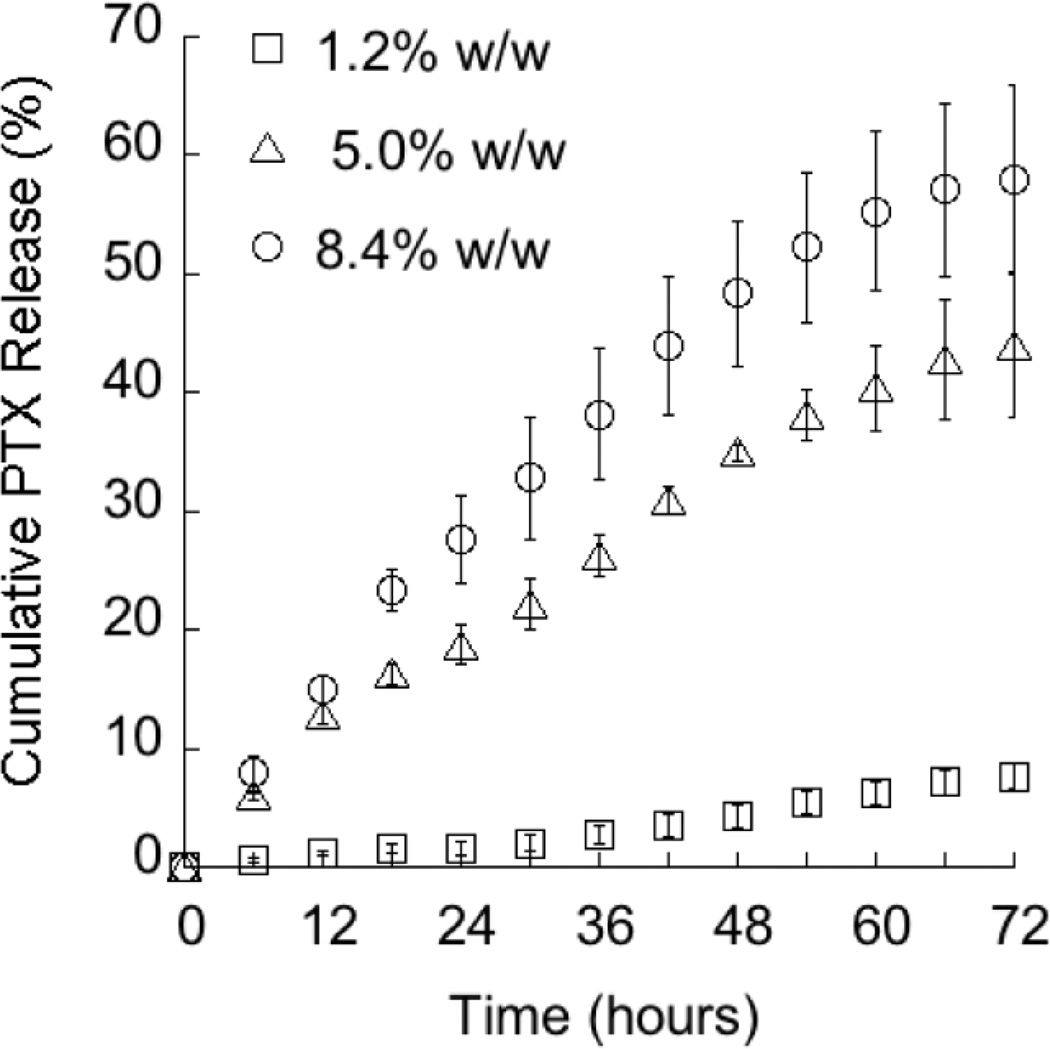
Comparison of the release measured by two methods (Table 2) revealed that the cumulative amounts of PTX released at each time-point are similar in both cases. This outcome confirms that the Franz cell method, commonly used in topical and transdermal in vitro permeation studies, is reliable for investigating the release behavior of TyroSpheres under conditions mimicking topical applications. To evaluate the impact of drug content on release, three PTX loadings (1.2%, 5.0%, and 8.4% (w/w)) were evaluated in Franz cell diffusion studies. The initial formulations were diluted so that the amount of PTX was kept constant resulting in the following formulations: 10 µg PTX in 2.3, 0.5, and 0.3 mg of TyroSpheres, respectively. Results, depicted in Figure 2, suggest that the amount of PTX input is directly related to the rate of drug release. At the end of 72 hours, approximately 8%, 44%, and 58% of the drug was released from the 1.2%, 5.0%, and 8.4% (w/w) PTX-TyroSpheres, respectively. This phenomenon can be potentially explained by the different binding affinities of PTX with the available “hot spots” in TyroSpheres [20]. Investigations by Costache et al. suggest that as the drug input amount increases, the sites that bind stronger are saturated and excess drug then binds to sites that have weaker affinities [20]. Hence, the less preferred binding sites would most likely release PTX at a faster rate, explaining the observed relationship between release rate and drug loading.
Table 2.
Comparison of PTX release from PTX-TyroSpheres using Franz cell method and dialysis cassette and jar method.
| Time-point (hours) | PTX (µg/mL) |
|
|---|---|---|
| Franz Cell | Cassette and Jar | |
| 6 | 6.0 ± 0.3 | 5.9 ± 0.3 |
| 12 | 12.9 ± 0.9 | 12.1 ± 0.4 |
| 24 | 18.8 ± 1.7 | 19.4 ± 0.9 |
The results are presented as mean ±SE (n=3).
Figure 2.
The effect of PTX content on release from PTX-TyroSpheres.
Cumulative PTX release measured using Franz cell method from (□) 1.2%, (Δ) 5.0%, and (○) 8.4% w/w PTX-TyroSpheres. The results are presented as mean ± SE (n = 3).
3.3 Cytotoxicity of free PTX and PTX-loaded TyroSpheres to HaCaT
As over-proliferation of keratinocytes is one of the key characteristics of psoriatic skin, the cytotoxicity of free PTX and PTX-TyroSpheres to HaCaT cells was studied. HaCaT was selected as it is the first permanent epithelial cell line from adult human skin that exhibits normal proliferation and differentiation behavior [28]. AlamarBlue® assay has been used to quantitatively analyze the cell viability conversion of fluorescent Resazurin in the Alamar Blue® to Resorufin occurs in response to cell metabolic activity [29, 30].
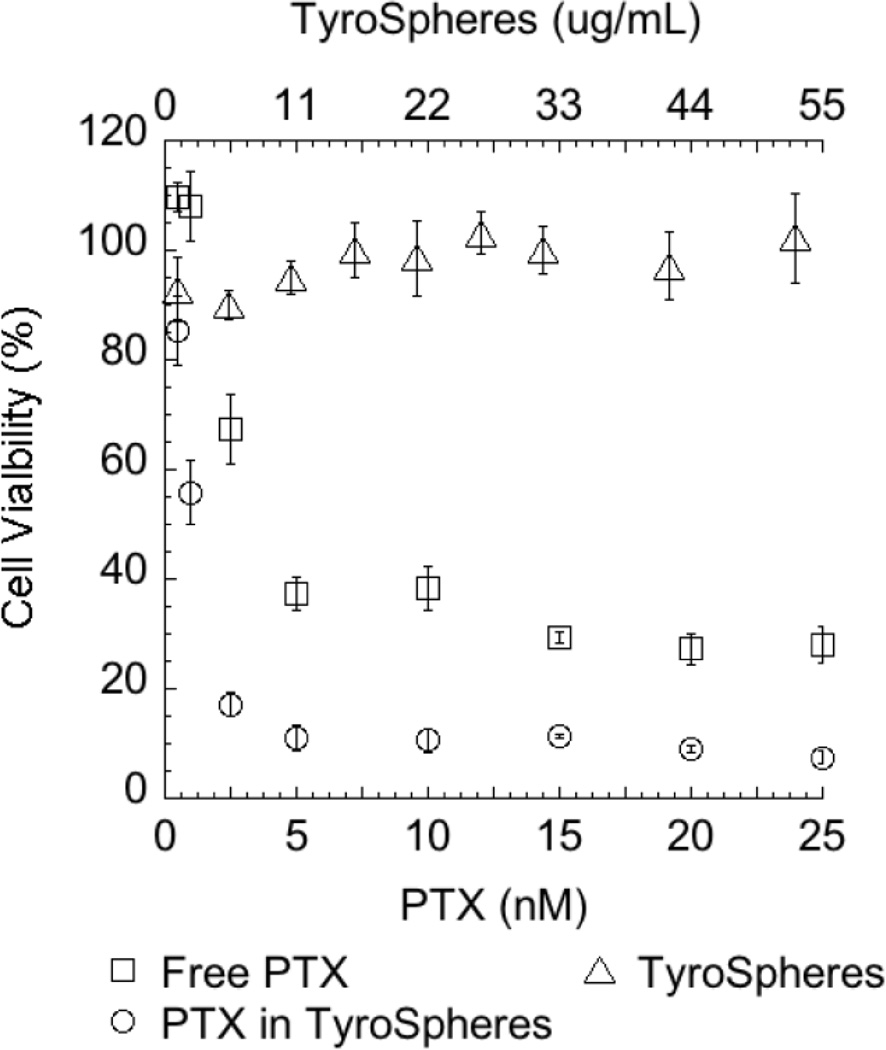
Figure 3 shows the cytotoxicity of TyroSpheres, free PTX and PTX-TyroSpheres to HaCaTs. The HaCaTs treated with drug-free TyroSpheres (concentration range 1 – 50 µg/mL) showed no significant decrease in metabolic activity (cell viability between 90 – 103%), confirming that TyroSpheres do not induce any short-term cytotoxicity. The difference in the cytotoxicity of free PTX and PTX delivered via TyroSpheres is evident: applied at the same drug concentration, PTX-TyroSpheres resulted in lower viability of HaCaTs as compared to free PTX. To confirm the significance and validation of the data, three independent experiments were performed and the IC50 of PTX-TyroSpheres (1.7 ± 0.3 nM, 1.5 ± 0.3 ng/mL) was approximately 45% lower than that of free PTX (3.3 ± 0.5 nM, 2.8 ± 0.4 ng/mL). These results suggest that TyroSpheres, a non-toxic vehicle themselves, improved the anti-proliferative effect of PTX to HaCaTs.
Figure 3.
Viability of HaCaT epithelial cell line from adult human skin exposed to (□) free PTX (n=6), (○) PTX-TyroSpheres (n=3), and (Δ) TyroSpheres (n=6).
Cell viability was measured after 72 h by AlamarBlue® cell proliferation indicator assay. Cell culture medium, cell culture medium with 10% DMSO, and 10% AlamarBlue® containing medium were applied as negative control, positive control, and blank, respectively. Cell viability data from a typical single experiment were plotted and the error bars represent the SD of the replicates. The upper x-axis represents TyroSphere concentration tested in this study, while the lower x-axis represents the concentrations of free PTX and PTX in TyroSpheres.
3.4. Gel-like PTX-TyroSpheres formulation
3.4.1. Homogeneity and short-term stability
TyroSpheres are an aqueous suspension and therefore their flow characteristics are very similar to that of water. Flowing formulations are not ideal for topical administration as they do not stay in the area of administration decreasing the contact time between formulation and site of application. In this study, hydroxypropyl methylcellulose (HPMC) has been chosen to increase the formulation viscosity since it has previously demonstrated (a) effectiveness in dispersing TyroSpheres uniformly and (b) lack of retardation in the deposition of lipophilic compounds in the skin [15]. To further investigate the effect of HPMC presence on the TyroSphere formulation with and without PTX, formulation homogeneity and stability (expressed as PTX stability), PTX release, and skin distribution have been evaluated. Homogeneity of the gel-like PTX-TyroSphere formulation stored at 4 °C was evaluated by determining PTX content from different areas of the formulation. Results from 10 samplings, established concentrations of 141.9 ± 0.9 µg and 143.0 ± 6.6 µg of PTX in 1 g of formulation at times 0 and 8 weeks, respectively. The small standard deviation in both measurements is an indication of formulation homogeneity while no significant change in PTX concentration over the 8 week time-period is an indication of formulation stability upon 4 °C storage.
3.4.2. In Vitro PTX Release from gel-like PTX-TyroSpheres
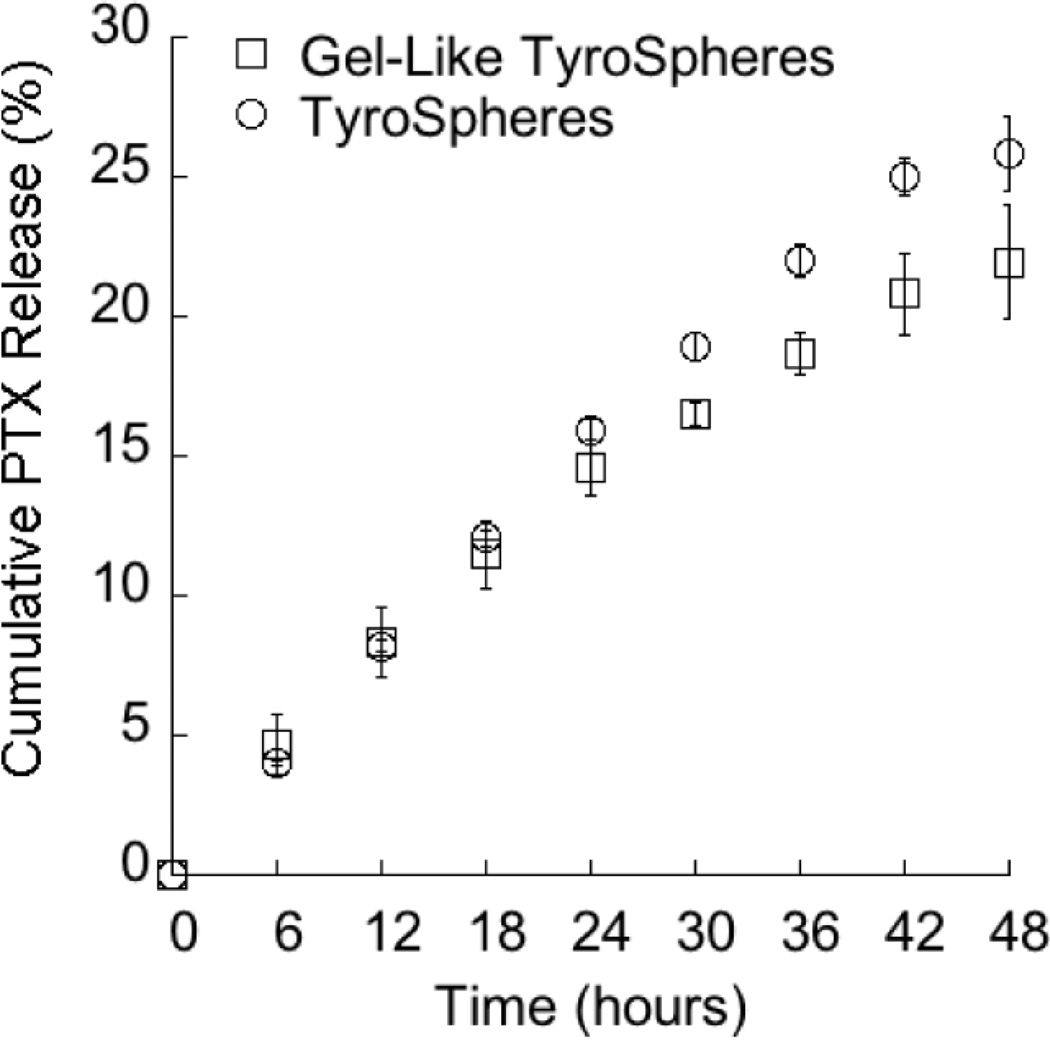
Release of PTX from gel-like PTX-TyroSpheres (1% w/v HPMC) is presented in Figure 4 and is similar to the release of PTX from TyroSpheres without HPMC at the same concentration of PTX and TyroSpheres. This finding provides evidence that the presence of the thickening agent is a suitable approach to reduce the flow properties of the base TyroSpheres.
Figure 4.
Release of PTX from TyroSphere formulations with and without HPMC as a thickening agent.
Cumulative PTX release from 5 % w/w (□) gel-like PTX-TyroSpheres and (○) PTX-TyroSpheres. The results are presented as mean ±SE (n = 3).
3.5. Skin Distribution
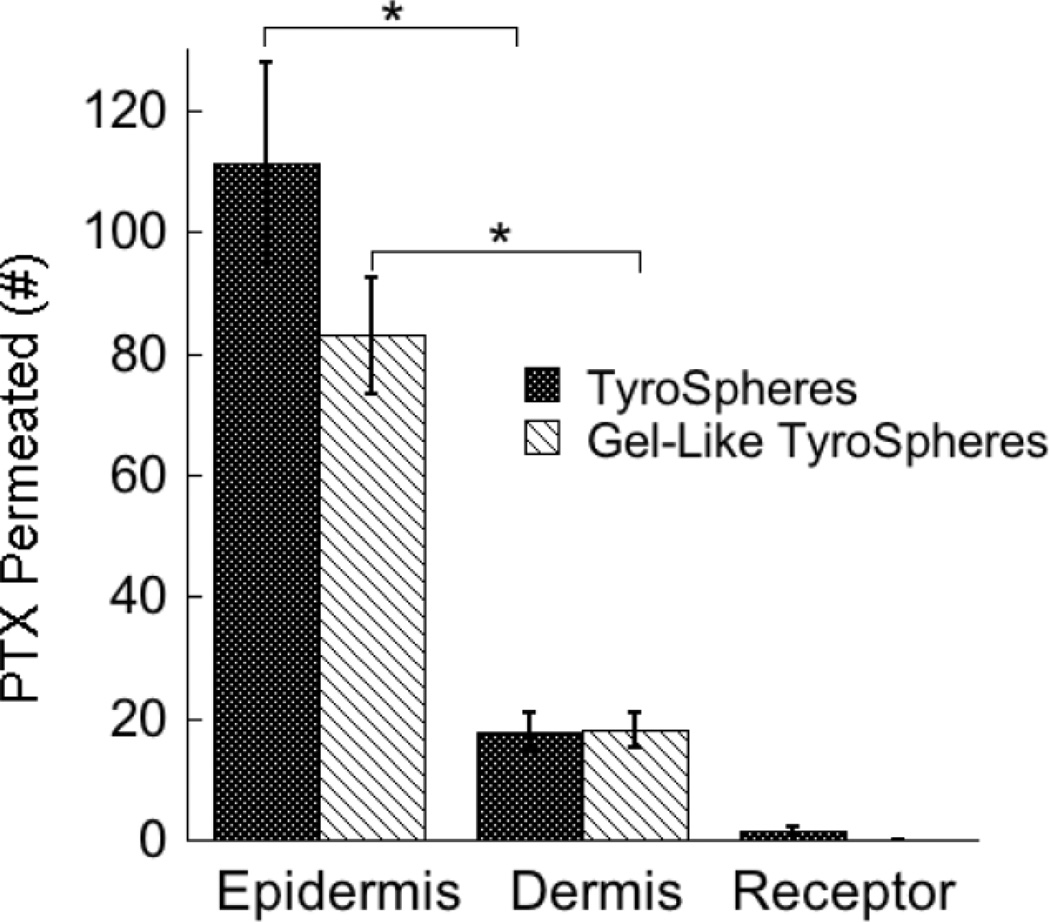
The ability of TyroSpheres with (gel-like) and without 1% w/v HPMC to deliver PTX into human cadaver skin was evaluated. Liquid chromatography–mass spectrometry was used to quantify PTX content in the epidermis, dermis, and receptor fluid in skin distribution studies. As shown in Figure 5, TyroSpheres deliver significant amounts of PTX into the epidermal layer of skin, the location of the over-proliferation of keratinocytes and the development of psoriasis.
Figure 5.
Skin distribution of PTX delivered via TyroSpheres (0% HPMC) and gel-like TyroSpheres (1% HPMC).
LC-MS was used as a detection method to quantify PTX deposited into different skin strata and receptor fluid. On the Y-axis, the units of PTX permeation (#) are: (ng/cm2) of tissue surface area for PTX in the epidermis and dermis, and (ng/mL) for PTX measured in the receptor compartment. The statistical data is expressed as mean ±SE (n = 8); p < 0.001 (*) was considered to be statistically significant between the amount of PTX present in the epidermis and dermis for both TyroSpheres and gel-like TyroSpheres.
Equally as important, minimal PTX was found in the dermis and in the receptor phase of the Franz cell, indicating that treatment with PTX-TyroSpheres would result in limited systemic escape of the cytotoxic drug. The limited amounts of PTX found in the dermis and receptor phase were expected as it is commonly accepted that in vitro skin distribution analysis, though a good tool for screening, frequently over-estimates the permeation when compared to an in vivo model. As an example, van de Sandt et al. have reported that in vitro permeation data with the pesticide propoxur over-estimated in vivo permeation data by up to a factor of three [31]. Additionally, the skin distribution results showed that the increased viscosity of gel-like TyroSpheres have no significant impact on PTX skin deposition at each evaluated skin layer confirming again that the addition of the thickening agent is not altering TyroSphere properties. The skin distribution results demonstrate that both TyroSphere formulations address the requirements of an efficient topical drug delivery system with minimal transdermal delivery and/or systemic escape and can be considered as an appropriate vehicle for the delivery of PTX as a topical psoriasis therapy.
Elucidation of the mechanism by which TyroSpheres deliver their payload into the skin is currently under investigation. Based on the skin distribution data reported here, we hypothesize that PTX delivery to the skin can be explained by the increased solubility of drug in the TyroSphere matrix, which in turn leads to an increased concentration gradient at the skin surface. Based on the recent review by Prow et al., nanoparticles larger than 10 nm are unlikely to penetrate into the viable epidermis [32], hence we assume that PTX release will most likely occurs at the interface of the TyroSphere and the skin surface and/or hair follicle. Due to the lipid-rich environment of the stratum corneum, lipophilic compounds, like PTX, may remain in the stratum corneum intercellular space (as a drug depot), allowing for slow permeation into the lower viable epidermis where psoriasis originates.
Similar findings were reported by Sachdeva et al. for the dermal delivery of terbinafine hydrochloride [33]. In the TyroSphere system, PTX largely remains in the epidermis (topical delivery) because the increased hydrophilicity of the dermis restricts significant partitioning of hydrophobic drug. Additionally, it is well-known that disease-state skin, and in particular psoriatic skin, is more permeable than healthy skin [34]. Therefore, in patients, we expect even more deposition of PTX in the epidermis thereby making PTX-TyroSpheres more effective.
4. Conclusions
The aim of this study was to develop and evaluate the potential of tyrosine-derived nanospheres (TyroSpheres) as a topical delivery system for paclitaxel (PTX). Non-cytotoxic TyroSpheres provide greatly enhanced solubility of PTX and PTX-TyroSpheres successfully combine the benefits of: (a) a therapeutic to control the over-proliferation of keratinocytes thereby bringing the system back into equilibrium, (b) dose-controlled delivery of PTX, and (c) preferential deposition of PTX into the epidermis. Selective accumulation of PTX in the epidermis should eliminate adverse side effects associated with systemic exposure. Further, TyroSpheres can be incorporated into a gel-like formulation that allows enhanced skin contact and ease of application without altering the release or skin distribution characteristics of TyroSpheres. Although additional studies are needed in order to evaluate the therapeutic potential of PTX-TyroSpheres in clinically relevant models, current results suggest that PTX-TyroSpheres could be a promising modality for psoriasis treatment.
Acknowledgements
The authors wish to thank Dr. Qing Ren (Department of Radiation Oncology at Thomas Jefferson University) for her generous gift of HaCaT cells. We appreciate Dr. Haiyan Zheng (Biological Mass Spectrometry Facility of the UMDNJ-Robert Wood Johnson Medical School and Rutgers, The State University of New Jersey) for fruitful discussions on and assistance with LC-MS work. We acknowledge Ms. Lulu Wang (New Jersey Center for Biomaterials) for her valuable technical contributions and we thank skilled Rutgers University undergraduates Yangmin Chen, Diane Kim, David Winters, and Jimmy Winters for their assistance with various release studies, solubility measurements, and biological assays. The project described was supported by Grant Number 5R01AR056079 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS - NIH).
Glossary of abbreviations
- PTX
paclitaxel
- TyroSpheres
tyrosine-derived nanosphere(s)
- PTX-TyroSpheres
tyrosine-derived nanospheres containing paclitaxel
- DTO-SA/5K
PEG5K-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-PEG5K
- HPMC
hydroxypropyl methylcellulose
- BE
binding efficiency
- LE
loading efficiency
- HPLC
reverse-phase high performance liquid chromatography
- SD
standard deviation
- SE
standard error
- PDI
polydispersity index
- LC-MS
liquid chromatography–mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danilenko DM. Review paper: preclinical models of psoriasis. Vet. Pathol. 2008;45:563–575. doi: 10.1354/vp.45-4-563. [DOI] [PubMed] [Google Scholar]
- 2.Clark RA, Kupper TS. Misbehaving macrophages in the pathogenesis of psoriasis. J. Clin. Invest. 2006;116:2084–2087. doi: 10.1172/JCI29441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae YS, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Young M, Bebo B, Jr, Kimball AB. Review of treatment options for psoriasis in pregnant or lactating women: From the Medical Board of the National Psoriasis Foundation. J. Am. Acad. Dermatol. 2011 doi: 10.1016/j.jaad.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Koziara JM, Lockman PR, Allen DD, Mumper RJ. Paclitaxel nanoparticles for the potential treatment of brain tumors. J. Control. Release. 2004;99:259–269. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Liggins RT, Hunter WL, Burt HM. Solid-state characterization of paclitaxel. J. Pharm. Sci. 1997;86:1458–1463. doi: 10.1021/js9605226. [DOI] [PubMed] [Google Scholar]
- 6.Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47:2486–2493. [PubMed] [Google Scholar]
- 7.Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release. 2003;86:33–48. doi: 10.1016/s0168-3659(02)00320-6. [DOI] [PubMed] [Google Scholar]
- 8.Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV., 3rd Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol. Oncol. 2006;100:437–438. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Bartoli MH, Boitard M, Fessi H, Beriel H, Devissaguet JP, Picot F, Puisieux F. In vitro and in vivo antitumoral activity of free, and encapsulated taxol. J. Microencapsul. 1990;7:191–197. doi: 10.3109/02652049009021832. [DOI] [PubMed] [Google Scholar]
- 10.Tarr BD, Sambandan TG, Yalkowsky SH. A new parenteral emulsion for the administration of taxol. Pharm. Res. 1987;4:162–165. doi: 10.1023/a:1016483406511. [DOI] [PubMed] [Google Scholar]
- 11.Ong BYS, Ranganath SH, Lee LY, Lu F, Lee H-W, Sahinidis NV, Wang C-H. Paclitaxel delivery from PLGA foams for controlled release in post-surgical chemotherapy against glioblastoma multiforme. Biomaterials. 2009;30:3189–3196. doi: 10.1016/j.biomaterials.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich A, Booher S, Becerra Y, Borris DL, Figg WD, Turner ML, Blauvelt A. Micellar paclitaxel improves severe psoriasis in a prospective phase II pilot study. J. Am. Acad. Dermatol. 2004;50:533–540. doi: 10.1016/j.jaad.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Sheihet L, Piotrowska K, Dubin RA, Kohn J, Devore D. Effect of tyrosine-derived triblock copolymer compositions on nanosphere self-assembly and drug delivery. Biomacromolecules. 2007;8:998–1003. doi: 10.1021/bm060860t. [DOI] [PubMed] [Google Scholar]
- 14.Sheihet L, Garbuzenko OB, Bushman J, Gounder MK, Minko T, Kohn J. Paclitaxel in tyrosine-derived nanospheres as a potential anti-cancer agent: In vivo evaluation of toxicity and efficacy in comparison with paclitaxel in Cremophor. Eur. J. Pharm. Sci. 2012;45:320–329. doi: 10.1016/j.ejps.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release. 2011;149:159–167. doi: 10.1016/j.jconrel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Sheihet L, Chandra P, Batheja P, Devore D, Kohn J, Michniak B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int. J. Pharm. 2008;350:312–319. doi: 10.1016/j.ijpharm.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Sheihet L, Dubin RA, Devore D, Kohn J. Hydrophobic drug delivery by self-assembling triblock copolymer-derived nanospheres. Biomacromolecules. 2005;6:2726–2731. doi: 10.1021/bm050212u. [DOI] [PubMed] [Google Scholar]
- 18.Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. J. Cell Biol. 2007;177:7–11. doi: 10.1083/jcb.200611141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourke SL, Kohn J. Polymers derived from the amino acid L-tyrosine: polycarbonates, polyarylates and copolymers with poly(ethylene glycol) Adv. Drug Deliv. Rev. 2003;55:447–466. doi: 10.1016/s0169-409x(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 20.Costache AD, Sheihet L, Zaveri K, Knight DD, Kohn J. Polymer-drug interactions in tyrosine-derived triblock copolymer nanospheres: a computational modeling approach. Mol. Pharm. 2009;6:1620–1627. doi: 10.1021/mp900114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003;258:141–151. doi: 10.1016/s0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release. 2004;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001;14:101–114. doi: 10.1016/s0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 24.Mathew AE, Mejillano MR, Nath JP, Himes RH, Stella VJ. Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J. Med. Chem. 1992;35:145–151. doi: 10.1021/jm00079a019. [DOI] [PubMed] [Google Scholar]
- 25.Tarr BD, Yalkowsky SH. A new parenteral vehicle for the administration of some poorly water soluble anti-cancer drugs. J. Parenter. Sci. Technol. 1987;41:31–33. [PubMed] [Google Scholar]
- 26.Swindell CS, Krauss NE, Horwitz SB, Ringel I. Biologically active taxol analogues with deleted A-ring side chain substituents and variable C-2' configurations. J. Med. Chem. 1991;34:1176–1184. doi: 10.1021/jm00107a042. [DOI] [PubMed] [Google Scholar]
- 27.Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seleverstov O, Zabirnyk O, Zscharnack M, Bulavina L, Nowicki M, Heinrich JM, Yezhelyev M, Emmrich F, O'Regan R, Bader A. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 2006;6:2826–2832. doi: 10.1021/nl0619711. [DOI] [PubMed] [Google Scholar]
- 30.Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007;22:1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 31.van de Sandt JJ, Meuling WJ, Elliott GR, Cnubben NH, Hakkert BC. Comparative in vitro-in vivo percutaneous absorption of the pesticide propoxur. Toxicol. Sci. 2000;58:15–22. doi: 10.1093/toxsci/58.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, Wurm EM, Yoong C, Robertson TA, Soyer HP, Roberts MS. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011;63:470–491. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Sachdeva V, Kim HD, Friden PM, Banga AK. Iontophoresis mediated in vivo intradermal delivery of terbinafine hydrochloride. Int. J. Pharm. 2010;393:112–118. doi: 10.1016/j.ijpharm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Roberts MS. Targeted drug delivery to the skin and deeper tissues: role of physiology, solute structure and disease. Clin. Exp. Pharmacol. Physiol. 1997;24:874–879. doi: 10.1111/j.1440-1681.1997.tb02708.x. [DOI] [PubMed] [Google Scholar]