Abstract
INTRODUCTION
Adoptive immunotherapy for patients with metastatic melanoma has yielded encouraging results. However, methods to expand melanoma-specific T-cells from Stage III are limited. The objective of this study is to determine whether melanoma-specific T-cells could be generated from the melanoma-draining lymph nodes (MDLN) of Stage III patients.
METHODS
Stage III patients undergoing completion lymphadenectomy were enrolled onto an IRB-approved protocol. MDLN cells were tested for ability to undergo cryopreservation, expand ex vivo in IL-2 or IL-2 and IL-7 and mediate melanoma-specific antitumor responses in vitro.
RESULTS
Cryopreservation produced no significant differences from fresh cultures in terms of cell growth and cellular phenotype. IL-2 and IL-2/IL-7 cultures resulted in similar growth rates, and functional studies revealed the presence of T cells which secreted interferon gamma in response to melanoma antigen peptides. Both IL-2 and IL-2/IL-7 cultured MDLN cells mediated significant apoptosis of human melanoma cell lines as compared to breast and brain tumor lines in vitro. Overall there did not seem to be a benefit of adding IL-7. Both CD4+ and CD8+ T-cells appear to mediate tumor cell apoptosis.
CONCLUSION
This study demonstrates that melanoma antigen-specific T-cells can be generated from regional melanoma-draining lymph nodes and expanded ex vivo from patients with Stage III disease.
Introduction
The incidence of melanoma has been steadily increasing for both men and women for over a decade. It is currently the 5th leading cause of cancer in men and the 7th in women1. Currently, the five year survival for stage III and stage IV patients is ~60% and ~15%, respectively2. Interestingly, the death rate from patients older than 65 years is increasing, which suggests the need to investigate treatments with lower toxicity profiles3. Immunotherapy using high dose intravenous interleukin-2 (HD IL-2) has demonstrated modest response rates (~16%) in patients with metastatic disease, but many who undergo a complete response will have durable responses beyond 10 years4. HD IL-2 in combination with infusion of tumor-infiltrating lymphocytes (TILs) has increased the objective response rate to as high as 72% and durable complete response in up to 16% of patients with metastatic melanoma5. These studies demonstrate proof-of-concept that immunotherapy can be efficacious in select patients. However, there are significant limitations related to IL-2 toxicity and challenges surrounding isolation and expansion of TILs in vitro that have limited the translation of this approach outside of a relatively few investigational sites. In addition, since many patients with Stage III melanoma do not have a significant volume of tumor, TIL therapy is not feasible in this patient population.
It has been established in mice models that lymph nodes draining a progressively growing tumor contain antigen-specific pre-effector T cells6, 7. After a short expansion and activation in vitro using IL-2, these tumor-draining lymph nodes (TDLN) mediate specific eradication of established tumors in murine models8. Of interest is that the therapeutic effect of the adoptively transferred TDLN cells does not require the administration of systemic IL-2, presumably because of the short exposure of the TDLN cells to low concentrations of IL-2 during the culture process and the significant proportion of CD4+ T-helper cells within the final cell product9, 10. Despite the very robust antitumor efficacy of TDLN in murine models, human clinical trials of activated lymph nodes cells draining either autologous irradiated melanoma cell vaccines or gene-modified melanoma cell vaccines have demonstrated only modest clinical responses11, 12. One reason for this divergence might be that in the murine model, lymph nodes are draining progressively growing tumors over a prolonged period of time (months or years), whereas in the human clinical trials the lymph nodes were derived from sites draining artificially constructed melanoma vaccines over a 10–14 day period.
Based upon these observations both in animal and human models, we sought to determine if tumor-specific T-cells could be cultured and expanded from melanoma-draining regional lymph nodes in Stage III patients undergoing completion lymph node dissection after a positive sentinel node biopsy.
Methods
Melanoma-draining lymph node (MDLN) samples
All patients had informed consent on a protocol that was approved by the Case Western Reserve University IRB (CASE 3610). Adult patients underwent completion lymphadenectomy as part of their standard care for stage III melanoma determined by a positive sentinel lymph node biopsy. A tangential portion of three lymph nodes were removed from the lymphadenectomy specimen and mechanically separated creating a single cell suspension (SSC). Cell counts and viability were measured using Improved Neubauer Hemocytometer and trypan blue staining.
MDLN cells which were scheduled to be cryopreserved were first cultured overnight at a concentration of 1×106 cells/ml in complete media (CM, AIM-V media (Gibco, Grand Island, NY) with 5% pooled human AB serum (Innovative Research, Novi, MI)) and 30 U/ml interleukin-2 (IL-2, Invitrogen) at 37°C with 5% CO2. The following day cells were centrifuged and resuspended in AIM-V media with 25% human pooled AB serum and 10% DMSO at a concentration of 10–20×106 cells/ml. Cells were then frozen using a controlled-rate freezer and stored in liquid nitrogen until further analysis.
MDLN cell culture activation in vitro
Freshly harvested or thawed-cryopreserved MDLN cells were activated with anti-CD3/anti-CD28 beads (Dynal AS, Oslo, Norway) in the presence of 100U/ml IL-2 or IL-2 with 10ng/ml interleukin-7 (IL-7, Invitrogen) at a cell density of with 2×106 cells/well in a 24-well plate. Cells were split to a concentration of 0.25×106 cells/ml by adding fresh CM containing cytokines on days 4, 7, 11, and 14.
Tumor and antigen-presenting cell lines
Human melanoma cell lines A375, Sk-mel-28 (Sk-mel), breast cancer cell line MDA-231, and glioma cell line U87 were donated by Dr. Bingcheng Wang, Case Western Reserve University, Cleveland, OH. Cells were cultured in DMEM with 5% FBS (Gibco). T2 antigen-presenting cell line was donated by Dr. Pierre Triozzi, Cleveland Clinic Foundation, Cleveland, OH. Cells were cultured in RPMI with 10% FBS (Gibco).
Flow cytometry analyses
MDLN samples were stained with CD3-PE, CD4-FITC, CD8-FITC, CD56 and CD19-FITC (Invitrogen) and fixed with 1% paraformaldehyde prior to being analyzed on LSR II flow cytometer (BD Biosciences, San Jose, Ca) and data was processed using WinList (Verity Software House, Topsham, ME)
Interferon-γ ELISA
Day 14 MDLN cultures were plated in a 96-well plate with A375 cells or melanoma peptide-labeled T2 cells at an effector:stimulator ratio of 2:1 and no IL-2. Cells were co-cultured for 72 hours prior to assay of supernatant for Interferon-γ (IFN-γ) levels using ELISA (Thermo Scientific, Rockford, IL).
Apoptosis assays
Day 14 MDLN cultures were incubated with human tumor cells lines in 24-well plate with 2 ml of CM per well and no IL-2. MDLN/tumor cell mixture was stained with CD3-PE in 100μl staining buffer (BD Pharmingen, San Diego, CA) and then Annexin V-FITC Apoptosis Stain Kit and 7-AAD (BD Pharmingen) according to the manufacturer’s instructions. Cells were analyzed using Winlist software and gating on the CD3- population to determine early and late apoptosis of the tumor cells13.
MDLN cell fractionation
Day 14 MDLN cultures were labeled with anti-CD8 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and then separated using LD Magnetic Separation Column (Miltenyi Biotec) into CD8 enriched and CD8 depleted cell populations. The isolation procedure resulted over 90% purity in each cell population. Confocal fluorescent microscopy
Day 14 MDLN cells and A375 cells were seeded on a cover slide in a 6-well culture plate at a T cell:tumor cell ratio of 4:1 for 24 hours. The cells were then labeled with anti-CD4-pacific blue, anti-CD8-pacific blue, Annexin-FITC, anti-CD3-PE, anti-TCR-FITC, and/or anti-CD43-PE. The fluorescence images were acquired on a Zeiss LSM 510 and processed by Zeiss LSM Image software (Zeiss, Dublin, Germany).
Results
Cryopreservation of MDLN does not significantly alter phenotype and function
The ability to cryopreserve patient samples effectively can alleviate issues related to timing of cell infusion and allow for multiple cell infusions. Therefore, we tested the impact of cryopreservation on MDLN cell survival, phenotype, on growth potential. Cell counts throughout the cryopreservation process (after creation of a fresh single-cell suspension (SCS), after overnight culture prior to cryopreservation, and after cells were cryopreserved and then thawed at a later date) show that on average 65% (95% CI 45.1–84.9%) of cells are recovered (Figure 1A). Cell loss occurred almost exclusively during the thawing of the cells and not during overnight culture. In order to determine whether specific cell subsets were lost during the cryopreservation process, FACS analysis was performed. A small but significant decrease in B-cells from 20.5% to 16.7% (p=0.03) was observed after thawing cryopreserved cells, but there was no significant change in the percentage of CD3, CD4, or CD8 T-cells (Figure 1B). Finally, to determine if the growth potential of MDLN cells was affected by cryopreservation, cell samples were cultured with IL-2 either fresh or after cryopreservation and no obvious difference in growth rates were observed (Figure 1C).
Figure 1.
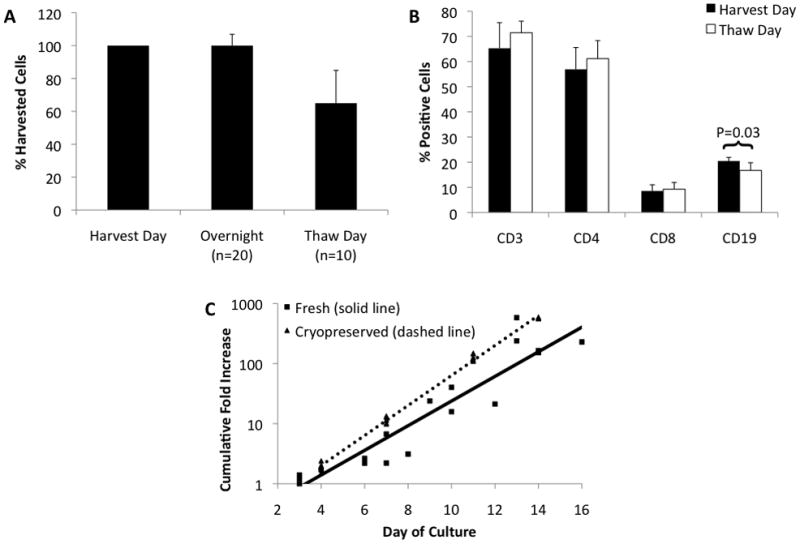
A. Percentage of cells retained through cryopreservation process. Single cell suspensions were counted on the day of harvesting, after overnight culture, and after cryopreservation. Overnight culture demonstrated no significant loss of cells from harvest, 100% recovery (95% CI 93.1–106.9). Approximately 65% (95% CI 45.1–84.9) of cells were recovered after cryopreservation. N=20 for overnight, N=10 for after cryopreservation. B. Phenotype of cells before and after cryopreservation. Single cells suspensions were evaluated for B-cell marker (CD19) and T-cell marker (CD3, CD4 and CD8) expression by flow cytometry. CD3+, CD4+, CD8+ cells were 63.5, 56.9, 8.6, respectively, prior to cryopreservation and 71.5, 61.2, 9.3, respectively, after cryopreservation (p=0.18, 0.32, 0.43, respectively). CD19+ cells decrease from 20.5 to 16.7 after cryopreservation (p=0.03) Error bars indicate 95% CI. N=4. C. Cumulative fold increase of T-cells grown fresh from harvest or cultured after cryopreservation. Cryopreserved MDLN grown with IL-2 expanded at least as well as fresh MDLN grown under the same culture conditions. MDLN cultures achieved over 100-fold growth after 14 days in culture. R2 ≥ 0.90 for all trendlines. N=5.
IL-2 and IL-2 with IL-7 produce similar MDLN cultures
Although most regimens of T cell expansion in vitro involve the use of IL-2, we also were interested in investigating the potential benefit of adding IL-7. IL-7 has been shown to support the growth of tumor-specific cytotoxic T-cell during long term culture14. IL-2 alone and IL-2/IL-7 resulted in nearly identical growth rates of MDLN cultures (Figure 2A), with most cultures achieving greater than 100-fold increase in 14 days. Both IL-2 and IL-2/IL-7 cultures started from the same MDLN and had 60% (95% CI 48.2–71.8%) CD3+ T cells. At the end of the 14-day culture period, the percent of CD3+ cells in IL-2 cultures increased to 96% while for IL-2/IL-7 the increase in CD3+ cells was to 93% (p=0.03, Figure 2B). Day 0 MDLN samples had a CD4/CD8 ratio of 6 (95% CI 4–8), which decreased to 3 (95% CI 1.9–4.1) for IL-2 and 2 (95% CI 1.3–2.7) for IL-2/IL7 by the end of the culture period (p=0.43 for IL-2 versus IL-2/IL-7) (Figure 2C). Staining was also performed for CD56 on day 14 on a few patients and was consistently below 5% (data not shown).
Figure 2.
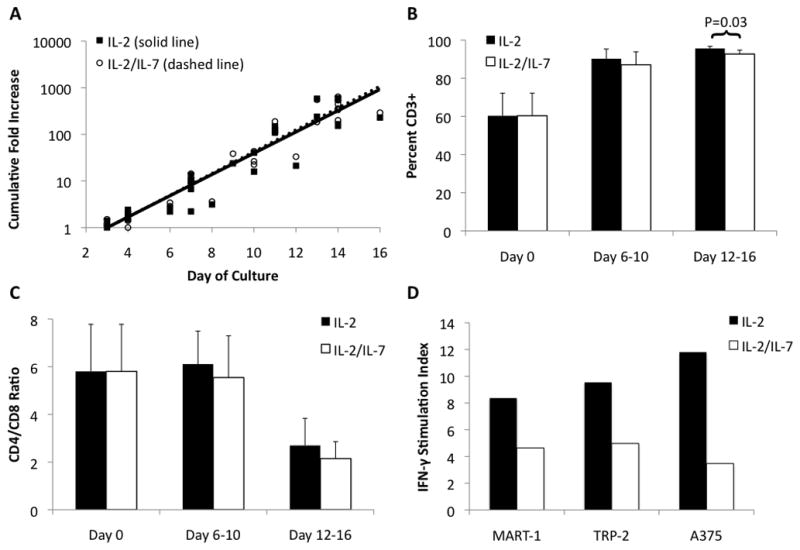
A. Growth curves for MDLN grown under two culture conditions. MDLN were incubated with anti-CD3/anti-CD28 beads with either IL-2 or IL-2 + IL-7. MDLN growth with either IL-2 or IL-2/IL-7 showed no difference in expansion, achieving over 100-fold expansion by 14 days. R2 ≥ 0.90 for both trendlines. N=10. B. Percent of MDLN cells expressing CD3. MDLN cultures started with 60% CD3+ cells, which increased to 96% CD3+ for IL-2 culture and 93% for IL-2/IL-7 culture (p=0.03 for IL-2 vs. IL-2/IL-7). Error bars indicate 95% CI. N=10. C. Ratio of CD4+ T-cells to CD8+ T-cells. MDLN cultures started with CD4+/CD8+ ratio of 6, which decreased to 3 for IL-2 and 2 for IL-2/IL7 (p=0.43 for IL-2 vs. IL-2/IL-7). Error bars indicate 95% CI. N=10. D. Antigen-specific IFN-γ production. MDLN cells from single patient were incubated alone, with melanoma peptides (MART-1 or TRP-2), or with melanoma cell line (A375) for 72 hours. The stimulation index (SI) for MART-1, TRP-2 and A375 for MDLN cultured with IL-2 was 8.3, 9.5, 11.8, respectively. This was greater than the SI for IL-2/IL-7 (4.6, 5.0, 3.5, respectively). Stimulation index (SI = stimulated sample/MDLN alone sample).
The functional capacity of the Day 14 MDLN cell cultures was evaluated first by cytokine production and then by in vitro assays of tumor cell apoptosis. Co-culture of Day 14 MDLN cells with T2 antigen presenting cells pulsed with human melanoma peptides MART-1 and TRP-2 as well as whole live human melanoma A375 cells resulted in an IFN-γ stimulation index of 8.3, 9.5, and 11.8, respectively for IL-2 cultures (Figure 2D). For MDLN cells grown in IL-2/IL-7 the stimulation index was 4.6, 5.0 and 3.5, respectively. These results suggest that both culture types contain peptide-reactive T cells with the potential for significant “helper” function.
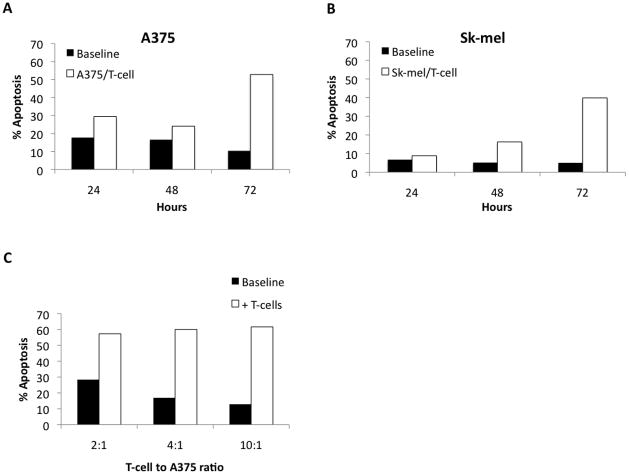
In order to determine whether culture-activated T cells had the ability to mediate tumor cell apoptosis in vitro, we performed kinetics and dose response experiments analyzing Day 14 MDLN cells co-cultured with A375 and Sk-mel melanoma cell lines (Figure 3A and B). For A375, MDLN cells increased apoptosis of tumor cells over baseline (tumor cells alone) by 11.7%, 7.5%, and 42.4% for 24, 48, and 72 hours, respectively. For Sk-mel, the increase over baseline was 2.1%, 11% and 34.9%, respectively. We also observed a dose-response of increase in tumor cell apoptosis with increasing MDLN cell to tumor cell ratio (Figure 3C). Using MDLN cells with the A375 cell line at ratios of 2:1, 4:1, and 10:1 the increase of apoptosis over baseline was 28.9%, 43.1%, and 48.7%. These results were used to perform all subsequent apoptosis experiments with a T cell:tumor cell ratio of 2:1 assayed at 72 hours.
Figure 3.
A and B. Determining the optimal time frame for apoptosis assays with MLDN cultures. Day 14 MDLN cultures were incubated with two human melanoma tumor lines (A375 and Sk-mel, respectively) at a MDLN cell to tumor cell ratio of 2:1 for 24, 48 and 72 hours. Tumor cells without MDLN cells were used for baseline apoptosis. Baseline apoptosis was 17.8%, 16.6%, and 10.4% for A375 and 6.8%, 5.2%, and 5.0% for Sk-mel at 24, 48, and 72 hours, respectively. MDLN cells increased apoptosis 11.7%, 7.5%, and 42.4% over baseline for A375 and 2.1%, 11% and 34.9% for Sk-mel at 24, 48 and 72, hours, respectively. C. Apoptosis increases as MDLN cell to tumor cell ratio increases. Day 14 MDLN cultures were incubated with human melanoma cell line (A375) for 72 hours and increasing MDLN cell to tumor cell ratios (2:1, 4:1, 10:1). Apoptosis increased 28.9%, 43.1%, and 48.7% over baseline with increasing MDLN cell ratio.
Day 14 MDLN cells from four different patients cultured in IL-2 and IL-2/IL-7 resulted in similar levels of tumor cell apoptosis for human melanoma lines A375 and Sk-mel (Figure 4). Day 14 MDLN cells cultured in IL-2 alone increase apoptosis over baseline by 42.3% and 50.6%, respectively (p<0.001 for both) whereas those cultured in IL-2/IL-7 increased apoptosis by 43.2% and 46.6%, respectively (p<0.03 for both). When incubated with human brain tumor line U87, IL-2 cultures increased apoptosis by 17.5% (p=0.71) and IL-2/IL-7 increased apoptosis by 33.1% (p=0.06). These results suggest that both culture types result in T cells that mediate melanoma-specific apoptosis in vitro. Based upon these data, we saw no benefit from adding IL-7.
Figure 4. Apoptosis from MDLN generated by two culture conditions.
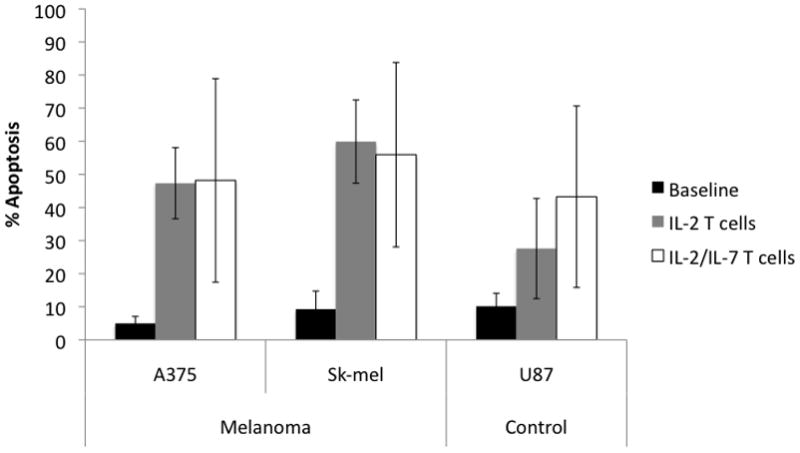
Day 14 MDLN cells from IL-2 or IL-2/IL-7 cultures were incubated with two human melanoma lines (A375, Sk-mel) and one human brain cancer line (U87) at a ratio of 2:1 for 72 hours. IL-2 cultures increase apoptosis 42.3%, 50.6%, and 17.5% over baseline for A375, Sk-mel, and U87, respectively (p= <0.001, <0.001 and 0.71). IL-2/IL-7 cultures increase apoptosis 43.2%, 46.6%, and 33.1% over baseline for the 3 tumors lines respectively (p=0.03, 0.02, 0.06). There was no significant difference between IL-2 and IL-2/IL-7 cultures. Error bars indicated 95% confidence interval. N=4.
In order to determine if Day 14 MDLN cells from a large number of patients demonstrated melanoma-specific antitumor activity in vitro, apoptosis assays were performed with two human melanoma cell lines (A375 and Sk-mel), one human breast cancer cell line (MDA-231) and one human brain cancer cell line (U87) (Figure 5). Day 14 MDLN cells mediated significant increase in apoptosis over baseline on average of 27.1% and 29.1% for A375 and Sk-mel, respectively (p<0.001). By contrast, MDLN cells resulted in non-significant increases over baseline of 14.6% and 14.8% for MDA-231 (p=0.25), and U87 (p=0.18), respectively, confirming that the cultures contain melanoma-specific T cells.
Figure 5. MDLN cultured in IL-2 results in melanoma specific apoptosis.

MDLN cells were incubated with two human melanoma cell lines (A375 and SK-Mel-28), one human breast cancer line (MDA-231) and one human brain cancer line (U-87). Baseline apoptosis for A375, Sk-mel-28, MDA-231, and U-87 were 8.0%, 9.0%, 7.7%, 7.3%, respectively. Addition of MDLN cells increased apoptosis over baseline by 27.1%, 29.1%, 14.6%, 14.8%, respectively. Increase in apoptosis for A375 and Sk-mel-28 were statistically significant (p<0.001) while increases in MDA-231 and U-87 were not (p=0.25 and p=0.18, respectively). Error bars indicate 95% CI. N=9.
In order to determine if MDLN cells physically interacted with tumor cells we employed confocal fluorescent microscopy. Figure 6A shows Annexin V positive apoptotic A375 tumor cells interacting with T-cells labeled with anti-CD3. To further characterize this interaction, MDLN cultures were labeled with either anti-CD4 or anti-CD8 antibodies, T-cell receptor (TCR) antibodies and CD43 antibodies (Figures 6B and C). These images show CD4+ or CD8+ T-cells interacting with unlabeled A375 tumor cells. At the center of the interaction is the TCR, which has been concentrated at the point of interaction, indicating that this is an immunologic synapse. Also, noted is that CD43 (a ligand for E-selectin that can also down regulate cell interactions15) is excluded from the T cell-tumor cell interaction.
Figure 6.

A. Confocal fluorescent microscopy showing apoptosis of A375 by CD3+ lymphocytes. MDLN cells were incubated with melanoma cell line (A375) for 24 hours at a ratio of 4:1. Cells were stained with anti-annexin (green) and anti-CD3 (red). CD3+ T-cells are closely associated with A375 tumor cells that are undergoing apoptosis (white arrow). 40x magnification. B and C. Formation of immunological synapse between T-cells and melanoma tumor line. MDLN cells were stained with pacific blue-conjugated anti-CD4 or pacific blue-conjugated anti-CD8 antibodies to identify T-cells. T-cell Receptor (TCR) and CD43 were stained with FITC-conjugated and PE-conjugated antibodies, respectively. Interactions of CD4+ or CD8+ T-cells (blue) with A375 melanoma tumor lines are associated with enrichment of TCR (green) and exclusion of CD43 (pink). 63x magnification.
As both CD4+ and CD8+ T-cells can create an immunologic synapse with A375, the contribution to tumor cell apoptosis from each T-cell population was evaluated. CD8+ T-cells were depleted from the standard MDLN culture on day 14 and the tumor cell apoptosis assays were performed (Figure 7). Unfractionated MDLN cells were capable of increasing A375, Sk-mel and U87 apoptosis by 44.1%, 56.9% and 23% over baseline, respectively (p=0.012, 0.003, 0.017). The CD8+ fraction of MDLN was able to increase apoptosis of the tumor lines by 18.3%, 31.8%, and 20.5%, respectively (p=0.018, 0.06, 0.015). Interestingly, the CD8 depleted fraction consisting primarily of CD4+ cells was able to increase apoptosis of tumor lines by 23%, 39.1% and 15.9%, respectively (p=0.001, 0.021, 0.006).
Figure 7. Depletion of CD8 cells from MDLN.
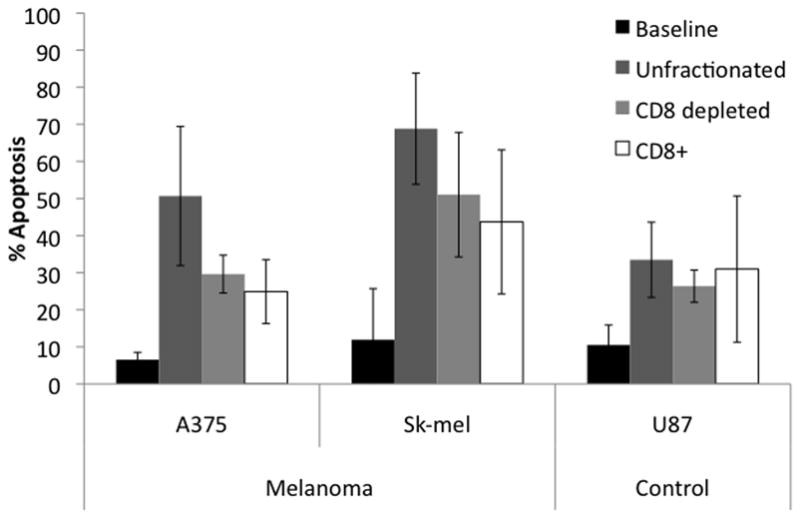
Day 14 MDLN cultures were labeled with anti-CD8 magnetic beads and depleted via magnetic column. Flow-through cells were collected (CD8 depleted sample). The anti-CD8 cells were then washed from the column and collected (CD8+ sample). Apoptosis assays were performed as described above using A375, Sk-mel and U87 tumor cell lines. The ratio of CD8 depleted cells and CD8+ cells to tumor reflected the proportion of these respective samples in the unfractionated sample. Unfractionated MDLN cells increase apoptosis 44.1%, 56.9%, and 23% over baseline, for each tumor line respectively. CD8 depleted MDLN cells increased apoptosis 23%, 39.1%, and 15.9% over baseline, while CD8+ MDLN cells increased apoptosis 18.3%, 31.8%, and 20.5% over baseline, for each tumor line respectively. All increases over baseline were significant (p<0.02) except CD8+ with Sk-mel and U87 (p= 0.06 and 0.15, respectively). Error bars indicated 95% CI. N=4.
MDLN cultures do not result in cell exhaustion phenotype
It was been reported that T cells exhibiting surface co-expression of PD-1 and Tim-3 may represent an “exhausted” phenotype with decreased ability to proliferate and perform effector function in response to antigen16. Figure 8 shows that on Day 0 of the culture there is minimal exhausted T-cells, 0.2% for both CD4+ and CD8+ subsets. Over 14 days in culture the percentage for CD8+ and CD4+ exhausted T-cells increases to 1.2% (p=ns) and 2.6% (p=0.003) respectively. However, these levels are well below the clinically significant levels seen in tumor bearing mice which can approach 50% of T-cells16.
Figure 8. PD-1+/TIM-3+ lymphocytes at the beginning and end of culture.
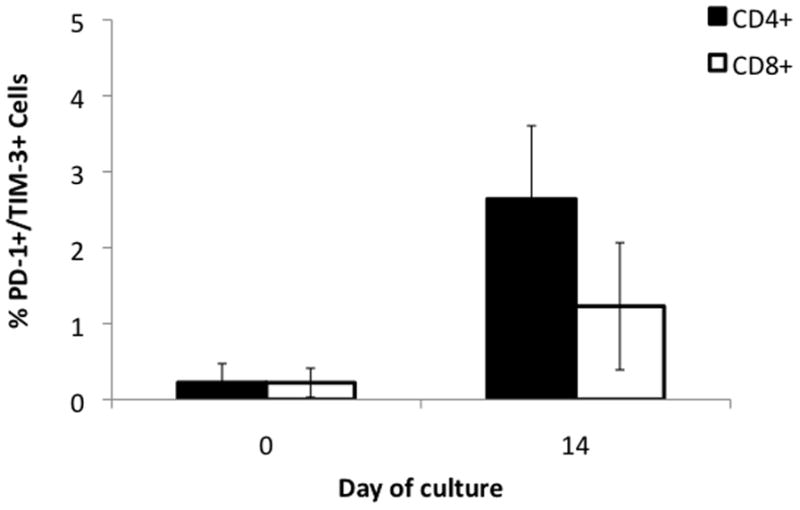
Cells were stained with antibodies to PD-1 and Tim-3, markers of T-cell exhaustion. At the start of MDLN culture both CD4+ and CD8+ had exhausted phenotype for <1% of cells. At the end of culture exhausted phenotype increased to 2.6 (p=0.003) for CD4+ and 1.2 (p=0.061) for CD8+. Error bars indicate 95% CI. N=4.
Discussion
In this study we describe a method of reproducibly generating melanoma-specific T cells from regional lymph nodes. To our knowledge this is the first publication utilizing tumor-draining lymph node cells primed in vivo by a progressively growing primary cutaneous melanoma in stage III patients. This MDLN culture method is unique in that it does not rely on ex vivo priming with antigen or in vivo vaccination. T-cells from MDLN are naturally primed against defined as well as undefined tumor antigens, which in theory should lead to a broad repertoire of antigen-specific responses.
Cryopreservation is another important aspect of this process. Several studies have shown that cryopreservation does not result in major changes to T-cells from sentinel lymph node and leukapheresis samples17, 18. Those results were borne out in our study. There was no major change to T-cell subsets and cryopreserved cells retained their growth potential. The ability to cryopreserve these cells allows for flexibility in when cultures can be started and makes multiple infusions over a prolonged period of time feasible, which may enhance the duration of the anti-tumor effect.
These results demonstrated that both CD4+ and CD8+ T cells are involved in targeting tumor cells. Both cell types are capable of forming an immunologic synapse with tumor cells and mediating apoptosis. While these results are from in vitro experiments, there is evidence of the effectiveness of both CD4+ and CD8+ T cell subsets from in vivo mouse models19, 20 as well as human case studies21. Using a combination of CD4+ as well as CD8+ T cells in adoptive immunotherapy applications may have several advantages. First, the direct cytotoxic effects of CD8+ T-cells are well known, however, CD4+ may open another non-redundant pathway for tumor rejection22. Second, CD4+ T cells may produce the cytokines that support CD8+ T cells without the need for exogenous cytokines23. With the elimination of exogenous HD IL-2, adoptive immunotherapy has been performed safely in the outpatient setting24.
This study does have limitations. It relies on in vitro testing as opposed to in vivo models. However, given this limitation we obtained MDLN samples from a diverse patient population with heterogeneous disease and obtained surprisingly consistent trends in antitumoral response. In this study we describe a relatively simple method of MDLN culturing for stage III melanoma patients. This method uses low doses of IL-2, short culture duration, and no gene modification. This protocol has the potential to be widely adopted if proven effective in human clinical trials of patients who develop metastatic disease following completion lymph node dissection or in the adjuvant therapy of Stage III melanoma patients.
Acknowledgments
Funded in part by National Institutes of Health (K23 CA109115-01A5 to J.A.K.) and the University Hospitals Seidman Cancer Center Immunogene Therapy Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF. Update on the melanoma staging system: the importance of sentinel node staging and primary tumor mitotic rate. J Surg Oncol. 2011 Sep;104(4):379–385. doi: 10.1002/jso.21876. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011 Nov;65(5 Suppl 1):S17–25. e11–13. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000 Feb;6 (Suppl 1):S11–14. [PubMed] [Google Scholar]
- 5.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008 Nov 10;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shu S, Sussman JJ, Chang AE. In vivo antitumor efficacy of tumor-draining lymph node cells activated with nonspecific T-cell reagents. J Immunother Emphasis Tumor Immunol. 1993 Nov;14(4):279–285. doi: 10.1097/00002371-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura T, Krinock RA, Chang AE, Shu S. Cross-reactivity of anti-CD3/IL-2 activated effector cells derived from lymph nodes draining heterologous clones of a murine tumor. Cancer Res. 1993 Sep 15;53(18):4315–4321. [PubMed] [Google Scholar]
- 8.Yoshizawa H, Chang AE, Shu S. Specific adoptive immunotherapy mediated by tumor-draining lymph node cells sequentially activated with anti-CD3 and IL-2. J Immunol. 1991 Jul 15;147(2):729–737. [PubMed] [Google Scholar]
- 9.Li Q, Furman SA, Bradford CR, Chang AE. Expanded tumor-reactive CD4+ T-cell responses to human cancers induced by secondary anti-CD3/anti-CD28 activation. Clin Cancer Res. 1999 Feb;5(2):461–469. [PubMed] [Google Scholar]
- 10.Li Q, Yu B, Grover AC, Zeng X, Chang AE. Therapeutic effects of tumor reactive CD4+ cells generated from tumor-primed lymph nodes using anti-CD3/anti-CD28 monoclonal antibodies. J Immunother. 2002 Jul-Aug;25(4):304–313. doi: 10.1097/00002371-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chang AE, Aruga A, Cameron MJ, et al. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J Clin Oncol. 1997 Feb;15(2):796–807. doi: 10.1200/JCO.1997.15.2.796. [DOI] [PubMed] [Google Scholar]
- 12.Meijer SL, Dols A, Urba WJ, et al. Adoptive cellular therapy with tumor vaccine draining lymph node lymphocytes after vaccination with HLA-B7/beta2-microglobulin gene-modified autologous tumor cells. J Immunother. 2002 Jul-Aug;25(4):359–372. doi: 10.1097/00002371-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995 Jul 17;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 14.Lynch DH, Miller RE. Interleukin 7 promotes long-term in vitro growth of antitumor cytotoxic T lymphocytes with immunotherapeutic efficacy in vivo. J Exp Med. 1994 Jan 1;179(1):31–42. doi: 10.1084/jem.179.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostberg JR, Barth RK, Frelinger JG. The Roman god Janus: a paradigm for the function of CD43. Immunol Today. 1998 Dec;19(12):546–550. doi: 10.1016/s0167-5699(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 16.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010 Sep 27;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Pul KM, Vuylsteke RJ, Bril H, Stockmann HB, de Gruijl TD. Feasibility of flowcytometric quantitation of immune effector cell subsets in the sentinel lymph node of the breast after cryopreservation. J Immunol Methods. 2011 Oct 26; doi: 10.1016/j.jim.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Tramsen L, Koehl U, Tonn T, et al. Clinical-scale generation of human anti-Aspergillus T cells for adoptive immunotherapy. Bone Marrow Transplant. 2009 Jan;43(1):13–19. doi: 10.1038/bmt.2008.271. [DOI] [PubMed] [Google Scholar]
- 19.Wang LX, Huang WX, Graor H, et al. Adoptive immunotherapy of cancer with polyclonal, 108-fold hyperexpanded, CD4+ and CD8+ T cells. J Transl Med. 2004 Nov 26;2(1):41. doi: 10.1186/1479-5876-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagamu H, Shu S. Purification of L-selectin(low) cells promotes the generation of highly potent CD4 antitumor effector T lymphocytes. J Immunol. 1998 Apr 1;160(7):3444–3452. [PubMed] [Google Scholar]
- 21.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008 Jun 19;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen PA, Peng L, Plautz GE, Kim JA, Weng DE, Shu S. CD4+ T cells in adoptive immunotherapy and the indirect mechanism of tumor rejection. Crit Rev Immunol. 2000;20(1):17–56. [PubMed] [Google Scholar]
- 23.Aruga A, Aruga E, Cameron MJ, Chang AE. Different cytokine profiles released by CD4+ and CD8+ tumor-draining lymph node cells involved in mediating tumor regression. J Leukoc Biol. 1997 Apr;61(4):507–516. doi: 10.1002/jlb.61.4.507. [DOI] [PubMed] [Google Scholar]
- 24.To WC, Wood BG, Krauss JC, et al. Systemic adoptive T-cell immunotherapy in recurrent and metastatic carcinoma of the head and neck: a phase 1 study. Arch Otolaryngol Head Neck Surg. 2000 Oct;126(10):1225–1231. doi: 10.1001/archotol.126.10.1225. [DOI] [PubMed] [Google Scholar]