Abstract
Objective
To examine the differentiation-related expression of CB1-R mRNA and protein in endometrial tissue obtained from women with and without endometriosis and to determine the impact of acute TCDD exposure on CB1-R gene expression in isolated endometrial stromal cells.
Design
Laboratory-based study
Setting
University-affiliated medical center
Patients
Women with and without endometriosis undergoing volunteer endometrial biopsies after informed consent.
Interventions
None
Main Outcome Measures
Analysis of in vivo CB1-R mRNA and protein expression in human endometrial tissues and mRNA expression in isolated stromal cells following exposure to TCDD or a progesterone receptor antagonist (Onapristone).
Results
CB1-R mRNA and protein expression was highest during the progesterone-dominated secretory phase in control women, while expression was minimal in endometrial tissues acquired from women with endometriosis, regardless of the cycle phase. Although progesterone was found to induce CB1-R mRNA expression in endometrial stromal cells from control donors, steroid-induced expression of this gene was inhibited by co-treatment with either TCDD or Onapristone.
Conclusions
Our studies reveal a role for the anti-inflammatory actions of progesterone in regulating endometrial cannabinoid signaling, which is disrupted in women with endometriosis. Significantly, our studies demonstrate, for the first time, that acute TCDD exposure disrupts cannabinoid signaling in the human endometrium.
Keywords: Cannabinoid receptor CB1-R, progesterone, endometriosis, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD;dioxin)
INTRODUCTION
Endometriosis, characterized by the presence of endometrial glands and stroma at extrauterine sites, is a common and debilitating reproductive disease of uncertain etiology. Although typically considered an endocrine disorder, women with endometriosis frequently exhibit immune system dysfunctions that not only affect the initial development of the disease but may contribute to the impaired fertility and severe pelvic pain that are frequently noted as co-morbidities [1–2]. Although estrogen has long been considered to represent the most important steroid in the pathogenesis of endometriosis [2–3], research over the past decade suggests that reduced progesterone sensitivity may play an equally relevant role in the initiation and progression of this disease [4–7]. For example, we previously demonstrated an important role for progesterone in suppressing endometrial expression of matrix metalloproteinases (MMPs), enzymes which both regulate extracellular matrix remodeling during the menstrual cycle in healthy women [8–9] and impact the function of the innate immune system [10]. However, in women with endometriosis, an elevated pattern of MMP-3 and MMP-7 expression persisted in the progesterone dominant secretory phase [11]; an alteration which was also associated with the establishment of ectopic disease in a chimeric mouse model of human endometriosis [12–13]. Thus, adequate progesterone response within the stromal compartment of the endometrium is critical for regulating the expression of multiple proteins by various cell types during endometrial maturation in preparation for pregnancy. Equally important, continued progesterone action is critical in mediating the complex interactions that occur between immune and specialized stromal/decidual cells at the maternal-fetal interface throughout pregnancy. Therefore, reduced responsiveness to the anti-inflammatory actions of this steroid within the reproductive tract may contribute to the numerous immunological alterations noted among women with endometriosis-related infertility [10].
During endometrial maturation in healthy women, progesterone principally regulates differentiation-related gene expression following binding to functionally distinct progesterone receptor (PR) isoforms, A and B. These isoforms are selectively produced from a single gene by alternative transcription from two different promoter start sites [14–15]. Importantly, the full-length PR-B is primarily responsible for mediating the anti-inflammatory effects of progesterone [16], while the truncated PR-A isoform can act as a dominant repressor of PR-B activity [17–18]. In women with endometriosis, a predominance of PR-A expression relative to PR-B has been noted in both eutopic and ectopic endometrium, promoting the progesterone resistant endometrial phenotype identified in these patients [4, 7, 19].
While progesterone is the most important endocrine signal for successful initiation and maintenance of pregnancy, emerging evidence suggests a significant role for endometrial endocannabinoid signaling in maintaining fertility [20–23]. Nevertheless, endometrial expression and activation of the endocannabinoid system (ECS) must be carefully orchestrated, since pregnancy failure and other reproductive disorders can result from either the absence or an excess of endocannabinoids and their respective metabolic enzymes [21, 23–31]. The ECS is comprised of the endocannabinoids, anandamide (N-arachidonoylethanolamide or AEA) and 2-arachidonoyl glycerol (2-AG) as well as their receptors and various regulatory enzymes [32–33]. The membrane bound cannabinoid receptors (CB1-R and CB2-R) are members of the G protein-coupled receptor superfamily [21, 34]. CB1-R is highly expressed in testis, placenta and uterus as well as multiple non-reproductive tissues [29, 35–43]. Within the reproductive tract, the uterus contains the highest concentrations of AEA, the primary endogenous ligand for the CB1-R [36, 44–46]. Although the extent to which the ECS contributes to normal reproductive function has yet to be elucidated, this system has been suggested to direct immune cell migration into the endometrium and homing of the embryo to the site of uterine attachment [29, 44–45]. Not unexpectedly, studies in humans and animals indicate that inappropriate activation of the ECS via exogenous cannabinoids (i.e., Cannabis) is associated with reproductive malfunctions including early pregnancy failure and preterm birth [23, 47–49].
Sex steroids have been shown to impact circulating and local endocannabinoid levels as well as the availability of the enzymes required for their synthesis and metabolism [28, 50–52]. Progesterone, in particular, has been shown to play a significant role in regulating activation and suppression of a number of ECS constituents. Specifically, CB1-R mRNA is induced during decidualization of isolated human endometrial stromal cells in response to progesterone treatment [53], and temporal CB1-R mRNA and protein expression has been reported in the human fallopian tube, with the highest expression levels correlating to the progesterone dominant secretory phase [27].
At this juncture, the origin(s) of reduced endometrial responsiveness to progesterone relative to the initiation and progression of endometriosis remain unclear. Based on a review of evidence from multiple laboratories, we recently proposed that exposure to dioxin-like environmental toxicants, including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin), may trigger inflammation-related changes within the reproductive tract that promote the progesterone resistant endometriosis phenotype [5, 54–55]. Additionally, using a murine model, we found that an early life exposure to TCDD can lead to infertility and pregnancy loss among female offspring which correlates with a reduced responsiveness to the anti-inflammatory action of progesterone [5, 19, 56]. Although a similar prospective study of early life human exposure to this toxicant is not possible, we have examined the effects of TCDD on primary, adult endometrial stromal cells acquired by biopsy from disease-free women. Within an in vitro study, we demonstrated that short-term TCDD treatment of isolated stromal cells led to development of a progesterone resistant cellular phenotype as a consequence of reduced PR-B expression [7]. Furthermore, this same study also revealed that the TCDD mediated loss of PR-B was associated with a failure of progesterone to suppress stromal cell expression of MMP-3 protein. Though several studies indicate that progesterone may play a fundamental role in ECS signaling in the human reproductive tract, whether the progesterone resistant phenotype noted in vivo in endometriosis patients or in vitro following experimental toxicant exposure affects the ECS has not previously been examined.
In the current study, we evaluated the cyclic expression of CB1-R mRNA and protein in endometrial tissues obtained from women with and without endometriosis. We also utilized an established in vitro model of endometrial stromal cell differentiation to examine the relative influence of TCDD, interleukin 1α (IL-1α) and the anti-progestin, Onapristone, on progesterone-regulated expression of CB1-R mRNA. We found that progesterone exposure in vivo during the secretory phase was associated with increased CB1-R mRNA and protein expression in endometrial samples obtained from healthy control women while minimal gene and protein expression was observed in similar tissues obtained from women with endometriosis. In vitro studies revealed that acute TCDD exposure of isolated primary endometrial stromal cells obtained from healthy control women resulted in a failure of progesterone to up-regulate CB1-R mRNA expression or reduce MMP-3 mRNA expression. The results of our TCDD treatment studies mirror the results we obtained by treating the cells with the PR antagonist, Onapristone, and further support a role for progesterone in regulating CB1-R expression. Moreover, we demonstrated a synergistic ability of IL-1α and TCDD treatments to synergistically reduce expression levels of PR-B and progesterone mediated CB1-R mRNA expression in isolated stromal cells. Taken together, our current studies identify a previously unrecognized link between the loss of progesterone's anti-inflammatory action in the endometrium and dysregulation of the ECS through a loss of CB1-R expression.
MATERIAL AND METHODS
Acquisition of Human Endometrial Tissue
The use of human endometrial tissues was approved by the Vanderbilt University Institutional Review Board for the Protection of Human Subjects. After obtaining informed consent, tissues were collected from donors (ages 18–45) with predictable menstrual cycles. Exclusion criteria for all donors included recent (<3 months) hormonal therapy (i.e., oral contraceptives) and other medications that could impact the results. Additional exclusion criteria for the disease-free, control population (N=20) included history of adhesions, polycystic ovarian disease or endometrial disorders, including fibroid uterus and endometriosis. Finally, inclusion criteria of patients with endometriosis (N=15) required a previous surgical confirmation with histopathological diagnosis of endometriosis and no hormonal treatment for the disease. Tissues were obtained by Pipelle biopsy (Unimar, Inc, Wilton, CT) at Vanderbilt University Medical Center. Proliferative phase samples (days 9–12; control N=9; endometriosis N=7) were confirmed by a serum progesterone level of ≤1.5 ng/mL. Secretory phase samples (days 14–35; control N=11; endometriosis N=8) were timed from the LH surge, using ovulation predictor kits. Following biopsy, endometrial tissues were washed immediately in pre-warmed, phenol red-free DMEM/F-12 (Sigma, St. Louis, MO) medium. A portion of each sample was formalin-fixed for histological confirmation of cycle phase and an additional portion flash frozen and stored at −80°C for further analysis.
Endometrial Stromal Cell Isolation and Culture
Isolated endometrial stromal cells were obtained by enzymatic digestion and filter separation as previously described [57]. Two hundred thousand stromal cells were seeded onto 1.9-cm2 wells coated with type I collagen and maintained until approximately 75% confluent (18–24 hours) in phenol red-free DMEM/F-12 with 5% charcoal-stripped calf serum, 1nM 17-β estradiol (E) and 1× antibiotic-antimycotic solution at 37°C and 5% CO2. Subconfluent cells were removed to serum-free medium with 1nM E with and without 500nM progesterone (P). Some cultures also received 10nM TCDD (in nonane; Cerilliant Corp, Round Rock, TX), Interleukin-1α (IL-1α; 10ng/mL; R&D Systems, Minneapolis, MN) or 5μM Onapristone (Ona; a gift from Schering-AG, Berlin, Germany). Triplicate groups were maintained for either 48h (Ona) or 5 days with media changes every 48h. Terminated cultures were flash-frozen and stored at −80°C until analysis. Steroids and remaining chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Quantitative Real-Time PCR (qRT-PCR)
RNA was extracted from cultured endometrial stromal cells using Qiagen RNeasy kit (Qiagen, CA) while RNA from endometrial biopsies was extracted with TRIzol (Invitrogen, Carlsbad, CA). Reverse transcription was performed using BioRad iScript cDNA Synthesis Kit (BioRad, CA) in duplicate reactions of: 1μl cDNA, 0.5μl (10pmol/μl) each of forward and reverse primers, 5 μl of BioRad SsoFast™ EvaGreen® Supermix (BioRad, CA) and 3μl nuclease free water (Ambion, Austin TX). Human ribosomal protein, large P0 (RPLP0), was used as the internal reference gene [58]. Primer sequences:
hRPLP0-F 5'-TCGACAATGGCAGCATCTAC-3'
hRPLP0-R 5'-GCCTTGACCTTTTCAGCAAG-3'
hCB1-F 5'-TCCTAGATGGCCTTGCAGAT-3'
hCB1-R 5'-CCCACACTGGATGTCTCCT-3'
hPR-B-F 5'-ACACCTTGCCTGAAGTTTCG-3
hPR-B-R 5'-TCCAAGACACTGTCCAGCAG-3
hMMP-3-F 5'-GCAGTTTGCTCAGCCTATCC-3'
hMMP-3-R 5'-GAGTGTCGGAGTC CAGCTTC-3'
Negative controls included duplicate wells in which cDNA was omitted (volume adjusted with nuclease-free water). qRT-PCR was performed using a BioRad CFX96 Real-Time System™, followed by melt curve to ensure single amplicon. Data was analyzed by ΔΔct method [59].
Immunohistochemical Localization of CB1-R protein
Immunohistochemical staining of human endometrial tissues obtained by biopsy was performed by standard methodology for formalin-fixed, paraffin-embedded tissues. Antigen retrieval was performed on sections using 1× Antigen Retrieval Citra Solution (BioGenex, Fremont, CA) and endogenous peroxidase activity blocked by incubating sections in 3% hydrogen peroxide. To prevent non-specific binding of the antibody, sections were blocked in 3% Bovine Serum Albumin (BSA; Sigma-Aldrich, St. Louis, MO) in PBS-Tween (PBST) for 1 hr at room temperature (RT). The primary antibody (rabbit anti-human CB1-R; Santa Cruz, Santa Cruz, CA), diluted 1:200 in BSA/PBST, was added to the sections which were incubated overnight at 4°C. After washing with PBST, a biotinylated universal secondary antibody (Dako LSAB kit, Dako, Carpinteria, CA) was added to the sections for 30 min at RT, followed by incubation with Streptavidin-HRP (DakoCytomation LSAB+ System-HRP). Color detection was performed using 3,3'-diaminobenzidine (DAB; DAB Substrate kit, Vector Laboratories, Burlingame, CA). The sections were then lightly counterstained with Gill's hematoxylin, dehydrated and coverslipped. Slides were viewed with an Olympus BX51 microscope system and images captured at 1000× using an Olympus DP71 digital camera.
Statistical Analysis
Analyses were performed with GraphPad Prism©5 software and presented as mean ±SEM. The statistical difference between samples was determined using unpaired two-tailed t-test (Figures 3 and 4) and one-way analysis of variance (ANOVA) followed by Bonferroni posthoc test (Figures 1A, 2 and Supplemental Figure 1). P<0.05 were considered significant.
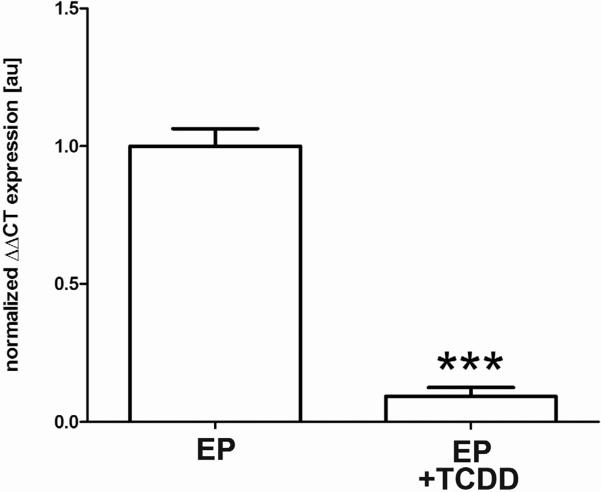
Figure 3. In vitro TCDD exposure blocks progesterone induction of CB1-R mRNA expression in endometrial stromal cells obtained from control donors.
Stromal cells were isolated from control endometrial biopsies taken during the secretory phase (N=5) and cultured 4 days with estradiol + progesterone (E; 1nM; P; 500nM) with or without TCDD (10nM). CB1-R mRNA expression was robust in cells maintained in EP, while those cells treated with TCDD showed significantly reduced expression of this transcript (P< 0.001). qRT-PCR data, normalized to the housekeeping gene, was analyzed using the ΔΔCT [59] method and *** is indicative of P< 0.001.
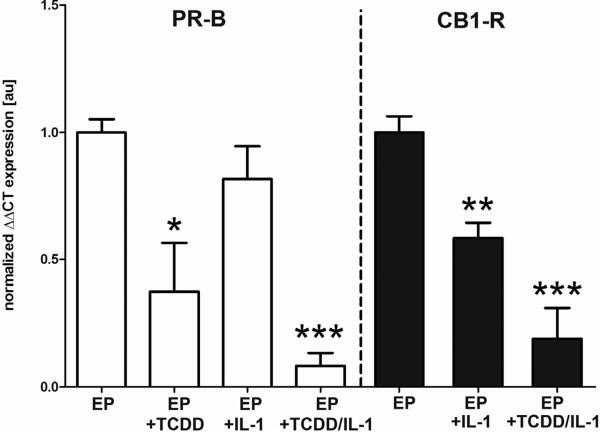
Figure 4. Synergistic effect of TCDD and IL-1α in isolated stromal cells.
Control endometrial stromal cells were isolated from the secretory phase and cultured 5 days with estradiol + progesterone (E; 1nM; P; 500nM) in the presence and absence of TCDD (10nM). Some cultures were challenged with IL-1α (10ng/mL) during the last 24 hrs of culture. Expression of PR-B (left panel) and CB1-R mRNA (right panel) was robust in cells treated with EP, while cells treated with TCDD showed a marked reduction in PR-B mRNA expression levels (P< 0.05), similar to CB1-R mRNA levels observed in Figure 3. Treatment with IL-1α alone led to a non-significant reduction in PR-B mRNA expression while CB1-R mRNA levels were significantly reduced (P< 0.01). Combinatorial treatment with EP, TCDD and IL-1 led to the lowest expression levels of both genes (P< 0.001). qRT-PCR data, normalized to the housekeeping gene, was analyzed using the ΔΔCT method [59]. **P<0.01; *** P< 0.001.
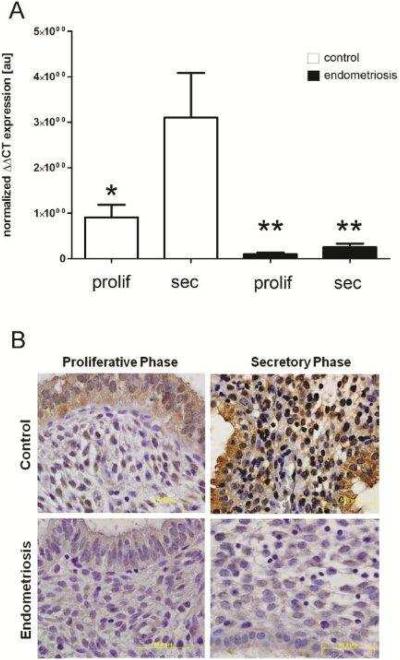
Figure 1. Progesterone-mediated endometrial CB1-R mRNA and protein expression is disrupted in women with endometriosis.
Human endometrial tissues were obtained by biopsy during the proliferative or secretory phase from women with or without endometriosis and a portion flash-frozen for mRNA analysis and a portion formalin-fixed and paraffin embedded for immunohistochemical analysis. Quantitative RT-PCR analysis of CB1-R mRNA expression (A) revealed that in samples obtained from disease-free women, CB1-R mRNA expression was dramatically increased in the secretory phase (N=11) compared with samples obtained during the proliferative phase (N=9). Endometrial tissues obtained from women with endometriosis expressed minimal CB1-R mRNA in both the proliferative (N=7) and secretory (N=8) phases of the cycle. qRT-PCR data, normalized to the housekeeping gene (ribosomal protein large P0, RPLP0), was analyzed using the ΔΔCT method [59] and * is indicative of P<0.05. Immunohistochemical localization of CB1-R (B) was similar to mRNA expression patterns in that protein expression was weak in control samples obtained during the proliferative phase, but robust staining was observed in secretory phase samples. However, CB1-R immunolocalization was minimal in both proliferative and secretory phase samples from women with laparoscopically-confirmed endometriosis. All slides were photographed at 1000× and results representative of at least 5 samples in each group.
Figure 2. Progesterone-mediated CB1-R mRNA expression in isolated stromal cells is blocked by Onapristone.
Stromal cells were isolated from control endometrial biopsies taken during the proliferative phase (N=5) and cultured for 48hr in estradiol (E; 1nM) with or without progesterone (P; 500nM). Some cultures additionally received the progesterone receptor antagonist, Onapristone (Ona; 5μM). CB1-R mRNA expression was low in cells maintained in E alone, but was significantly increased in cells additionally exposed to P (P<0.01). Co-treatment with Ona abolished progesterone induction of CB1-R mRNA (P<0.01). qRT-PCR data, normalized to the housekeeping gene, was analyzed using the ΔΔCT method [59] and ** is indicative of P<0.01.
RESULTS
CB1-R mRNA and Protein Expression in Human Endometrial Biopsies
Following RNA extraction from flash frozen human endometrial biopsies, we examined CB1-R mRNA expression levels in tissues obtained from healthy women and women with surgically-confirmed endometriosis during the late proliferative and midsecretory phases of the menstrual cycle. In disease-free women, CB1-R mRNA expression was significantly elevated in secretory tissues compared to proliferative phase samples (Figure 1A), suggesting an important role for progesterone in the regulation of this gene. In contrast, CB1-R mRNA expression was minimal in endometrial tissues obtained from women with endometriosis, regardless of the cycle phase (Figure 1A).
Formalin-fixed, paraffin-embedded endometrial tissues from women with and without endometriosis were further examined for immunolocalization of CB1-R protein. These studies revealed that expression of CB1-R protein is minimal in tissues obtained during the proliferative phase from healthy women (Figure 1B), but is abundant in both stromal and epithelial cells in endometrial tissues obtained during the secretory phase (Figure 1B). In contrast, immunolocalization of CB1-R protein, associated with either stromal or epithelial cells, is virtually absent in proliferative and secretory phase samples obtained from women with endometriosis (Figure 1B); an expression pattern which mirrors the mRNA data.
Onapristone Prevents Progesterone-Mediated CB1-R mRNA Expression in Isolated Human Endometrial Stromal Cells
The in vivo data described above suggest a role for progesterone in endometrial CB1-R regulation. To more definitively link progesterone treatment to the induction of endometrial CB1-R expression, we isolated stromal cells from the proliferative phase, thereby avoiding in vivo exposure of cells to progesterone or downstream mediators of progesterone action. As shown in Figure 2, endometrial stromal cells cultured with estradiol alone (E) exhibited virtually no expression of CB1-R mRNA while a dramatic (>100-fold) increase was observed in the presence of progesterone (EP) (P<0.001). Cells exposed to EP and Onapristone, a pure PR antagonist, expressed a significantly lower level of CB1-R mRNA (P<0.001) similar to cultures treated only with E.
TCDD Inhibits Progesterone-Dependent Induction of CB1-R mRNA
We have previously demonstrated that acute, in vitro exposure of isolated human endometrial cells to the environmental endocrine disruptor, TCDD, reduces the subsequent responsiveness of the cells to progesterone [7, 55]. As shown in Figure 3, the current study demonstrates that TCDD treatment of isolated endometrial stromal cells obtained from control women during the progesterone dominated secretory phase leads to a significant (P<0.001) reduction in CB1-R mRNA expression despite continuous exposure to progesterone. Specifically, cells were cultured for 5 days in media supplemented with estradiol and progesterone (EP) in the presence and absence of TCDD. These data further implicate progesterone as a key regulator of the ECS and suggest cannabinoid signaling during endometrial maturation is equally sensitive to disruption by TCDD.
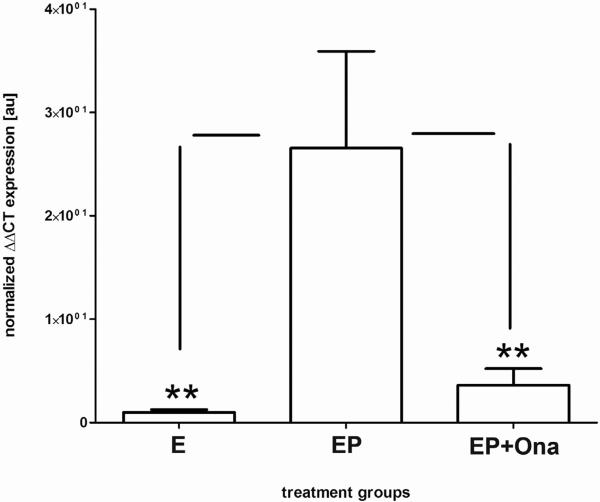
TCDD or Onapristone Prevents Progesterone Suppression of MMP-3 mRNA
Reduced expression of multiple MMPs has been demonstrated during the progesterone dominant secretory phase of healthy women, while persistent expression has been noted in women with endometriosis, regardless of the cycle phase [8, 11, 16]. Using isolated endometrial stromal cells from the proliferative phase of control donors, Supplemental Figure 1 demonstrates that either TCDD treatment (which suppresses endometrial PR-B expression) or treatment with Onapristone (a pure PR antagonist) effectively blocks the ability of progesterone to down-regulate stromal cell MMP-3 mRNA expression. Thus, impeding progesterone action not only leads to a failure to induce CB1-R mRNA expression (Figures 2 and 3), but also prevents regulation of a gene known to be suppressed by this steroid (Supplemental Figure 1) [11, 60–62].
Loss of PR-B Correlates with Reduced CB1-R mRNA Induction in vitro
As noted above, both in vivo and in vitro studies suggest that the ability of TCDD exposure to decrease the expression of PR within the reproductive tract correlates with a heightened sensitivity to inflammation [5, 7, 19, 55–56]. Therefore, we explored whether acute TCDD exposure in combination with a pro-inflammatory cytokine might act synergistically to disrupt progesterone-mediated regulation of endometrial CB1-R mRNA expression. Isolated human endometrial stromal cells obtained from endometrial biopsies taken from disease-free women during the secretory phase were cultured for a total of 5 days in media supplemented with estradiol and progesterone (EP) with and without TCDD (10nM), interleukin-1α (10ng/mL) or a combination of these agents. Consistent with our previous studies, TCDD exposure of secretory phase stromal cells leads to a significant decrease in PR-B mRNA expression (Figure 4; left panel). Although only a non-significant decrease in PR-B mRNA expression was observed following stromal cell exposure to IL-1α alone, the levels of this gene were significantly decreased in cells treated with both TCDD and IL-1α (Figure 4; left panel), suggesting a synergistic effect of these compounds. Exposure of cells to TCDD and IL-1α led to a similar pattern of diminished progesterone-mediated expression of CB1-R mRNA (Figure 4; right panel), further linking the loss of progesterone's anti-inflammatory actions to aberrations in cannabinoid signaling within the human endometrium.
DISCUSSION
A woman's exposure to progesterone is recognized as an important negative risk factor for the development of endometriosis [6, 16]; therefore, it is not surprising that recent research suggests that reduced sensitivity to this steroid plays a key role in the pathophysiology of this disease [6–7, 19]. Since the anti-inflammatory action of progesterone is required for normal immune cell migration and function during the menstrual cycle and pregnancy, loss of normal progesterone responsiveness is likely a component of the immunological alterations noted in endometriosis [10]. Importantly, pregnancy establishment requires a controlled inflammatory event, which allows for implantation to occur, yet prevents widespread tissue destruction and spontaneous pregnancy loss. To this end, activation and function of the ECS is known to be required for successful pregnancy establishment and maintenance [20, 63], perhaps due to its ability to mediate a controlled inflammatory response which limits collateral tissue damage [64–65]. Thus, progesterone and local ECS signaling must act in concert to regulate the complex inflammatory microenvironment that allows establishment and maintenance of an appropriate maternal-fetal interface.
In the current study, we explored whether a loss of progesterone action, due to endometriosis [4, 6, 11, 66] or following acute exposure to TCDD [7], would affect stromal cell expression of CB1-R mRNA, the receptor which primarily mediates endometrial response to cannabinoids. Initially, we demonstrated that the expression of CB1-R mRNA and protein in healthy human endometrium is significantly increased during the progesterone-dominant secretory phase (Figure 1A and 1B). These findings suggest that progesterone is involved in regulating endometrial CB1-R expression and agree with the findings of Horne et al, who noted the highest levels of CB1-R mRNA and protein expression in the human fallopian tube during the secretory phase [27]. In contrast, examination of endometrial samples exhibiting the progesterone resistant endometriosis phenotype revealed minimal CB1-R expression, regardless of the cycle phase (Figure 1A and 1B). Thus, our current study indicates that endometriosis patients exhibit alterations in cannabinoid responsiveness, potentially contributing to the infertility and immune dysregulation which is frequently associated with this disease [10].
Additionally, while it is known that acute exposure of women and other primates to TCDD induces pregnancy failure [56, 67], the specific role that this ubiquitous environmental toxicant may play in the etiology of endometriosis remains uncertain. Using cultures of endometrial stromal cells obtained from disease-free women, we previously found that acute exposure to TCDD leads to a dose-dependent loss of PR-B expression and subsequent failure of progesterone to down-regulate MMP-3 expression [7], mimicking the endometrial phenotype observed in women with endometriosis [4, 6, 19]. In the present study, endometrial stromal cells were similarly obtained from disease-free women during both the proliferative and secretory phases of the menstrual cycle in order to develop a more comprehensive view of TCDD's effects on CB1-R mRNA expression and regulation. As shown in Figure 2, in the absence of TCDD exposure, we found that endometrial stromal cells obtained during the proliferative phase and cultured only with estradiol expressed minimal levels of CB1-R mRNA while progesterone treatment led to a significant increase in expression of this gene, mirroring the cyclic regulation of CB1-R mRNA and protein in endometrial biopsies from control donors (Figure 1A and 1B). In contrast, treatment of proliferative phase stromal cells with the PR antagonist, Onapristone, largely prevented progesterone-mediated induction of CB1-R mRNA (Figure 2) and additionally blocked the suppressive impact of this steroid on MMP-3 mRNA expression (Supplemental Figure 1). The use of proliferative phase samples to obtain stromal cells without a prior exposure to progesterone in vivo coupled with the use of Onapristone to block progesterone action in vitro confirm progesterone's involvement in regulating CB1-R expression in human endometrial stromal cells.
We also examined whether exposure to TCDD disrupts the ability of progesterone to induce CB1-R mRNA expression involved a loss of this steroid's anti-inflammatory action. For this study, secretory phase endometrial stromal cells were maintained in the presence of both estradiol and progesterone and some cultures were further treated with either TCDD (Figure 3), IL-1α or a combination of these agents (Figure 4). These studies were designed to determine whether an inflammatory challenge in cells treated with TCDD further disrupts the ability of progesterone to regulate the differentiation-related expression of CB1-R mRNA. As expected, addition of TCDD to progesterone-treated stromal cells significantly reduced the ability of this steroid to up-regulate CB1-R mRNA (p<0.001) compared to sister cultures which were not exposed to TCDD. Interestingly, while exposure of stromal cells to estradiol and progesterone plus IL-1α only modestly affected expression levels of PR-B, exposure to this cytokine significantly (p<0.01) inhibited CB1-R mRNA expression (Figure 4), potentially indicating an important intermediary between progesterone and CB1-R gene expression. Regardless, the lowest level of CB1-R mRNA expression was observed following combined treatment with TCDD and IL-1α, an exposure paradigm that also led to a significant reduction of PR-B mRNA expression (Figure 4). Relative to our findings linking loss of CB1-R to inflammation, it is interesting to note that CB1-R/CB2-R knockout mice exhibit an exacerbated inflammatory response following influenza infection [65] and loss of CB1-R in the urinary bladder promotes hypersensitivity to inflammation in humans [68]. These recent studies, taken together with studies presented herein, suggest an important inter-relationship between the anti-inflammatory action of progesterone and the regulation of endometrial endocannabinoid signaling via CB1-R.
To our knowledge, these studies demonstrate for the first time that the TCDD-mediated loss of PR-B expression correlates with the loss of stromal cell expression of CB1-R mRNA. Therefore, these data not only support an important role of progesterone in the regulation of endometrial CB1-R mRNA expression, but demonstrate an ability of a persistent, environmental toxicant to disrupt normal endometrial ECS signaling. However, our studies do not rule out the possibility that progesterone-mediated regulation of CB1-R expression is indirect, via a mediator of progesterone action. Indeed, the promoter of the human CB1-R gene does not contain a known progesterone response element (promoter search 3KB, 5' of transcriptional start site, data not shown), suggesting progesterone regulation of this gene may be indirect. For example, we and others have previously identified retinoic acid as an important mediator of progesterone-associated endometrial action [66, 69–70], and retinoid signaling has been shown to directly regulate CB1-R mRNA expression in the murine liver [71].
It is important to note that our in vivo findings in the endometrium of endometriosis patients are in conflict with two previous studies examining endometrial CB1-R expression. Taylor and colleagues [72] reported that endometrial CB1-R immunoreactivity is “unrelated to the phase of the cycle” while Leconte and coworkers concluded that CB1-R expression is similar in endometrial cells obtained from women with and without endometriosis [73]. However, in contrast to the aforementioned studies, control tissue donors used for this study were carefully screened in an effort to avoid medications or occult gynecologic diseases that may alter endometrial steroid action.
In summary, we demonstrate an important role for the anti-inflammatory actions of progesterone in the regulation of endometrial endocannabinoid response which is dysregulated in women with endometriosis and by in vitro exposure to TCDD. Since both progesterone and the ECS are critical to regulating the appropriate inflammatory response during early pregnancy establishment, understanding the mechanisms which lead to a failure of steroid-regulated CB1-R expression may provide a better understanding of endometriosis-associated infertility. Our studies further support the examination of agents which target these systems, potentially providing new and much needed avenues for additional treatment strategies.
Supplementary Material
ACKNOWLEDGEMENTS
We greatly appreciate and acknowledge the women who donated endometrial biopsies for these studies as well as the physicians within the Vanderbilt Reproductive Medicine Clinic for performing these critical biopsies. We also acknowledge the U54 tissue bank at Yale University (U54 HD052666) which provided additional human endometrial samples for RNA analysis.
Financial support has been provided by the following entities:
Natl. Institute for Environmental Health Sciences (NIH): 5 R01ES014942
Natl. Institute for Child Health/Human Development (NIH): 5 U54HD052666
Endometriosis Association
Natl. Ctr. for Research Resources (NIH): Clinical Translational Scientific Award to Vanderbilt University: 1 UL 1 RR024975 (Database resources)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272–80. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587–9. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 4.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 5.Bruner-Tran KL, Ding T, Osteen KG. Dioxin and endometrial progesterone resistance. Semin Reprod Med. 2010;28(1):59–68. doi: 10.1055/s-0029-1242995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83(3):529–37. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-pdioxin. Fertil Steril. 2005;84(1):67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, Svitek C, et al. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94(3):946–53. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodgers WH, Osteen KG, Matrisian LM, Navre M, Giudice LC, Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am J Obstet Gynecol. 1993;168(1 Pt 1):253–60. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- 10.Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol. 2011;7(5):611–26. doi: 10.1586/eci.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87(10):4782–91. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 12.Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99(12):2851–7. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: the nude mouse as a xenographic host. Ann N Y Acad Sci. 2002;955:328–39. doi: 10.1111/j.1749-6632.2002.tb02793.x. discussion 340–2, 396–406. [DOI] [PubMed] [Google Scholar]
- 14.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5'-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7(12):1603–16. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- 16.Osteen KG, Bruner-Tran KL, Keller NR, Eisenberg E. Progesterone-mediated endometrial maturation limits matrix metalloproteinase (MMP) expression in an inflammatory-like environment: a regulatory system altered in endometriosis. Ann N Y Acad Sci. 2002;955:37–47. doi: 10.1111/j.1749-6632.2002.tb02764.x. discussion 86–8, 396–406. [DOI] [PubMed] [Google Scholar]
- 17.Vegeto E, Cocciolo MG, Raspagliesi F, Piffanelli A, Fontanelli R, Maggi A. Regulation of progesterone receptor gene expression. Cancer Res. 1990;50(17):5291–5. [PubMed] [Google Scholar]
- 18.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 19.Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol. 2007;23(3):326–36. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci. 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- 21.Maccarrone M. Endocannabinoids: friends and foes of reproduction. Prog Lipid Res. 2009;48(6):344–54. doi: 10.1016/j.plipres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Xie H, Dey SK. Endocannabinoid signaling directs periimplantation events. AAPS J. 2006;8(2):E425–32. doi: 10.1007/BF02854916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Xie H, Dey SK. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS One. 2008;3(10):e3320. doi: 10.1371/journal.pone.0003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battista N, Rapino C, Di Tommaso M, Bari M, Pasquariello N, Maccarrone M. Regulation of male fertility by the endocannabinoid system. Mol Cell Endocrinol. 2008;286(1–2 Suppl 1):S17–23. doi: 10.1016/j.mce.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Habayeb OM, Taylor AH, Finney M, Evans MD, Konje JC. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA. 2008;299(10):1135–6. doi: 10.1001/jama.299.10.1135. [DOI] [PubMed] [Google Scholar]
- 26.Habayeb OM, Taylor AH, Bell SC, Taylor DJ, Konje JC. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149(10):5052–60. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- 27.Horne AW, Phillips JA, 3rd, Kane N, Lourenco PC, McDonald SE, Williams AR, et al. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS One. 2008;3(12):e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacCarrone M, De Felici M, Bari M, Klinger F, Siracusa G, Finazzi-Agro A. Down-regulation of anandamide hydrolase in mouse uterus by sex hormones. Eur J Biochem. 2000;267(10):2991–7. doi: 10.1046/j.1432-1033.2000.01316.x. [DOI] [PubMed] [Google Scholar]
- 29.Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci U S A. 1995;92(21):9460–4. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276(23):20523–8. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AH, Ang C, Bell SC, Konje JC. The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum Reprod Update. 2007;13(5):501–13. doi: 10.1093/humupd/dmm018. [DOI] [PubMed] [Google Scholar]
- 32.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2–3):101–21. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura T, Waku K. Cannabinoid receptors and their endogenous ligands. J Biochem. 2002;132(1):7–12. doi: 10.1093/oxfordjournals.jbchem.a003200. [DOI] [PubMed] [Google Scholar]
- 34.Boyd ST. The endocannabinoid system. Pharmacotherapy. 2006;26(12 Pt 2):218S–221S. doi: 10.1592/phco.26.12part2.218S. [DOI] [PubMed] [Google Scholar]
- 35.Chang MC, Berkery D, Schuel R, Laychock SG, Zimmerman AM, Zimmerman S, et al. Evidence for a cannabinoid receptor in sea urchin sperm and its role in blockade of the acrosome reaction. Mol Reprod Dev. 1993;36(4):507–16. doi: 10.1002/mrd.1080360416. [DOI] [PubMed] [Google Scholar]
- 36.Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci U S A. 1995;92(10):4332–6. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–34. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminski NE, Abood ME, Kessler FK, Martin BR, Schatz AR. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992;42(5):736–42. [PMC free article] [PubMed] [Google Scholar]
- 39.Kenney SP, Kekuda R, Prasad PD, Leibach FH, Devoe LD, Ganapathy V. Cannabinoid receptors and their role in the regulation of the serotonin transporter in human placenta. Am J Obstet Gynecol. 1999;181(2):491–7. doi: 10.1016/s0002-9378(99)70583-1. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346(Pt 3):835–40. [PMC free article] [PubMed] [Google Scholar]
- 41.Schatz AR, Kessler FK, Kaminski NE. Inhibition of adenylate cyclase by delta 9-tetrahydrocannabinol in mouse spleen cells: a potential mechanism for cannabinoid-mediated immunosuppression. Life Sci. 1992;51(6):PL25–30. doi: 10.1016/0024-3205(92)90414-k. [DOI] [PubMed] [Google Scholar]
- 42.Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci U S A. 1994;91(16):7678–82. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiura T, Kodaka T, Nakane S, Kishimoto S, Kondo S, Waku K. Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator? Biochem Biophys Res Commun. 1998;243(3):838–43. doi: 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]
- 44.Paria BC, Deutsch DD, Dey SK. The uterus is a potential site for anandamide synthesis and hydrolysis: differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Mol Reprod Dev. 1996;45(2):183–92. doi: 10.1002/(SICI)1098-2795(199610)45:2<183::AID-MRD11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 1997;94(8):4188–92. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vercelli CA, Aisemberg J, Billi S, Wolfson ML, Franchi AM. Endocannabinoid system and nitric oxide are involved in the deleterious effects of lipopolysaccharide on murine decidua. Placenta. 2009;30(7):579–84. doi: 10.1016/j.placenta.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109(1):21–7. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 48.Fride E, Gobshtis N, Dahan H, Weller A, Giuffrida A, Ben-Shabat S. The endocannabinoid system during development: emphasis on perinatal events and delayed effects. Vitam Horm. 2009;81:139–58. doi: 10.1016/S0083-6729(09)81006-6. [DOI] [PubMed] [Google Scholar]
- 49.Mendelson JH, Mello NK, Ellingboe J, Skupny AS, Lex BW, Griffin M. Marihuana smoking suppresses luteinizing hormone in women. J Pharmacol Exp Ther. 1986;237(3):862–6. [PubMed] [Google Scholar]
- 50.Klinger FG, Battista N, De Felici M, Maccarrone M. Stage-variations of anandamide hydrolase activity in the mouse uterus during the natural oestrus cycle. J Exp Clin Assist Reprod. 2006;3:3. doi: 10.1186/1743-1050-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazzarin N, Valensise H, Bari M, Ubaldi F, Battista N, Finazzi-Agro A, et al. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecol Endocrinol. 2004;18(4):212–8. doi: 10.1080/09513590410001692492. [DOI] [PubMed] [Google Scholar]
- 52.Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem. 2003;278(35):32726–32. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- 53.Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S. Cannabinoid receptor I activation markedly inhibits human decidualization. Mol Cell Endocrinol. 2005;229(1–2):65–74. doi: 10.1016/j.mce.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Bruner-Tran KL, Osteen KG. Dioxin-like PCBs and endometriosis. Syst Biol Reprod Med. 2010;56(2):132–46. doi: 10.3109/19396360903381023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruner-Tran KL, Yeaman GR, Crispens MA, Igarashi TM, Osteen KG. Dioxin may promote inflammation-related development of endometriosis. Fertil Steril. 2008;89(5 Suppl):1287–98. doi: 10.1016/j.fertnstert.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31(3):344–50. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osteen KG, Hill GA, Hargrove JT, Gorstein F. Development of a method to isolate and culture highly purified populations of stromal and epithelial cells from human endometrial biopsy specimens. Fertil Steril. 1989;52(6):965–72. doi: 10.1016/s0015-0282(16)53160-4. [DOI] [PubMed] [Google Scholar]
- 58.Stern-Straeter J, Bonaterra GA, Hormann K, Kinscherf R, Goessler UR. Identification of valid reference genes during the differentiation of human myoblasts. BMC Mol Biol. 2009;10:66. doi: 10.1186/1471-2199-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, et al. Transforming growth factor beta mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci U S A. 1995;92(16):7362–6. doi: 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hampton AL, Nie G, Salamonsen LA. Progesterone analogues similarly modulate endometrial matrix metalloproteinase-1 and matrix metalloproteinase-3 and their inhibitor in a model for long-term contraceptive effects. Mol Hum Reprod. 1999;5(4):365–71. doi: 10.1093/molehr/5.4.365. [DOI] [PubMed] [Google Scholar]
- 62.Keller NR, Sierra-Rivera E, Eisenberg E, Osteen KG. Progesterone exposure prevents matrix metalloproteinase-3 (MMP-3) stimulation by interleukin-1alpha in human endometrial stromal cells. J Clin Endocrinol Metab. 2000;85(4):1611–9. doi: 10.1210/jcem.85.4.6502. [DOI] [PubMed] [Google Scholar]
- 63.Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Reprod Update. 2011;17(3):347–61. doi: 10.1093/humupd/dmq058. [DOI] [PubMed] [Google Scholar]
- 64.Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108(1–2):169–90. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 65.Karmaus PW, Chen W, Crawford RB, Harkema JR, Kaplan BL, Kaminski NE. Deletion of cannabinoid receptors 1 and 2 exacerbates APC function to increase inflammation and cellular immunity during influenza infection. J Leukoc Biol. 2011;90(5):983–95. doi: 10.1189/jlb.0511219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 67.Couture LA, Abbott BD, Birnbaum LS. A critical review of the developmental toxicity and teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: recent advances toward understanding the mechanism. Teratology. 1990;42(6):619–27. doi: 10.1002/tera.1420420606. [DOI] [PubMed] [Google Scholar]
- 68.Walczak JS, Cervero F. Local activation of cannabinoid CB(1) receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol Pain. 2011;7:31. doi: 10.1186/1744-8069-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osteen KG, Bruner-Tran KL, Ong D, Eisenberg E. Paracrine mediators of endometrial matrix metalloproteinase expression: potential targets for progestin-based treatment of endometriosis. Ann N Y Acad Sci. 2002;955:139–46. doi: 10.1111/j.1749-6632.2002.tb02774.x. discussion 157–8, 396–406. [DOI] [PubMed] [Google Scholar]
- 70.Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26(8):2157–64. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukhopadhyay B, Liu J, Osei-Hyiaman D, Godlewski G, Mukhopadhyay P, Wang L, et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J Biol Chem. 2010;285(25):19002–11. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor AH, Abbas MS, Habiba MA, Konje JC. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem Cell Biol. 2010;133(5):557–65. doi: 10.1007/s00418-010-0695-9. [DOI] [PubMed] [Google Scholar]
- 73.Leconte M, Nicco C, Ngo C, Arkwright S, Chereau C, Guibourdenche J, et al. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am J Pathol. 2010;177(6):2963–70. doi: 10.2353/ajpath.2010.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.