Abstract
This task-switching ERP study of 16 young participants investigated whether increased RT slowing on stay trials and faster RTs on switch trials for frequent than infrequent switching are explained by an activation or preparation account. The activation account proposes that task sets are maintained at a higher baseline activation level for frequent switching, necessitating increased task-set updating, as reflected by a larger and/or longer lasting early parietal positivity. The preparation account assumes advance (pre-cue) switch preparation (i.e., task-set reconfiguration), preceding stay and switch trials for frequent switching, as reflected by pre-cue and post-cue late parietal positivities. By and large, the data support the activation account. However, we also found increased, pre-cue task-set updating on frequent stay trials and pre-cue, task-set reconfiguration prior to predictable, frequent switches. These results lead us to propose an extended activation account to explain the effects of switch probability on the executive processes underlying task-switching behavior.
Keywords: Task switching, switch probability, switch predictability, task-set updating, task-set reconfiguration, cue-locked ERP, response-locked ERP
Introduction
The ability to rapidly and effectively adjust one’s behavior in response to changes in the environment requires executive control processes. The executive processes involved in switching between tasks have been examined with the task-switching paradigm (Allport et al., 1994; Meiran, 1996; Rogers and Monsell, 1995). This paradigm typically requires participants to perform one of two different task sets (e.g., parity, odd/even, or magnitude, more/less than 5) in pure and mixed blocks. While pure blocks require the execution of a single task set, mixed blocks require switching between two (or more) different task sets. There is evidence that people use environmental cues, such as switch probability, to select strategies that will allow them to switch more effectively to the alternative task (Pashler, 1999; Shiffrin, 1998). Hence, the present study is focused on examining how executive processes respond to changes in switch probability.
The presence of executive processes in task-switching paradigms has been inferred from two different reaction-time (RT) costs, “general” and “specific.” General switch costs are evident when participants respond in accord with the same task set as on the previous trial (i.e., on “stay” trials in mixed blocks). This cost is believed to occur because updating processes for the current task set are necessary, which also includes inhibition of the alternative task set (Mayr, 2001; Rubin and Meiran, 2005). Hence, these general switch costs, which are expressed in longer RTs, reflect the task-set updating processes for stay trials in mixed blocks compared to trials in pure blocks. Costs can also be observed when a different task than on the previous trial must be performed (i.e., “switch” trials). Making the transition from performing one task to performing another task, which has been subsumed under the term “task-set reconfiguration” (e.g., Monsell, 2003), requires a series of steps, each with their own executive processes. These steps include preparing for the upcoming task by shifting attention toward the new task set (top-down control), overcoming the inhibition of the now relevant task set (Mayr and Keele, 2000), inhibiting elements of the prior task set, and retrieval of the new task set’s rules (e.g., Arrington et al., 2007; Meiran, 1996; Monsell, 2003; Rogers and Monsell, 1995). Task-set reconfiguration processes have been inferred from longer RTs on switch than on stay trials in mixed blocks, which have been labeled “specific” switch costs.
Although detailed experimental examinations are missing, research has indicated that task-set updating and reconfiguration processes are affected by switch probability. In one of the few studies of this type, Monsell and Mizon (2006) manipulated switch probability (25%, 50% or 75%; Experiment 4) and showed that specific switch costs decreased as switches became more frequent. Moreover, increased specific switch costs for infrequent switches appeared to be due to both a RT increase for switch trials and a RT decrease for stay trials (Monsell and Mizon, 2006, see Fig.7 page 502). That is, the RT increase for stay trials in the frequent switch condition suggests that general switch costs decrease directly with switch probability (see also Monsell et al., 2003; Dreisbach and Haider, 2006). Hence, increased switch probability appears to be associated with increased general switch costs, suggesting increased recruitment of task-set updating processes, but decreased specific switch costs, suggesting decreased engagement of task-set reconfiguration processes.
The fact that increased switch probability is associated with increases in general switch costs but decreased specific RT switch costs might be explained by two different hypotheses, which could be added to existing task switching models (e.g., Monsell, 2003) that do not currently account for the effects of switch probability. One idea posits that frequent switching benefits performance on switch trials because both task sets remain highly activated (i.e., higher baseline activation). This hypothesis, which we therefore label the “activation account,” predicts increased demand for task-set updating on stay trials when switches are frequent because more resources must be allocated to inhibit the alternate task set. In addition, maintaining both task sets at a higher baseline activation level for higher switch probabilities would reduce the demand for task-set reconfiguration processes on switch trials. Hence, this account predicts that frequent switching might encourage increased baseline activation of both task sets and explain the increased general and decreased specific RT switch costs.
An alternative hypothesis proposes that advanced preparation occurs when switches occur frequently (Monsell and Mizon, 2006). The preparation could take the form of dismissing the previous task set once a response has been made (i.e., going to a neutral state; see Brass and von Cramon, 2004), which predicts no differences between RTs on stay and switch trials. However, because specific RT switch costs are, in fact, also present during frequent switching (Monsell and Mizon, 2006), in this account preparation is thought of as advance reconfiguration on those trials that participants predict will be switch trials. When such predictions turn out to be wrong (participants encounter another stay trial), general switch costs would increase because a second task-set reconfiguration would be necessitated after the task information has been provided (task-set reconfiguration on stay trials). Hence, frequent switches might encourage advanced task-set reconfiguration on both stay and switch trials and explain the increased general and decreased specific RT switch costs, an idea that we label the ‘preparation account.’
The present study was undertaken to clarify whether the activation or preparation account provides the best explanation for the effects of switch probability on RT switch costs. To examine this question, event-related potentials (ERPs) had to be measured, as justified in the next paragraph. Moreover, because the effects of switch probability on the behavioral and ERP data are influenced by the factors of Cue Informativeness and Predictability, these two factors were added to the design as outlined below.
Behavioral investigations cannot disambiguate between the activation and preparation accounts because both predict similar behavioral effects on RT, albeit for different reasons (see above). However, the two accounts vary in their predictions about the influence of switch probability manipulations on task-set updating and reconfiguration processes. Consequently, the present study measured event-related potential (ERP) components that can provide precise temporal information of the engagement of task-set updating and reconfiguration processes. Prior research has been especially successful in identifying temporally different ERPs reflecting task-set updating and reconfiguration during the interval between an informative cue and the target (cue-target interval or CTI). Hence, an informative CTI was used in the present study. The requirement to update the task set has been associated with the presence of a relatively early parietal positivity, which is present at varying durations between 200 and 600 ms post-cue onset (e.g., Eppinger et al., 2007; Jamadar et al., 2010b; Kray et al., 2005; West, 2004). The early parietal positivity is usually elicited by both stay and switch trials after informative cues compared to pure trials (see also Karayanidis et al., 2010; Karayanidis et al., 2011). Task-set reconfiguration processes, such as retrieval of the new task set, have been associated with a later parietal positivity for switch compared to stay trials that has been found at varying durations between 400 and 1000 ms post cue (Eppinger et al., 2007; Jamadar et al., 2010a; Jamadar et al., 2010b; Jost et al., 2008; Karayanidis et al., 2003; Kieffaber and Hetrick, 2005; Nicholson et al., 2005; Rushworth et al., 2002; Travers and West, 2008).
Differentiating the activation and preparation accounts is possible by comparing the differential effects of switch probability on the amplitude and the duration of cue-locked early (task-set updating) and late (task-set reconfiguration) parietal positivities. That is, if greater switch probability increases task-set updating processes on stay trials, as predicted by the activation account, a larger and/or longer lasting early parietal positivity would be expected for stay compared to pure trials. In addition, if both task sets are more highly activated for frequent switches, this should lead to a reduction in task-set reconfiguration processes and thus a smaller and/or shorter lasting switch-minus-stay late parietal positivity. The preparation account also predicts a smaller and/or shorter switch-minus-stay late parietal positivity for frequent switches, because pre-cue reconfiguration (i.e., advance preparation before task information is provided) should decrease the demand for post-cue reconfiguration. However, only the preparation account predicts a cue-locked late parietal positivity on stay relative to pure trials when a switch is predicted incorrectly, indicating task-set reconfiguration processes on stay trials. Finally, if participants engage in pre-cue, task-set reconfiguration when switches are frequent (preparation account), then the presence of a late switch-minus-stay parietal response-locked positivity analog to the cue-locked late parietal positivity would be expected in the interval between the response on the previous trial and the cue on the current trial (response-cue interval, RCI) (see Table 1 for a summary of the predictions).
Table 1.
Summary of Predictions from the Activation and Preparation Accounts.
| Activation account | Preparation account | |||
|---|---|---|---|---|
| Both task sets have higher baseline activation when switches occur frequently. | Advanced preparation for switch trial when switches occur frequently. | |||
| Predictions for… | Stay-minus-Pure trials | Switch-minus-Stay trials | Stay-minus-Pure trials | Switch-minus-Stay trials |
| … in frequent compared to infrequent switch condition | ||||
| Involved processes | Increased demand for task set updating | Decreased demand for task-set reconfiguration | Task-set reconfiguration occurs when a switch is incorrectly prepared | Decreased demand for task-set reconfiguration |
| Behavioral effects (RT)* | Increased general switch costs | Decreased specific switch costs | Increased general switch costs | Decreased specific switch costs |
| CTI ERP effects** | Larger and/or longer lasting early parietal positivity | Smaller and/or shorter lasting late parietal positivity | Presence of late parietal positivity | Smaller and/or shorter lasting late parietal positivity |
| RCI ERP effects*** | no prediction | no prediction | Presence of late parietal positivity | Presence of late parietal positivity |
Notes: CTI = Cue-Target Interval; RCI = Response-Target Interval;
Behavioral effects are expected to be strongest for uninformative, unpredictable trials, as reflected by an interaction of Switch Probability, Cue Informativeness and Predictability;
ERP effects in the CTI are only expected for informative-cue trials;
ERP effects in the RCI are expected to be largest for predictable trials in the frequent switch condition.
Executive processes have usually been associated with activations of the frontal cortex (Sakai, 2008). Although ERPs lack neuroanatomical precision, a cue-locked frontal ERP positivity between 350–500 ms for switch compared to stay trials (Barcelo et al., 2002; Rushworth et al., 2002; see also West et al., 2011) might indicate frontal cortex activation. As part of task-set reconfiguration, the frontal switch-stay positivity might reflect top-down control of the task switch in the form of shifting attention to the new task set (Rushworth et al., 2002). By contrast, very few ERP studies have investigated whether stay relative to pure trials show frontal effects (for an example see Manzi et al., 2011). Hence, as a further aid in differentiating between the activation and preparation accounts, the current study also examined the presence of frontal stay-pure and switch-stay ERP effects.
Previous research has shown that the opportunity provided by an informative cue to prepare for the upcoming task modulates switch-probability RT effects. The results of Monsell and Mizon (2006) indicated, for instance, that the difference in specific switch costs between the frequent and infrequent switches was larger for a short CTI (140 ms) compared to a long CTI (790 ms). This was attributed to the fact that infrequent switches benefit more from the advance (endogenous) preparation provided by the long CTI (for evidence on endogenous reconfiguration see Meiran, 1996; Cepeda et al., 2001; Meiran et al., 2001). Hence, in the long CTI, the proposed increased recruitment of task-set reconfiguration processes for infrequent compared to frequent switches occurred before the target presentation, decreasing probability-related RT-switch-cost differences. Hence, to provide a pure assessment of the behavioral effect of switch probability, we created a Cue Informativeness factor by adding an “uninformative” condition in which the cue provided no information about the upcoming task.
Finally, if an informative cue is not presented, participants can also use the information inherent in predictable task sequences (e.g., AABBAABB or AAAABBBB) to prepare for the next trial (Monsell et al., 2003; Rogers and Monsell, 1995). However, it is conceivable that a predictable task sequence in frequent switch blocks (e.g., AABB) might be easier to remember and, thereby, be a greater aid to task performance than a predictable task sequence in infrequent switch blocks (e.g., AAAABBBB). Hence, to further enhance our ability to parse the behavioral effect of switch probability, we created an “unpredictable” condition with a random task sequence and compared it with a “predictable” condition (Predictability factor).
To summarize, the present study was designed to determine whether the recruitment of task-set updating, reflected by the amplitude and duration of the early parietal positivity, and task-set reconfiguration, reflected by the amplitude and duration of the late-parietal positivity, would differentially support the activation or preparation accounts under conditions of frequent switching. Frequent compared to infrequent switching was expected to be accompanied by larger general RT switch costs and decreased specific RT switch costs, especially in the uninformative-cue, unpredictable condition, as reflected by an interaction of Switch Probability, Cue Informativeness and Predictability (see Table 1). An association between larger general RT switch costs and a larger and/or longer lasting stay-pure early parietal positivity in the informative CTI of the frequent switch condition would indicate increased task-set updating and, thereby support the activation account. An association between larger general RT switch costs and the presence of a late-parietal, stay-pure positivity in the informative CTI of the frequent switching condition, indicating the presence of task-set reconfiguration on stay trials, would support the preparation account. The preparation account would also be supported if signs of pre-cue task-set reconfiguration were found in the RCI for frequent but not infrequent switches (see Table 1).
Methods
Participants
Twenty-four young adults participated after signing informed consent, and received payment for volunteering. Seven participants became fatigued and either chose not to continue or responded in an excessively long or inaccurate manner. Technical difficulties prevented one participant’s data from being used. The 16 remaining participants (18–30 years; M = 24.5), 12 women and four men, were right-handed, native-English speakers and had normal or corrected-to-normal vision. All reported themselves to be in good physical and mental health and free from medications known to affect the central nervous system. The study was approved by the New York State Psychiatric Institute’s Institutional Review Board.
Procedure
Participants were seated comfortably in a sound-damped and electrically-shielded room. They sat about 100 cm from a 17" computer monitor and held a small response box on their laps. A grey rectangular box (6.7° vertical × 7.4° horizontal) was shown centrally surrounded by a blank screen. On each trial, a single digit (72pt Arial, 1° × 1°) between 1 and 9 (except 5) was presented in the center of the grey box. Using their left and right index fingers, participants had to make speeded and accurate, choice button-press responses according to the instructions appropriate to one of two tasks. In the more/less task, participants decided whether the digit was more or less than 5. In the odd/even task they decided whether the digit was odd or even. Digits were presented with the constraint that the maximum number of consecutive trials that required responses on the same side was four. Throughout the experiment, response side indicators for both tasks (e.g., ‘more’ and ‘odd’ on the left and ‘less’ and ‘even’ on the right, 28pt Arial font) were displayed below the digit in the lowest quadrant of the grey box. Stimulus-response assignments for both tasks were counterbalanced across participants.
The experiment included pure and mixed blocks. During pure blocks, participants only performed one task. During mixed blocks, participants switched between performing two tasks. Eight pure and 16 mixed blocks were divided into a morning and afternoon session. Each session began with two pure blocks (one of each task) followed by eight mixed blocks, two each of four types (see below), and concluded with another two pure blocks (one of each task). The mixed blocks in one session required frequent switching while those in the other session required infrequent switching (factor of Switch Probability), as detailed below. Within each session, the mixed blocks differed with respect to the presence of informative or uninformative cues (factor of Cue Informativeness) and with respect to whether stay and switch trials were predictable or unpredictable (random) (factor of Predictability).
Pure blocks were composed of 30 trials with three additional fillers at the start and end of each block. The task order of each pair of pure blocks (i.e., first more/less or odd/even) was fixed within but counterbalanced across participants. The initial task in mixed blocks corresponded to the task that was performed in the first pure block.
The frequent-switching session contained mixed blocks in which the switch-to-stay trial ratio was 1:1 (equiprobable). Using task-run lengths of one, two, or three trials meant that a total of 12 trials was necessary to perform both tasks for all three run lengths (i.e., a full task-run sequence). That is, with one task represented by “A” and the other by “B”, one complete task-run sequence is ABBAAA BAABBB. Mixed blocks with frequent switches were comprised of eight full task-run sequences (96 trials). The infrequent-switching session contained mixed blocks in which the switch-to-stay trial ratio was 1:3 (i.e., task runs were three, four, or five trials long). Hence, a full task-run sequence was AAABBBBAAAAA BBBAAAABBBBB (24 trials). Mixed blocks with infrequent switches contained five full task-run sequences (120 trials). Both types of mixed blocks contained seven filler trials at the start and five at the end. The order of the frequent- and infrequent-switching sessions was counterbalanced across participants.
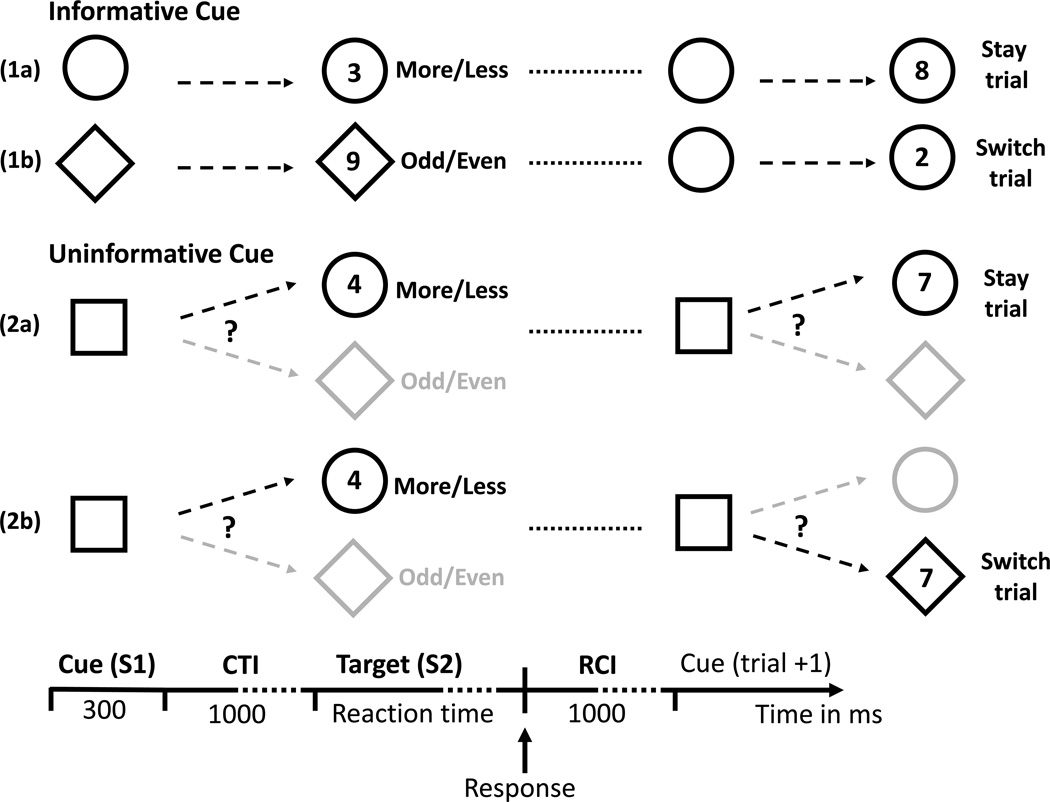
The trial structure (Figure 1) defined the Cue Informativeness variable. Specifically, a mixed-block trial began with a 300 ms visual cue (circle, triangle, or square; maximum vertical and horizontal visual angles, respectively, of 3.2° × 3.2°). One shape was assigned to indicate the more/less task and a second to indicate the odd/even task, representing informative cues. The third shape was uninformative because it did not indicate the task to be performed (see Figure 1). The task-shape assignments were counterbalanced across participants. To maintain the same trial structure in both pure and mixed blocks, the appropriate informative cue was presented for each pure-block trial. Half the mixed blocks in each session contained trials with informative cues and half contained trials with uninformative cues. After a 1300 ms cue-target interval (CTI), the target digit appeared within the shape assigned to the task for that trial. That is, at the time of target presentation an informative shape (cue) was provided for both the informative and uninformative cue conditions. The target remained on the screen until a response was made. A 1000 ms response-cue interval (RCI) then ensued (blank box).
Figure 1.
Experimental design: Schematic of the specific trial structure that defined the factor of Cue Informativeness. In the example given, the circle signals the more/less task (1a), the triangle signals the odd/even task (1b), and the square is uninformative (2a,b). Examples for stay and switch trials in mixed blocks are provided.
The Predictability variable was defined by the type of presentation of the full task-run sequences within mixed blocks. Half the mixed blocks had task runs that occurred in a fixed (i.e., predictable) order of ascending length (task runs of one, two and then three trials in frequent switch blocks, e.g., ABBAAA BAABBB etc., and task runs of three, four and then five trials in infrequent switch blocks, e.g., AAABBBBAAAAA BBBAAAABBBBB etc.). Task runs in the other half of mixed blocks were presented in random (i.e., unpredictable) order (e.g., ABAAA BBAABB etc.), with the constraint of a maximum of three consecutive identical task runs. Participants were informed at the start of each block whether task runs were predictable or unpredictable. Note, that even unpredictable blocks contained elements of predictability because participants were aware that task runs were between one and three trials in the frequent and between three and five trials in the infrequent session. As detailed below, these “predictable” trials in unpredictable blocks were removed from the analysis examining Predictability effects.
To summarize, combining the variables of Switch Probability, Cue Informativeness, and Predictability yielded eight types of mixed blocks. The four types resulting from varying Cue Informativeness and Predictability occurred randomly in both frequent and infrequent sessions. Two instances of each block type were presented, always consecutively, resulting in a total of eight mixed blocks in each session.
Participants performed practice versions of each pure (10 trials) and one mixed block (108 trials for frequent or 132 trials for infrequent blocks). The practice mixed block used the rules of the first mixed block to be encountered. Including electrode application and removal, the experiment lasted 4 to 5 hours. Small breaks were given if needed, with one longer break between the morning and afternoon sessions.
ERP Recordings
EEG activity was recorded with 62 sintered Ag/AgCl electrodes mounted in an elastic cap (Neuromedical Supplies) over scalp sites in accord with the extended ten-twenty system (ground: right forehead; electrode impedance < 5 kΩ; recording reference: nosetip; re-referenced offline: averaged mastoids). Vertical electrooculogram (EOG) was recorded from electrodes placed above and below the left eye and horizontal EOG from electrodes at the outer canthus of each eye. EEG and EOG (DC; 100 Hz high-frequency cutoff; 500 Hz digitization rate) were recorded continuously with Synamp amplifiers.
Data Processing and Analyses
Behavioral Data
Although no response deadline was imposed, only correct trials with RTs between 200 and 4000 ms were analyzed (cf., Karayanidis et al., 2003), which resulted in excluding a maximum of 0.3 % of trials across participants. The individual data were further trimmed to reject additional outliers because previous literature indicates that RTs for pure, stay and switch trials can vary considerably (e.g., Jost et al., 2008; Friedman et al., 2008; see also Monsell, 2003), and because the opportunity to prepare for the next trial, as after informative cues and long CTIs, shortens RTs markedly (Meiran, 1996; Jost et al., 2008). Hence, trials were rejected if they were more than 3 standard deviations (SDs) from the relevant individual mean RT (cf., Karayanidis et al., 2009) of one of the following five conditions: pure-block trials, switch (and stay) trials after informative cues, switch (and stay) trials after uninformative cues. Using this criterion, between 1.3 % and 2.5 % of trials were discarded across participants. The remaining data were used to create separate mean RTs and accuracy rates for pure blocks and stay and switch trials after informative and uninformative cues during frequent and infrequent sessions.
Because unpredictable blocks included trials that were predictable based on the knowledge of task-run length (see above), a second, restricted data set was created to examine the influence of Predictability on switch probability effects. In the restricted data set, these predictable trials were removed from unpredictable blocks. In addition, to ensure comparability between predictable and unpredictable blocks, trials not included for unpredictable blocks were also rejected from the data in predictable blocks. Using these criteria, behavioral data were averaged for predictable and unpredictable stay and switch trials for the different levels of Switch Probability and Cue Informativeness.
General switch costs for each participant were computed by subtracting mean RTs for pure from mean RTs for stay trials. Specific switch costs were computed by subtracting mean RTs for stay from mean RTs for switch trials. A first ANOVA examined the influence of the repeated-measures factors of Cue Informativeness and Switch Probability on general and specific RT switch costs. A second ANOVA on the restricted data set analyzed the effects of the repeated-measures factor of Predictability.
ERP Data
Only trials accepted for the behavioral analyses were included in the ERP averages. Eye-movement artifacts were corrected off-line (Semlitsch et al., 1986) and EEG epochs with artifacts were rejected. If single channels (up to four channels per trial) showed artifacts (e.g., spikes, drifts), the data from these electrodes were replaced by interpolation on a trial-by-trial basis using a spherical spline algorithm (Perrin et al., 1989). If more than four electrodes showed artifacts, then the given trial was rejected. The cue-locked ERP epochs included a 200 ms pre-cue baseline and the 1300 ms CTI. The response-locked epochs started 200 ms before the response and lasted until the end of the 1000 ms RCI. To provide a neutral baseline, the response-locked epoch for each trial was baseline corrected by using the 100 ms of activity preceding the target that elicited the response (e.g., Fiehler et al., 2005; Friedman et al., 2009; Johnson et al., 2003; Johnson et al., 2004). To determine if participants prepared for upcoming switch trials in the RCI, response-locked epochs were categorized according to the type of trial they preceded.
To examine the influence of Switch Probability on the timing, magnitude and duration of task-set updating and task-set reconfiguration processes, cue- and response-locked ERPs were computed for pure, stay and switch trials for each of the four conditions resulting from the 2 levels of Cue Informativeness and the 2 levels of Switch Probability. In addition, using the same restrictions as described for the behavioral data, cue- and response-locked ERPs were also computed for predictable and unpredictable stay and switch trials following (cue-locked) or preceding (response-locked) informative and uninformative cues (for trial numbers see Table 2). A 15-Hz low-pass filter was applied to all averages before measurement of the waveforms was undertaken and is reflected in the figures.
Table 2.
Ranges of trial numbers entering the cue-locked and response-locked ERP averages, with maximum possible trials in parentheses.
| ERP averages | Cue-locked | Response-locked | ||
|---|---|---|---|---|
| pure | 162–233 (240) | 177–233 (240) | ||
| Stay | Switch | Stay | Switch | |
| Informative cues | ||||
| frequent | 97–181 (192) | 68–177 (192) | 123–182 (192) | 119–183 (192) |
| infrequent | 222–348 (360) | 73–116 (120) | 257–347 (360) | 78–118 (120) |
| frequent, predictable | 56–91 (96) | 19–60 (64) | 60–94 (96) | 44–62 (64) |
| frequent, unpredictable | 41–91 (96) | 23–61 (64) | 48–92 (96) | 32–60 (64) |
| infrequent, predictable | 32–59 (60) | 18–38 (40) | 42–58 (60) | 21–39 (40) |
| infrequent, unpredictable | 35–59 (60) | 20–39 (40) | 47–59 (60) | 27–40 (40) |
| Uninformative cues | ||||
| frequent | 79–186 (192) | 86–189 (192) | 77–186 (192) | 100–190 (192) |
| infrequent | 195–342 (360) | 68–114 (120) | 242–345 (360) | 74–113 (120) |
| frequent, predictable | 47–95 (96) | 32–63 (64) | 57–95 (96) | 42–64 (64) |
| frequent, unpredictable | 27–92 (96) | 22–64 (64) | 27–92 (96) | 22–63 (64) |
| infrequent, predictable | 35–58 (60) | 18–40 (40) | 39–60 (60) | 23–39 (40) |
| infrequent, unpredictable | 32–59 (60) | 23–38 (40) | 43–59 (60) | 26–39 (40) |
For the analyses of task-set updating and reconfiguration processes associated with stay (stay vs. pure) and switch (switch vs. stay) trials, repeated-measures Trial Type by Electrode ANOVAs were performed on the mean-amplitude measures separately for each of the four (Cue Informativeness by Switch Probability) or eight conditions (Cue Informativeness by Switch Probability by Predictability). Following the procedures used in previous studies, six midline electrodes (Afz, Fz, Fcz, Cz, Cpz, Pz) were selected for the analyses of the effects (e.g., Manzi et al., 2011; Nicholson et al., 2005). To examine the amplitudes and durations of the early and late parietal effects presumably reflecting the amount of task-set updating and reconfiguration processes, repeated-measures ANOVAs were performed on mean-amplitude measures for 100-ms time intervals covering the entire CTI between 200 and 1300 ms and the entire RCI between 200 and 1000 ms (for a similar procedure see Manzi et al., 2011). Significant Trial Type by Electrode interactions were followed by testing the significance of stay-pure and switch-stay differences at each electrode using the modified Bonferroni procedure (Jaccard and Wan, 1996). If none of the differences at single electrodes using the modified Bonferroni procedure was reliable, then the Trial Type by Electrode interaction was considered unreliable and is not reported. The magnitude of Trial-Type effects for those time intervals and electrodes that revealed significant effects for both frequent and infrequent switching were compared with repeated-measures Switch Probability by Electrode ANOVAs on mean-amplitude differences (stay minus pure; switch minus stay). In accord with results from earlier studies cited above, Trial-Type differences between 200–600 ms after the cue or response will be labeled “early effects.” Trial-Type differences starting at or after 400 ms and lasting longer than 600 ms will be labeled “late effects.”
Data analyses
For all analyses, SPSS Version 18 for Windows (repeated-measures ANOVA) was used. Degrees of freedom and p values are reported using the Greenhouse-Geisser correction for violations of sphericity (when appropriate). The significance level was set at p ≤ .05. Partial eta squared (ηp2) is presented as an estimate of main and interaction effect sizes. Because of the large amount of data examined, F-, p-, and ηp2-values are only provided for reliable effects.
Results
Effect of Probability on task switch performance
Accuracy
Overall, performance was highly accurate (pure trials error rate = 2.1%; stay trials 3.1%; switch trials 4.1%), which left too few errors to assess meaningfully the effects of Cue Informativeness, Switch Probability or Predictability.
RT switch costs
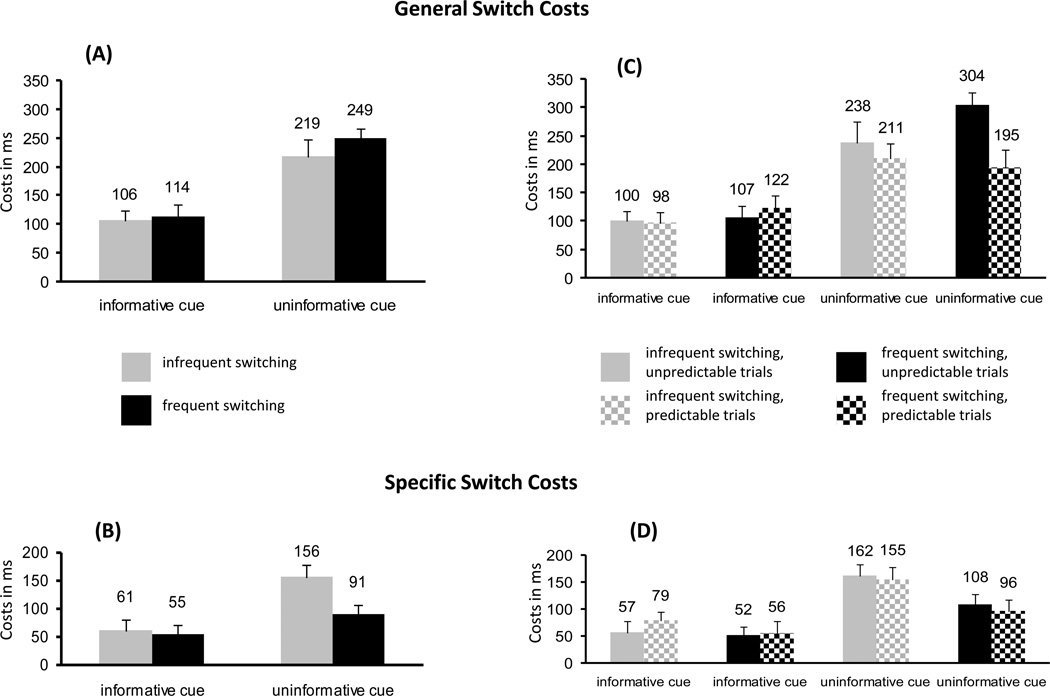
Table 3 displays mean RTs for all trial types and Figure 2 depicts costs in all conditions (all different from 0, ts > 2.5; ps < .05) (restricted data set including the factor of Predictability, Table 3B; Figures 2C, D). To examine patterns in these costs, ANOVAs with the repeated-measures factors of Cue Informativeness and Switch Probability (and Predictability, see below) were performed separately for general and specific switch costs. Informative cues decreased general switch costs compared to uninformative cues (110 ms vs. 234 ms; F(1, 15) = 66.0, p < .001, ηp2 = .82; Figure 2A). Contrary to prediction, Switch Probability did not affect general switch costs (frequent switching = 182 ms; infrequent switching = 163 ms; main effect of Switch Probability and interaction with Cue Informativeness Fs < 2.3, ps > .1). Specific switch costs were smaller following informative (58 ms) than uninformative (124 ms) cues (F(1,15) = 29.9, p < .001, ηp2 = .67; Figure 2 B). The main effect of Switch Probability [F(1,15) = 9.8, p < .01, ηp2 = .40] was modulated by the Cue Informativeness by Switch Probability interaction [F(1,15) = 5.3, p < .05, ηp2 = .26]. As predicted, this interaction reflected smaller specific switch costs after uninformative cues for frequent than infrequent blocks (F(1,15) = 10.8, p < .01, ηp2 = .42). Switch Probability had no effect on specific costs after informative cues (F < 1; Figure 2B).
Table 3.
Mean RTs (in ms, ±SE) on pure, stay and switch trials as a function of (A) Cue Informativeness and Switch Probability and (B) Cue Informativeness, Switch Probability and Predictability.
| (A) Cue Informativeness by Switch Probability | ||
|---|---|---|
| Reaction Times | ||
| pure task | 496 (17) | |
| Stay | Switch | |
| informative cue | ||
| infrequent switching | 602 (32) | 663 (45) |
| frequent switching | 611 (32) | 665 (34) |
| uninformative cue | ||
| infrequent switching | 715 (39) | 872 (41) |
| frequent switching | 745 (30) | 836 (35) |
| (B) Cue Informativeness by Switch Probability by Predictability | ||
|---|---|---|
| Reaction Times | ||
| pure task | 490 (18) | |
| Stay | Switch | |
| informative cue | ||
| infrequent, unpredictable | 596 (30) | 653 (43) |
| infrequent, predictable | 594 (32) | 673 (49) |
| frequent, unpredictable | 603 (30) | 656 (40) |
| frequent, predictable | 618 (34) | 673 (49) |
| uninformative cue | ||
| infrequent, unpredictable | 734 (47) | 896 (54) |
| infrequent, predictable | 707 (35) | 861 (33) |
| frequent, unpredictable | 800 (29) | 908 (41) |
| frequent, predictable | 691 (41) | 787 (43) |
Figure 2.
General and specific switch costs (in ms, ±SE) as a function of Cue Informativeness and Switch Probability (A, B) and Cue Informativeness, Switch Probability and Predictability (C, D).
The analyses performed on the restricted data set including the factor of Predictability revealed the same results with respect to Cue Informativeness and Switch Probability (not reported). In addition, for general switch costs, the ANOVA indicated significant Cue Informativeness by Predictability [F(1, 15) = 5.7, p < .05, ηp2 = .27] and, as predicted, Cue Informativeness by Switch Probability by Predictability interactions (F(1, 15) = 9.3, p < .01, ηp2 = .38; all other effects of Predictability Fs < 3.8, ps > .05). To explore the meaning of these interactions, separate ANOVAs were performed for informative and uninformative cues. As can be seen in Figure 2C, after informative cues general switch costs were not modulated by Predictability (M predictable = 110 ms; M unpredictable = 104 ms; Fs < 1.1, ps > .1). However, the ANOVA on general switch costs following uninformative cues revealed a main effect of Predictability [F(1,15) = 5.4, p < .05, ηp2 = .26] and a Switch Probability by Predictability interaction [F(1,15) = 6.3, p < .05, ηp2 = 30]. As indicated in Figure 2C for uninformative trials, while predictable task sequences decreased general switch costs in the frequent switch condition (t = 2.8, p < .05), no such effect was found in the infrequent switch condition (t = 1.0, p > .1). Importantly, additional paired t-tests on these data revealed that the predicted greater general switch costs in the frequent compared to the infrequent switch condition was confirmed for unpredictable trials after uninformative cues (t = 2.4, p < .05). However, this result did not obtain on predictable trials following uninformative cues (t < 1; cf. Figure 2C). By contrast, Predictability did not influence specific-switch costs (Fs < 1.5, ps > .1; see also Figure 2D)1. In sum, the behavioral data confirm the prediction that increasing switch probability increases general and decreases specific switch costs, but only following uninformative cues and only for unpredictable stay trials (general costs).
Effect of Switch Probability on ERP correlates of task-set updating and reconfiguration
Neural activity associated with general (stay vs. pure) and specific (switch vs. stay) switch costs was examined in the CTI and RCI. We first assessed the effect of Probability on the early and late ERP activity when the data were collapsed across Predictability and then, in a second step, queried the restricted data set (see methods) for the effects of Predictability. For uninformative cues, Trial type by Electrode ANOVAs only revealed ERP differences for predictable switch compared to stay trials preceding (RCI) uninformative cues in the frequent switch condition (see below). All other analyses did not show ERP differences and will not be reported. Hence, if not labeled as uninformative, all the results refer to informative trials.
Evidence for task-set updating and/or task-set reconfiguration processes after stay compared to pure trials (general switch costs) in the CTI (cue-locked data)
The activation accounts proposes that stay trials in the frequent switch condition require increased task-set updating (larger and/or longer lasting early parietal positivity), whereas the preparation account posits task-set reconfiguration on stay trials (presence of late parietal positivity). Therefore, mean-ERP amplitudes for stay and pure trials in the CTI were examined in Trial Type by Electrode ANOVAs, performed separately for frequent and infrequent switch conditions (collapsed across Predictability). Opposing the predications of the preparation account, these analyses revealed only early but no late frontal and parietal positivities (200–400 ms) for frequent switches, and an early parietal positivity (300–400 ms) for infrequent switches (Figure 3; Table 4A, bottom). A Switch Probability by Electrode (CPz, Pz) ANOVA performed on stay minus pure difference waveforms for frequent and infrequent switch conditions revealed no magnitude difference for the early parietal positivity between 300–400 ms, suggesting that the amount of task-set updating did not differ in this time interval.
Figure 3.
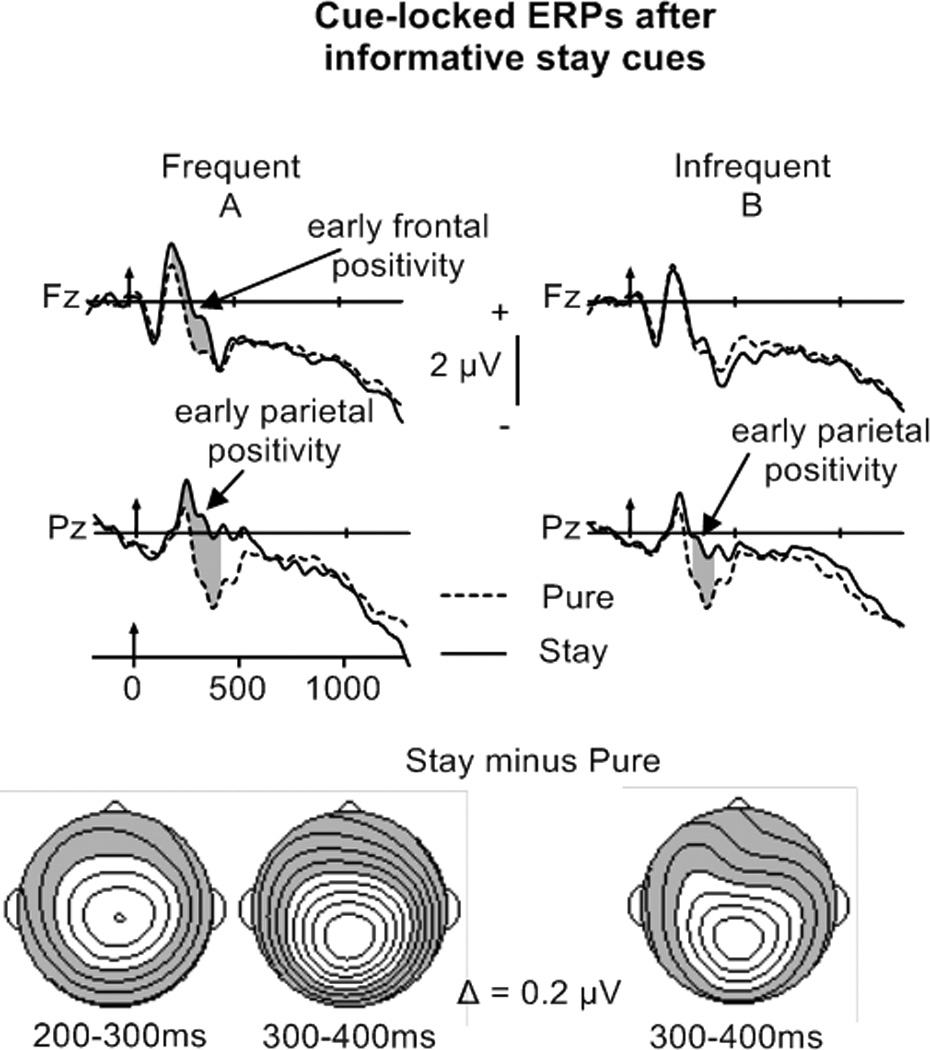
Top: Cue-locked grand–averaged ERP waveforms elicited by stay trials after informative cues (frequent switching in A, infrequent switching in B) at Fz and Pz. For comparison, the ERPs for pure trials are displayed for all conditions. For these and all other ERP waveforms, significant differences between conditions are shaded in grey.
Bottom: Potential maps illustrating the scalp topography of reliable cue-locked stay-pure differences. These and all other maps are 100° projections with the front of the head at the top. For these and all other maps, shaded areas = negativity; unshaded areas = positivity; isopotential lines are separated by 0.2µV.
Table 4.
Influence of Switch Probability on preparatory brain activity preceding (response-cue interval, RCI) and following (cue-target interval, CTI) informative cues. The RCI ANOVAs were performed on 100 ms time intervals between 200 and 1000 ms post response. The CTI ANOVAs were performed on 100 ms time intervals between 200 and 1300 ms post cue. Significant F-values are presented for Trial type by Electrode ANOVAs, separately performed for (A) stay vs. pure trials in RCI and CTI and (B) switch vs. stay trials in CTI (no significant differences between switch and stay trials were found in RCI). Effect sizes (partial eta squared, ηp2) are shown in parentheses. Non-significant effects have been omitted from the table.
| (A) Stay vs. pure trials | ||
|---|---|---|
| Effects in the preceding Response-Cue Interval (RCI) | ||
| Frequent | Infrequent | |
| 200–300ms | ||
| Trial type | F=16.4*** (.52) | 8.3* (.36) |
| 300–400ms | ||
| Trial type | 11.9** (.44) | 6.0* (.29) |
| 400–500ms | ||
| Trial type | 9.3** (.38) | |
| 500–600ms | ||
| Trial type | 6.1* (.29) | |
| Effects in the Cue-Target Interval (CTI) | ||
| Frequent | Infrequent | |
| 200–300ms | ||
| Trial type | 11.7** (.44) | |
| 300–400ms | ||
| Trial type | 20.7*** (.58) | |
| Trial type by Electrode | 9.8***(.40) | 26.3*** (.64) |
| follow-up | All electrodes significant, largest at CPz, Pz | Significant at CPz, Pz |
| (B) Switch vs. stay trials | ||
|---|---|---|
| Effects in the Cue-Target Interval (CTI) | ||
| Frequent | Infrequent | |
| 200–300ms | ||
| Trial type | F=13.1** (ηp2 =.47) | |
| 300–400ms | ||
| Trial type | 13.0** (.47) | |
| Trial type by Electrode | 4.8* (.24) | |
| follow-up | All electrodes significant, largest at Cpz, Pz | |
| 400–500ms | ||
| Trial type | 13.0** (.47) | 25.1*** (.63) |
| Trial type by Electrode | 4.6* (.23) | 7.0** (.32) |
| follow-up | All electrodes significant, largest at Cpz, Pz | All electrodes significant, largest at Cpz, Pz |
| 500–600ms | ||
| Trial type | 10.5** (.41) | 18.1*** (.55) |
| Trial type by Electrode | 12.6* (.46) | 10.8** (.42) |
| follow-up | Significant at Cz, CPz, Pz | Significant at Fz, Fcz, Cz, CPz, Pz |
| 600–700ms | ||
| Trial type | 10.5** (.41) | 11.0** (.42) |
| Trial type by Electrode | 18.0*** (.55) | 12.6*** (.46) |
| follow-up | Significant at Cz, CPz, Pz | Significant at Cz, CPz, Pz |
| 700–800ms | ||
| Trial type by Electrode | 14.8*** (.50) | 8.2** (.35) |
| follow-up | Significant at CPz, Pz | Significant at CPz, Pz |
Notes: Trial type degrees of Freedom (df) 1,15; Trial type by Electrode df 5,75;
= p ≤ .05;
= p ≤ .01;
= p ≤ .001.
The ANOVAs performed separately on predictable and unpredictable trials revealed similar durations of the early frontal and parietal stay-pure positivity (200–400 ms) in the frequent switch condition (Figure 4A, C; Table 5B). In the infrequent switch condition, the early parietal stay-pure positivity lasted longer for predictable trials (200–500 ms) than for unpredictable trials (300–400 ms)(Figure 4B, D; Table 5B). Probability by Electrode ANOVAs performed on stay minus pure difference waveforms failed to reveal differences between frequent and infrequent switch conditions in the magnitude of the early parietal effect between 200–400 ms for predictable trials (CPz, Pz) or between 300–400 ms for unpredictable trials (Cz, CPz, Pz).
Figure 4.
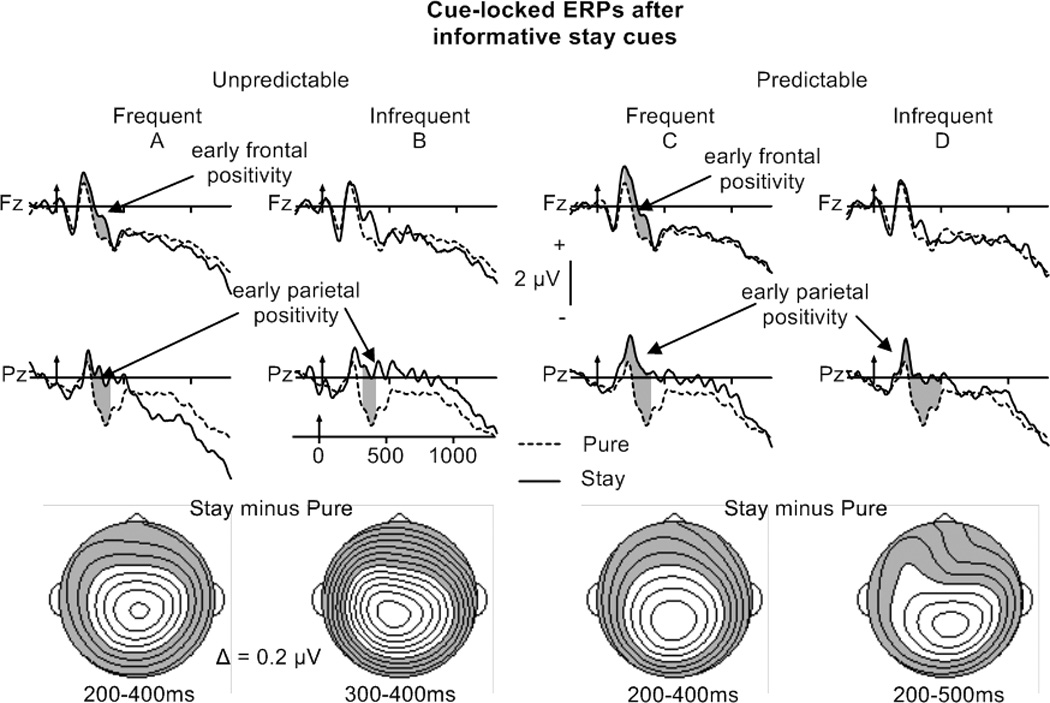
Cue-locked grand averaged ERPs after informative-cue, unpredictable (left) and predictable (right) stay trials (frequent switching in A and C, infrequent switching in B and D) at Fz and Pz. For comparison, the ERPs for pure trials are displayed for all conditions.
Bottom: Potential maps illustrating the scalp topography of reliable cue-locked stay-pure differences.
Table 5.
Influence of Predictability and Switch Probability on preparatory brain activity preceding (response-cue interval, RCI) and following (cue-target interval, CTI) informative cues. The RCI ANOVAs were performed on 100 ms time intervals between 200 and 1000 ms post response. The CTI ANOVAs were performed on 100 ms time intervals between 200 and 1300 ms post cue. Significant F-values are presented for Trial type by Electrode ANOVAs separately performed for (A) stay vs. pure trials in RCI (no significant effects were present in the infrequent switching condition) and (B) CTI and (C) switch vs. stay trials in RCI (only for predictable trials in the frequent switching condition) and (D) CTI. Effect sizes (partial eta squared, ηp2) are shown in parentheses. Non-significant effects have been omitted from the table.
| (A) Stay vs. pure trials in preceding Response-Cue Interval (RCI) | ||
|---|---|---|
| Frequent, Unpredictable |
Frequent, Predictable |
|
| 200–300ms | ||
| Trial type | F=16.8*** (.52) | 11.5** (.43) |
| Trial type by Electrode | 4.0*(.21) | |
| follow-up | Significant at Afz, Fz, Fcz, Cz, Cpz | |
| 300–400ms | ||
| Trial type | 12.6** (.46) | 8.1* (.35) |
| 400–500ms | ||
| Trial type | 8.9** (.37) | 6.5* (.30) |
| 500–600ms | ||
| Trial type | 6.8* (.31) | |
| (B) Stay vs. pure trials in Cue-Target Interval (CTI) | ||||
|---|---|---|---|---|
| Frequent, Unpredictable |
Frequent, Predictable |
Infrequent, Unpredictable |
Infrequent, Predictable |
|
| 200–300ms | ||||
| Trial type | F=4.8* (.24) | 12.0** (.44) | 4.7* (.24) | |
| Trial type by Electrode | - | 5.5* (.27) | ||
| follow-up | Significant at CPz, Pz | |||
| 300–400ms | ||||
| Trial type | 6.5* (.30) | 16.5*** (.52) | 6.5* (.30) | 7.8* (.34) |
| Trial type by Electrode | 8.4*** (.36) | 8.5** (.36) | 10.1*** (.40) | |
| follow-up | All electrodes significant, largest at Pz | Significant at Cz, CPz, Pz | Significant at CPz, Pz | |
| 400–500ms | ||||
| Trial type by Electrode | 5.7* (.28) | |||
| follow-up | Significant at Pz | |||
| (C) Switch vs. stay trials in preceding Response-Cue Interval (RCI) | |
|---|---|
| Frequent, Predictable |
|
| 300–400ms | |
| Trial type by Electrode | F=6.1* (.29) |
| follow-up | Significant at Pz |
| 400–500ms | |
| Trial type | |
| Trial type by Electrode | 4.2* (.22) |
| follow-up | Significant at Pz |
| 600–700ms | |
| Trial type | 5.1* (.25) |
| Trial type by Electrode | 4.3* (.22) |
| follow-up | Significant at Cz, Cpz, Pz |
| 700–800ms | |
| Trial type by Electrode | 6.0* (.29) |
| follow-up | Significant at Cz, Cpz, Pz |
| 800–900ms | |
| Trial type by Electrode | 7.1** (.32) |
| follow-up | Significant at Cz, Cpz, Pz |
| 900–1000ms | |
| Trial type | 4.9* (.25) |
| Trial type by Electrode | 8.3** (.36) |
| follow-up | Significant at Cz, Cpz, Pz |
| (D) Switch vs. stay trials in Cue-Target Interval (CTI) | ||||
|---|---|---|---|---|
| Frequent, Unpredictable |
Frequent, Predictable |
Infrequent, Unpredictable |
Infrequent, Predictable |
|
| 200–300ms | ||||
| Trial type | F=4.9* (.25) | |||
| 300–400ms | ||||
| Trial type | 4.5* (.23) | |||
| 400–500ms | ||||
| Trial type | 9.2** (.38) | 14.5** (.49) | 11.6** (.44) | |
| Trial type by Electrode | 4.3* (.22) | 5.6* (.27) | ||
| follow-up | Significant at Fz, Fcz, Cz, Cpz, Pz | Significant at Fcz, Cz, Cpz, Pz | ||
| 500–600ms | ||||
| Trial type | 13.3** (.47) | 14.4** (.49) | 11.2** (.43) | |
| Trial type by Electrode | 7.1** (.32) | 6.0* (.28) | 9.9*** (.40) | |
| follow-up | Significant at Fcz, Cz, Cpz, Pz | Significant at Fz, Fcz, Cz, Cpz, Pz | Significant at Fcz, Cz, Cpz, Pz | |
| 600–700ms | ||||
| Trial type | 8.4* (.36) | 8.0* (.35) | 7.5* (.33) | |
| Trial type by Electrode | 17.2*** (.53) | 7.6** (.34) | 16.2*** (.52) | |
| follow-up | Significant at Cz, Cpz, Pz | Significant at Cpz, Pz | Significant at Cz, Cpz, Pz | |
| 700–800ms | ||||
| Trial type | 4.9* (.25) | |||
| Trial type by Electrode | 18.9*** (.56) | 5.9* (.28) | 8.2** (.35) | |
| follow-up | Significant at Cpz, Pz | Significant at Cpz, Pz | Significant at Cpz, Pz | |
| 800–900ms | ||||
| Trial type by Electrode | 10.4*** (.41) | 4.4* (.23) | ||
| follow-up | Significant at Cpz, Pz | Significant at Cpz, Pz | ||
| 900–1000ms | ||||
| Trial type by Electrode | 6.3* (.30) | |||
| follow-up | Significant at Pz | |||
Notes: Trial type degrees of Freedom (df) 1,15; Trial type by Electrode df 5,75;
= p ≤ .05;
= p ≤ .01;
= p ≤ .001.
To summarize, the presence of an early and the absence of a late parietal effect for stay trials regardless of switch probability supports the activation but not the preparation account. The increased duration of the early parietal stay-pure positivity for frequent compared to infrequent switching (with the exception of predictable trials in the infrequent condition) might indicate increased task-set updating. An early frontal stay-pure positivity in the frequent but not in the infrequent condition might reflect increased executive processing in the frequent switch condition, further supporting the activation account (see discussion).
Evidence for task-set updating and/or task-set reconfiguration processes after switch compared to stay trials (specific switch costs) in the CTI (cue-locked data)
Mean amplitudes in the CTI were assessed to determine if, consistent with activation and preparation accounts, the amount of task-set reconfiguration processes (i.e., magnitude and duration of late parietal positivity) would increase as switch probability decreased. Trial Type by Electrode ANOVAs performed on the mean amplitudes for stay and switch trials in the 100 ms time intervals indicated an early frontal switch-stay positivity between 400 and 500 ms, and a late parietal switch-stay positivity between 400 and 800 ms (see Table 4B; maps Figure 5A) in the frequent switch condition. In the infrequent switch condition, the early frontal switch-stay positivity was reliable between 200 and 600 ms, while the late parietal positivity was significant between 400 and 800 ms. Moreover, an early parietal switch-stay positivity between 200–400 ms most likely reflects increased task-set updating processes for switch than stay trials in the infrequent switch condition (see Table 4B; maps Figure 5B).
Figure 5.
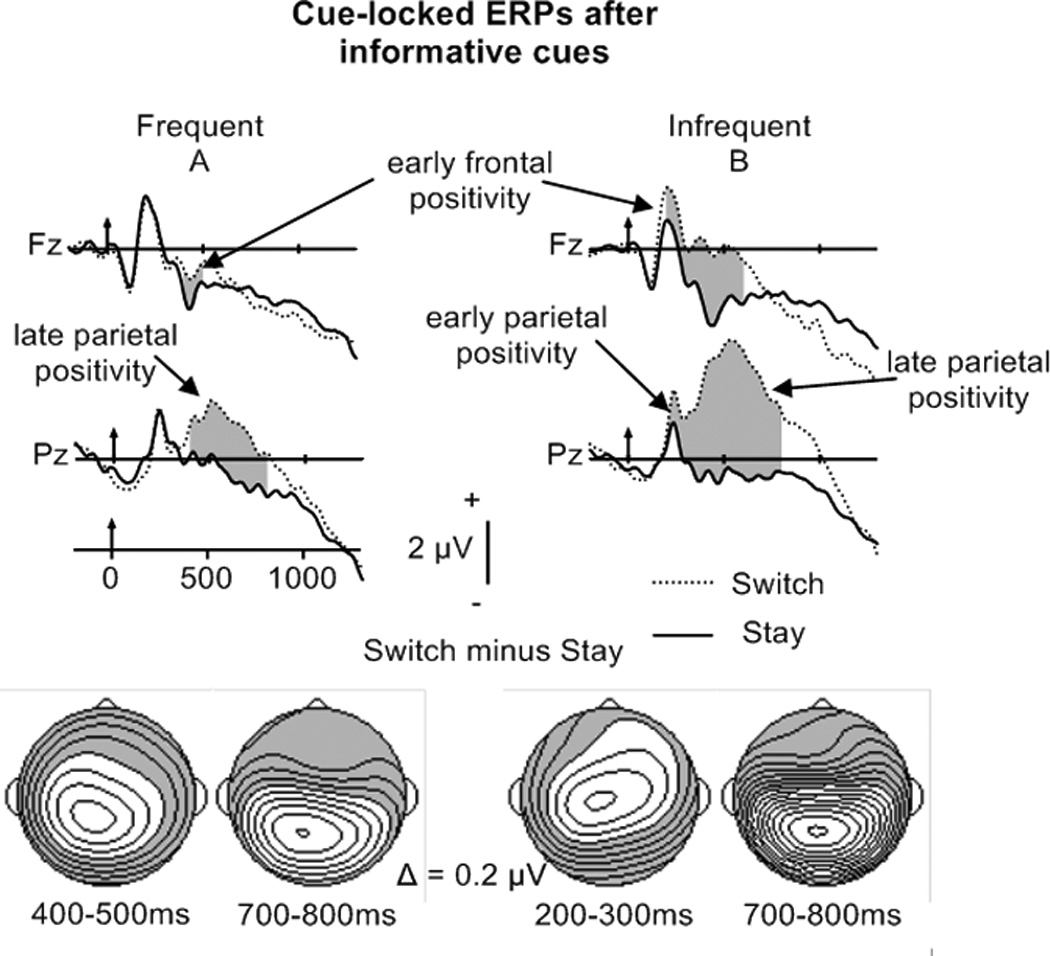
Top: Cue-locked grand–averaged ERP waveforms elicited by stay and switch trials after informative cues (frequent switching in A, infrequent switching in B) at Fz and Pz.
Bottom: Potential maps illustrating the scalp topography of reliable cue-locked switch-stay differences.
To determine whether Switch Probability modulated the magnitude of the early frontal and late parietal switch-stay positivities, Switch Probability by Electrode ANOVAs were performed on the mean amplitudes of the switch minus stay difference waveforms. The Switch Probability by Electrode (Afz, Fz, Fcz, Cz, CPz, Pz) ANOVA for the 400–500 ms interval revealed a significant main effect of Switch Probability (F(1,15) = 17.7, p = .001 η2 = .54; Switch Probability by Electrode interaction F(1,15) = 3.9, p > .05). This result indicates that the amplitude of both the early frontal and late parietal positivity were inversely related to switch probability. Similarly, Switch Probability by Electrode (Cz, Cpz, Pz) ANOVAs on 100 ms mean amplitudes between 500 and 800 ms also confirmed a larger late parietal positivity for infrequent switches (main effects of Switch Probability Fs > 5.0; ps < .05; Switch Probability by Electrode interactions Fs < 1), suggesting more task-set reconfiguration with decreased switch probability.
The Trial type by Electrode ANOVA performed on mean amplitudes of frequent, predictable trials did not reveal a significant switch-stay positivity (Figure 6C). However, an early frontal switch-stay positivity between 400 and 600 ms and a late parietal positivity between 400 and 900 ms were found in the frequent, unpredictable switch condition (Figure 6A, Table 5D). For infrequent switches, the early frontal and late parietal positivity were evident for both predictable and unpredictable task sequences (see Figure 6B and 6D, Table 5D). For predictable sequences, the early frontal switch-stay positivity was present between 400 and 600 ms, while the late parietal positivity was reliable between 400 and 800 ms (maps Figure 6D). For unpredictable switch trials in the infrequent condition, the early frontal positivity was reliable between 200 and 600 ms (increased duration compared to frequent, predictable switches), while the late parietal positivity lasted from 400 to 900 ms (same duration as found for frequent, predictable switches) (maps Figure 6B). An early parietal switch-stay positivity (200–400 ms) was only present for unpredictable switch trials in the infrequent condition, suggesting increased task-set updating for switch compared to stay trials. Switch Probability by Electrode ANOVAs performed on the switch minus stay mean-amplitude differences for unpredictable trials did not reveal any magnitude differences in either the frontal or parietal positivity.
Figure 6.
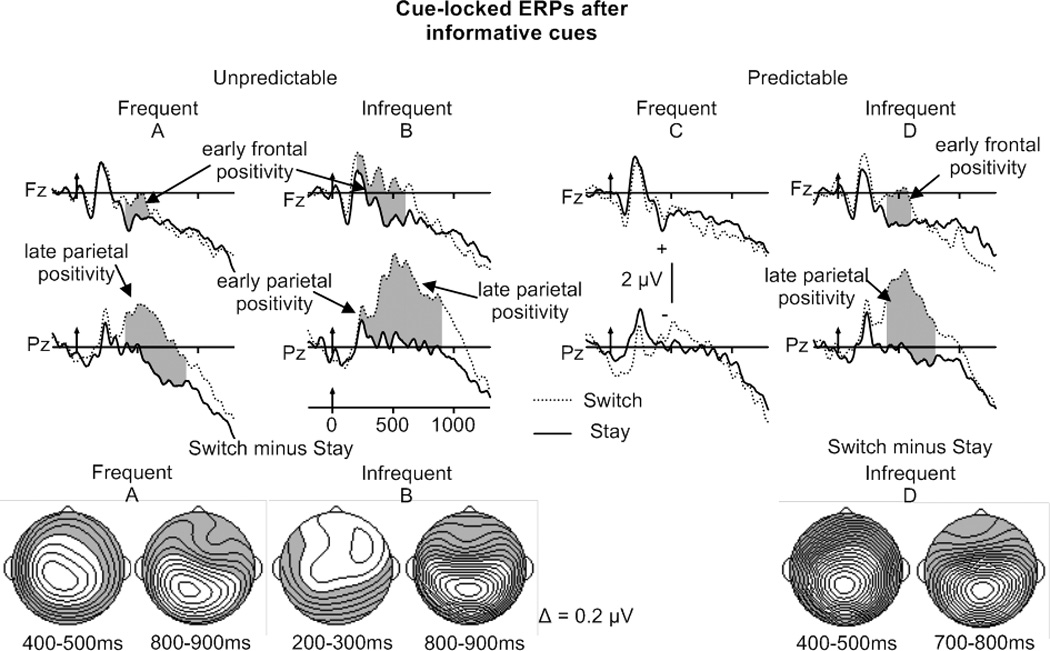
Cue-locked grand averaged ERPs after informative-cue, unpredictable (left) and predictable (right) stay and switch trials (frequent switching in A and C, infrequent switching in B and D) at Fz and Pz.
Bottom: Potential maps illustrating the scalp topography of reliable cue-locked switch-stay differences.
In sum, for predictable trials in the frequent-switch condition, neither a frontal nor a parietal switch-stay positivity was observed, suggesting that task-set reconfiguration following the cue was not engaged in this condition. Task-set updating processes, reflected by the early parietal positivity, and task-set reconfiguration, indicated by the duration of the early frontal, switch-stay positivity appeared to be increased for unpredictable, infrequent switch trials. However, task-set reconfiguration, reflected by the amplitude and duration of the late parietal positivity for unpredictable switch trials, did not differ between the two probability conditions.
Evidence for task-set updating and/or task-set reconfiguration processes preceding stay compared to pure trials (general switch costs) in the RCI (response-locked data)
To determine whether participants incorrectly prepared a switch to the alternate task set for at least some upcoming stay trials following the previous response, RT-locked activity in the RCI preceding stay trials was assessed. Only positive differences between stay vs. pure and switch vs. stay trials are reported for the RCI because the preparation account predicts an earlier engagement of those processes that are otherwise apparent in the CTI. Given that only positive stay-pure and switch-stay effects were present in the CTI, only positive effects are predicted to be present in the RCI.
For the frequent switch condition, Trial Type by Electrode ANOVAs on the mean amplitudes (collapsed across Predictability) revealed that greater positivity preceded informative stay relative to pure trials at both frontal and parietal locations between 200 and 600 ms post-RT (see Table 4A, top). These results suggest the presence of early frontal and early parietal positivities (Figure 7A), suggestive of pre-cue, advanced task-set updating processes. In the infrequent switch condition, Trial type by Electrode ANOVAs revealed an early frontal and early parietal stay-pure positivity only between 200 and 400 ms post-RT (Table 4A, top; Figure 7B). Switch Probability by Electrode ANOVAs on the mean amplitudes of the difference waveforms between 200 and 400 ms did not reveal any magnitude differences.
Figure 7.
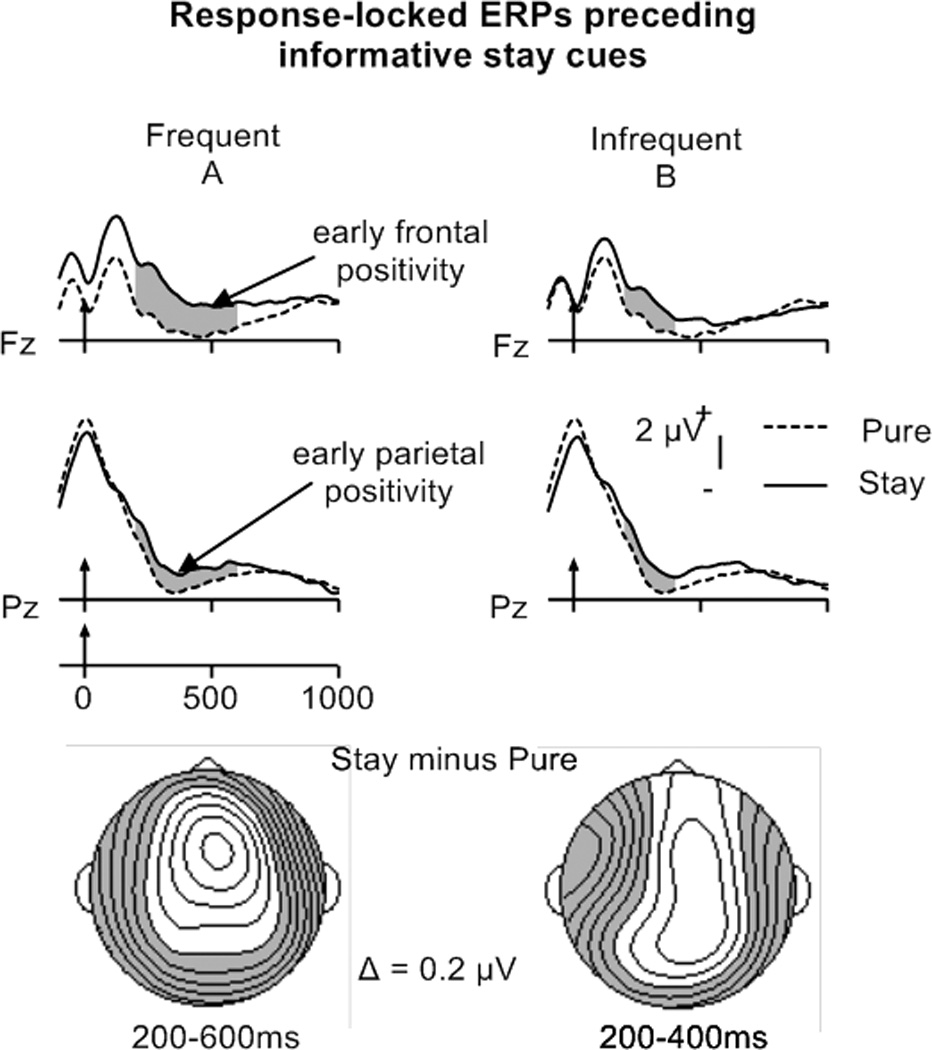
Top: Response-locked ERP grand averages preceding informative-cue stay trials (frequent switching in A, infrequent switching in B) at Fz and Pz. For comparison, the waveforms associated with pure trials are displayed for all conditions.
Bottom: Potential maps illustrating the scalp topography of reliable response-locked stay-pure positive differences.
The ANOVAs separately performed on mean amplitudes for predictable and unpredictable stay compared to pure trials did not reveal any significant effects for infrequent switching (Figure 8B, D). By contrast, evidence for an early frontal and early parietal positivity on stay trials in the frequent switch condition was found for both predictable (200–500 ms) and unpredictable trials (200–600 ms; see Table 5A, top; Figure 8A, C).
Figure 8.
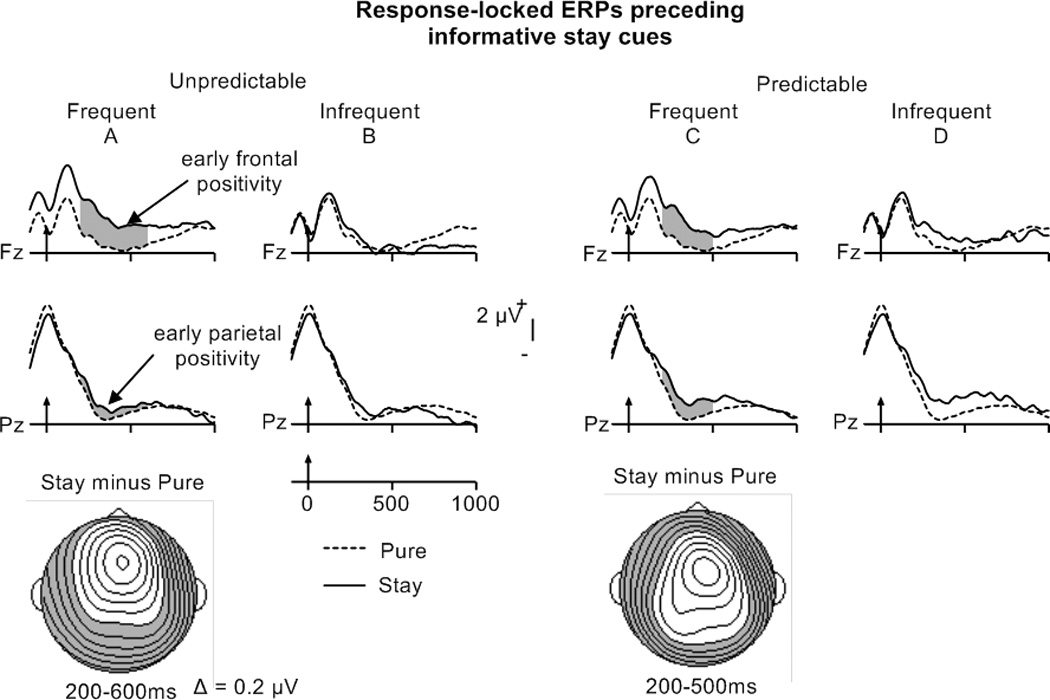
Response-locked ERP grand averages preceding informative-cue, unpredictable (left) and predictable (right) stay trials (frequent switching in A and C, infrequent switching in B and D) at Fz and Pz. For comparison, the ERPs for pure trials are displayed for all conditions.
Bottom: Potential maps illustrating the scalp topography of reliable response-locked stay-pure positive differences.
In sum, response-locked early frontal and parietal positivities were present for all frequent switch conditions but not for predictable and unpredictable stay trials in the infrequent switch condition. In addition, response-locked early effects lasted longer for the higher switch probability condition (cf. Table 6A, left), indicating increased task-set updating. There was no evidence of a late parietal positivity (associated with task-set reconfiguration), arguing against the preparation account. Rather, the presence of an early parietal positivity suggests that informative cues on stay trials are preceded by task-set updating processes, which are recruited to a greater extent in the frequent than infrequent switch condition.
Table 6.
Summary of all significant frontal (F) and parietal (P) ERP differences associated with (A) general and (B) specific switch costs in the response-cue interval (RCI, response-locked effects) and in the cue-target interval (CTI, cue-locked effects).
| (A) General Switch Costs (stay vs. pure) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response-locked effects | Cue-locked effects | ||||||||||||||
| Time intervals | 200– 300 |
300– 400 |
400– 500 |
500– 600 |
600– 700 |
700– 800 |
800– 900 |
900– 1000 |
200– 300 |
300– 400 |
400– 500 |
500– 600 |
600– 700 |
700– 800 |
800– 900 |
| across Predictability | |||||||||||||||
| informative, frequent; F | x | x | x | x | x | x | |||||||||
| informative, frequent; P | x | x | x | x | x | x | |||||||||
| informative, infrequent; F | x | x | |||||||||||||
| informative, infrequent; P | x | x | x | ||||||||||||
| only predictable trials | |||||||||||||||
| informative, frequent; F | x | x | x | x | x | ||||||||||
| informative, frequent; P | x | x | x | x | x | ||||||||||
| informative, infrequent; P | x | x | x | ||||||||||||
| only unpredictable trials | |||||||||||||||
| informative, frequent; F | x | x | x | x | x | x | |||||||||
| informative, frequent; P | x | x | x | x | x | x | |||||||||
| informative, infrequent; P | x | ||||||||||||||
| (B) Specific Switch Costs (switch vs. stay) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response-locked effects | Cue-locked effects | ||||||||||||||
| Time intervals | 200– 300 |
300– 400 |
400– 500 |
500– 600 |
600– 700 |
700– 800 |
800– 900 |
900– 1000 |
200– 300 |
300– 400 |
400– 500 |
500– 600 |
600– 700 |
700– 800 |
800– 900 |
| across Predictability | |||||||||||||||
| informative, frequent; F | x | ||||||||||||||
| informative, frequent; P | x | x | x | x | |||||||||||
| informative, infrequent; F | x | x | x | x | |||||||||||
| informative, infrequent; P | x | x | x | x | x | x | |||||||||
| uninformat., frequent; F | x | x | |||||||||||||
| uninformat., frequent; P | x | x | |||||||||||||
| only predictable trials | |||||||||||||||
| informative, frequent; P | x | x | x | x | x | x | |||||||||
| informative, infrequent; F | x | x | |||||||||||||
| informative, infrequent; P | x | x | x | x | |||||||||||
| uninformat., frequent; F | x | x | x | x | x | ||||||||||
| uninformat., frequent; P | x | x | x | x | x | ||||||||||
| only unpredictable trials | |||||||||||||||
| informative, frequent; F | x | x | |||||||||||||
| informative, frequent; P | x | x | x | x | x | ||||||||||
| informative, infrequent; F | x | x | x | x | |||||||||||
| informative, infrequent; P | x | x | x | x | x | x | x | ||||||||
Notes: F = frontal effects; P = parietal effects; uninformat. = uninformative.
Evidence for task-set updating and/or task-set reconfiguration processes preceding switch compared to stay trials (specific switch costs) in the RCI (response-locked data)
To determine whether participants prepared for an upcoming switch trial after the previous response, ERP activity in the RCI was examined. The results of Trial Type by Electrode ANOVAs on the mean amplitudes in the frequent switch condition preceding an uninformative cue revealed larger positivities for switch than stay trials at frontal and parietal locations between 600–700 ms and 800–900 ms (data not shown; main effects of Trial Type Fs > 4.7, ps < .05]. No differences were found preceding informative cues (data not shown).
Important insights are added by separate analyses on the mean amplitudes for predictable and unpredictable trials. Trial Type by Electrode ANOVAs for the frequent switch condition revealed late frontal and parietal switch-stay positivities between 400 and 900 ms preceding uninformative cues, but only for predictable switch trials (see Figure 9A; main effects of Trial Type Fs > 4.5, ps ≤ .05; no significant effects for unpredictable trials). Trial Type by Electrode ANOVAs on response-locked ERP activity for infrequent switches preceding predictable and unpredictable informative-cue switch compared to stay trials did not reveal any significant effects. However, both early (300–500 ms) and late parietal switch-stay positivities (600–1000 ms) preceding informative cues in the frequent condition were observed for predictable trials (see Figure 9B and Table 5C; no significant effects for unpredictable trials).
Figure 9.
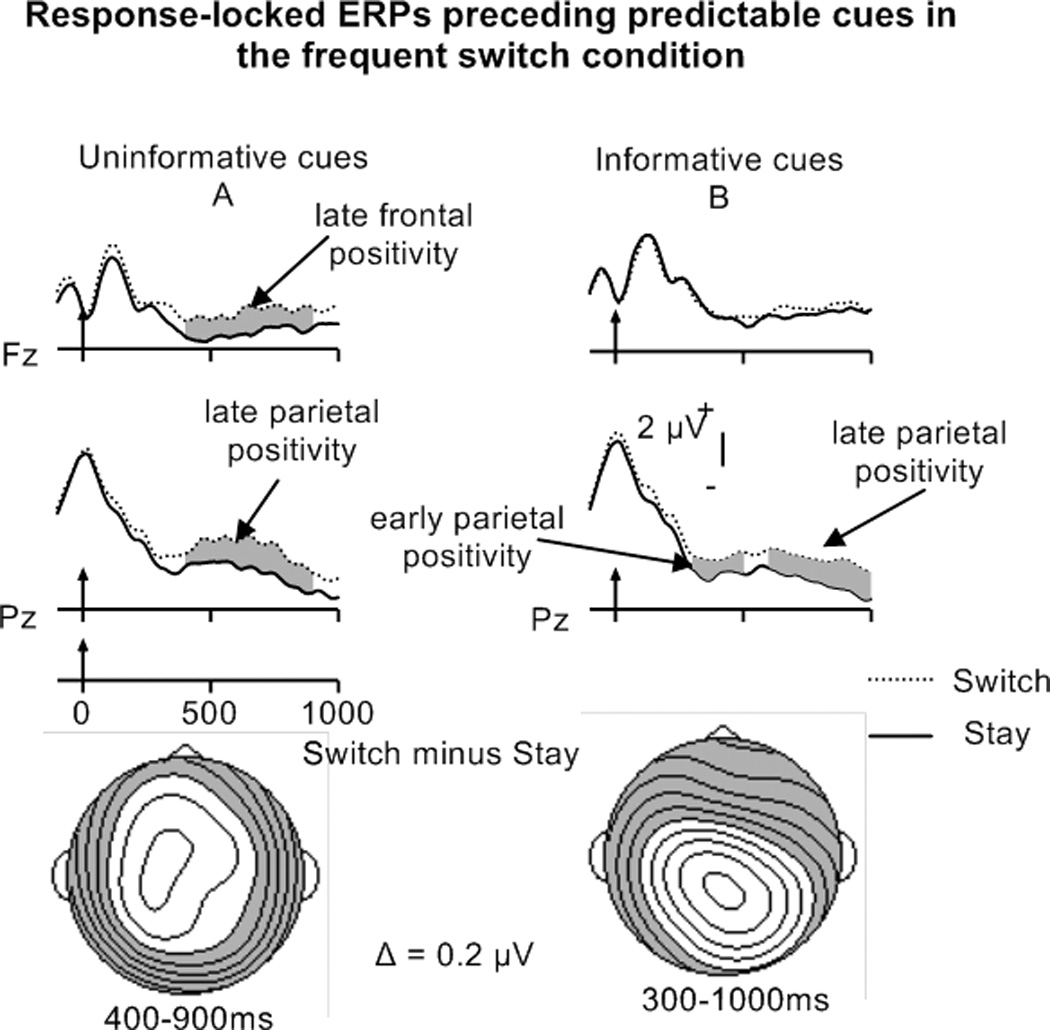
Response-locked grand means preceding predictable, uninformative cues (A) and informative cue (B) stay and switch trials in the frequent switching condition at Fz and Pz.
Bottom: Potential maps illustrating the scalp topography of reliable response-locked switch-stay positive differences.
To summarize, a response-locked late parietal switch-stay positivity, possibly reflecting advance task-set reconfiguration, was only reliable preceding informative- and uninformative-cue, frequent, predictable switches. These results suggest that participants used task-sequence information in the frequent condition to engage task-set reconfiguration processes in preparation for upcoming switch trials, supporting the preparation account. The analysis also revealed a late frontal positivity for predictable switches in the uninformative-cue, frequent-switch condition that might reflect increased executive processing (see discussion).
Discussion
The present study sought to examine whether switch probability RT differences in task switching can be explained better by the activation account, which assumes higher baseline task-set activation for frequent switching, or the preparation account, which assumes pre-cue task-set reconfiguration. The activation account is supported by results showing that task-set updating on stay trials, as reflected by the presence and the duration of the early parietal and frontal positivities, increased for the frequent switch condition. Advance, pre-cue reconfiguration for frequent switching, a prediction of the preparation account, only occurred on predictable switch trials. In addition, the results provide evidence for advance (pre-cue) task-set updating, but not for advance (pre-cue) task-set reconfiguration on stay trials for frequent switches. Hence, while the results support the predictions of the activation account, advanced, pre-cue preparation, for stay and switch trials also plays a role, as discussed below.
Switch probability effects on general switch costs
As predicted by both activation and preparation accounts, increases in switch probability increased general RT switch costs (cf. Table 1, Monsell and Mizon, 2006; Monsell et al., 2003, see also Dreisbach and Haider, 2006), but only for unpredictable trials following uninformative cues. Monsell and Mizon (2006) reported that probability-related RT differences were reduced for a long (790 ms) compared to a short (140 ms) informative CTI condition. In the present study, an informative CTI of 1300 ms was successful in removing all switch probability related differences in general RT costs, suggesting that all of the processes associated with switch probability RT differences can be performed prior to the target if there is sufficient time. Moreover, when an informative cue is missing, knowledge of the task sequence (predictable trials) similarly eliminated general RT cost differences between frequent and infrequent switching. Hence, the possibility of pre-target preparation for informative-cue and predictable trials implicates the examination of pre-target ERPs to aid conclusions concerning switch-probability related processing differences, which have been proposed by the activation and the preparation account.
Before we discuss the ERP evidence for (and against) the activation and preparation accounts following informative cues, it is interesting to note that uninformative cues (preceding stay and switch trials) did not elicit any ERP effects that differentiated them from pure trials (see also Karayanidis et al., 2010; Swainson et al., 2006). In a somewhat related study on attentional orienting processes in a modified Posner symbolic cueing task, Talsma and colleagues (2011) similarly report the absence of a cue-locked positive parietal ERP effect between 200–400 ms for uninformative cues compared to cues that indicated a shift in the location of the target (shift-inducing) and cues that indicated that the participant should maintain attention to the same location (stay-central). The authors suggested that the amplitude of their early parietal positivity might reflect the informational content of the cue, with more information leading to increased P300 amplitudes (see also Gratton et al., 1992). Alternatively, and in line with the interpretation in the current study, the early parietal positivity could reflect task-set updating even if there only is one task-set. However, uncertainty about the target location might hinder advanced task-set updating, as reflected in the failure to find an early parietal positivity after uninformative cues in Talsma and colleagues (2011). Hence, if future research can show that the ERP effects elicited in these different paradigms are similar, then the early parietal positivity might, at least partly, reflect a general preparation process after a specific task instruction2.
Most important, the presence of an early parietal positivity, associated with task-set updating (e.g., Jost et al., 2008; Kray et al., 2005; Manzi et al., 2011), but not a late parietal positivity, associated with reconfiguration (e.g., Jamadar et al., 2010a; Jost et al., 2008; Karayanidis et al., 2011), for stay trials after informative cues in both switch probability conditions (cf. Tables 1 and 6A, right columns) provides evidence against the validity of the preparation account. The preparation account assumes pre-cue task-set reconfiguration on those trials in frequent switch conditions that are deemed to be switch trials, necessitating additional task-set reconfiguration after the cue (back to the original task) if a stay trial is indicated (i.e., wrong prediction). However, stay compared to pure trials did not show a cue-locked late parietal positivity (> 500 ms) that might have reflected task-set reconfiguration. Instead, the longer lasting cue-locked early parietal positivity in the frequent (200 to 400 ms) compared to the infrequent switch condition (300 to 400 ms) suggests additional recruitment of task-set updating processes as predicted by the activation account.
Despite the imprecision of the topographical analysis, involvement of the frontal cortex is suggested by the presence of an early cue-locked frontal stay-pure positivity (200 to 400 ms) in the frequent but not in the infrequent switch condition (cf. Table 6A, right columns). An early switch-stay cue-locked frontal positivity between 360 and 520 ms has been previously associated with top-down control in the form of switching attention to the new task set (Rushworth et al., 2002). However, the frontal positivity for stay compared to pure trials might rather reflect increased inhibition of the alternative task set (i.e., higher demands on task-set updating). Wylie and colleagues (2008) and others have suggested that general and specific switch costs can be explained through a competition between the different stimulus-response mappings (i.e., task sets) (see also Gehring et al., 2003). According to this view, the response to a trial can only be produced when the competition from the alternative task set has been inhibited sufficiently. Higher activation of the alternative task set in the frequent compared to the infrequent switch condition (as suggested by the activation account) would, consequently, require increased inhibition, which might be reflected by the frontal stay-pure positivity. If, for frequent compared to infrequent switching, the increased frontal processing does, indeed, reflect an increment in inhibition, this finding would further support the activation account, although this effect was not predicted a priori.
Advanced processing most likely associated with task-set updating was also found for response-locked ERPs, as reflected by an early frontal positivity and an early parietal positivity preceding informative stay compared to pure trials (for related interpretations of response and cue-locked ERP effects see Karayanidis et al., 2009; Nicholson et al., 2006b; see also Karayanidis et al., 2003). Although the magnitude of the frontal and parietal positivities did not differ for frequent and infrequent stay trials between 200 and 400 ms, the effects lasted longer (until 600 ms) in the frequent switch condition, suggesting increased task-set updating. However, no late (>600 ms) parietal stay-pure positivity was evident, indicating that response-locked effects do not provide support for the preparation account (cf. Table 1).
For infrequent switching, response-locked correlates of task-set updating were not observed for the restricted set of data that compared predictable and unpredictable conditions after excluding those trials that were always predictable (cf. methods; Table 6A, left columns). Consequently, these always-predictable trials must have elicited the response-locked early frontal and parietal stay-pure positivities in the infrequent condition that were observed for the full set of data (Cue Informativeness by Switch Probability analysis). By contrast, pre-cue advanced task-set updating was evident for all frequent switching conditions (cf. Table 6A, left columns). Hence, increased task-set updating for stay trials in the frequent switching condition was evident preceding the cue.
It should be noted that we could not identify endogenous processes as possible explanations for all RT cost results. Specifically, predictability eliminated switch probability related differences in general RT costs after uninformative trials (see discussion above and Figure 2 top right). However, uninformative-cue trials did not show any significant cue- or response-locked frontal and/or parietal stay-pure positivities that could explain the behavioral benefit. It is possible, nevertheless, that advance pre-cue processing is not time-locked to the generation/production of the preceding response. This type of jittered effect might have weakened the differences between stay and pure trials.
To summarize, the behavioral and ERP results suggest that task-set updating for stay trials increases as switch probability increases, supporting the activation account. The ERP results do not confirm pre-cue task-set reconfiguration on stay trials, arguing against the preparation account. Instead, increased pre-cue task-set updating for stay trials in the frequent compared to the infrequent condition adds additional support to the idea of increased baseline task-set activation for frequent switch conditions as proposed by the activation account.
Switch probability effects on specific switch costs
As predicted by the activation and preparation accounts, decreased specific RT switch costs were found for frequent switching (cf. Table 1, Monsell and Mizon, 2006; Monsell et al., 2003, see also Dreisbach and Haider, 2006), but only for uninformative cues. Similar to the results for general switch costs, all processes associated with switch probability related differences in specific RT costs could be performed in the informative cue-target interval of 1300 ms (cf. Monsell and Mizon, 2006). In contrast to general RT costs, specific RT switch costs were unaffected by switch predictability.
At first glance, the cue-locked ERP results suggest, as predicted, that task-set reconfiguration after an informative cue was reduced as switch probability increased (no cue-locked switch-stay ERP differences after uninformative cues). This assertion is based on the finding that the late parietal switch-stay positivity, previously associated with task-set reconfiguration (e.g., Jost et al., 2008; Karayanidis et al., 2011; Lavric et al., 2008; Nicholson et al., 2006a) was smaller for frequent than infrequent switches in the Cue Informativeness by Switch Probability analysis (cf. Tables 1 and 6B, right, top). However, additional analyses suggested that the amplitude and duration of the late parietal switch-stay positivity did not differ as a function of switch probability for unpredictable switches. The differences in the late parietal positivity for the full-data analysis reflect the fact that only predictable, infrequent, but not predictable, frequent switches elicited task-set reconfiguration after an informative cue (see Figure 6C, D). Hence, only predictable, but not unpredictable, task sequences required less task-set reconfiguration in frequent compared to infrequent switch conditions.
The cue-related ERP results at frontal locations make a stronger case for the idea that the amount of task-set reconfiguration is inversely related to switch probability. A frontal switch-stay positivity, possibly reflecting top-down control in the form of shifting attention to the new task set (Rushworth et al., 2002), was present between 200 and 600 ms for infrequent switches but only between 400 and 500 ms for frequent switches (cf. Table 6B, right columns, top). Additional analyses showed that an early frontal, switch-stay positivity was not evident for predictable, frequent switches. For unpredictable trials, the early frontal positivity was shorter for frequent switches (400 – 600 ms) than for infrequent switches (200 – 600 ms) (cf. Table 6B, right). Hence, frequent switches might require less attention shifting either because task sets are maintained in a more active state (activation account) or because preparation for frequent switches has already begun prior to the cue (preparation account).
Nonetheless, similar to the interpretation advanced above for the stay-pure frontal positivity, it is also possible that the switch-stay frontal positivity, at least partly, reflects increased inhibition of the alternative task set in order to resolve competition between task sets (Wylie et al., 2008). A recent study using hemodynamic measurements indicated that attention shifting and inhibition share activations in the same regions of the dorsolateral prefrontal cortex, anterior cingulate and basal ganglia (Hedden and Gabrieli, 2010), making it difficult to identify a differential anatomy for these two sets of processes. Hence, based on current research, both increased attention shifting and/or inhibition provide valid interpretations for differences in the frontal switch-stay positivity between frequent and infrequent switches.
Infrequent switches might also require more task-set updating, as suggested by the cue-locked early parietal switch-stay positivity. This early positivity did not occur for frequent switches, suggesting the possibility that stay and switch trials required similar amounts of task-set updating (e.g., Eppinger et al., 2007). The activation account proposes reduced task-set updating on stay trials in the infrequent switch condition. However, when an infrequent switch occurs, the system might have to compensate for the low activation of the now relevant task set by engaging in additional task-set updating.
Validation for aspects of the preparation account was provided by response-locked data because neural events associated with pre-cue, advanced task-set reconfiguration were found preceding frequent but not infrequent switches (cf. Table 6B, left columns). Notably, pre-cue, advanced task-set reconfiguration for frequent switches occurred preceding informative and uninformative cues, but only when participants knew that a different task set would be required on the next trial (i.e., preceding predictable switches). Preceding informative cues, evidence was found for pre-cue task-set updating and reconfiguration in the form of response-locked early (300 to 500 ms) and late (600 to 900 ms) parietal switch-stay positivities. Moreover, the pre-cue advance preparation appeared to be complete, because cue-locked, switch-stay differences were not observed for predictable, frequent switches (see above). Pre-cue, advance preparation preceding uninformative cues was also associated with a late switch-stay parietal positivity between 400 and 900 ms, most likely reflecting task-set reconfiguration. However, it also appeared to be related to a late, frontal positivity in the same time interval (see Figure 9A, Table 6B, left column). The self-induced switch (i.e., preparing to switch before a cue- or target-related indication of the next task) to the other task set in the uninformative-cue condition, without pre-target verification as in the informative-cue condition, might require recruitment of additional executive processes that could be reflected by the late, frontal positivity. These processes might have interfered with response execution, because there was no RT benefit in the predictable compared to the unpredictable, uninformative-cue condition. While the current investigation cannot provide a specific functional interpretation for the late-frontal, switch-stay positivity, the response-locked results do provide some support for aspects of the preparation account.
The response-locked and cue-locked ERP results partly confirm the preparation account because pre-cue processing appears to decrease cue-locked task-set reconfiguration for predictable switches in the frequent compared to the infrequent switch condition. However, the validity of the preparation account as the only explanation for switch probability related differences seems questionable when results for unpredictable trials are taken into account. Specifically, a similar, cue-locked, late-parietal positivity and the lack of response-locked effects for unpredictable switches in the frequent and infrequent conditions do not support differential recruitment of task-set reconfiguration. However, the engagement of task-set updating increased as switch probability decreased, as suggested by the early parietal switch-stay positivity for infrequent, unpredictable switches. Moreover, decreased switch probability was also associated with an increase in and longer duration of the early frontal switch-stay positivity (cf. Table 6B, right, bottom). Hence, a full explanation of the present behavioral and ERP results appears to require lower baseline task-set activation for lower switch probabilities as predicted by the activation account. Specifically, the data suggest that lower baseline task-set activation for infrequent switches has to be overcome by increased task-set updating that precedes task-set reconfiguration for infrequent switches.
In summary, the behavioral and ERP results suggest pre-cue advance task-set reconfiguration for predictable switches in the frequent but not the infrequent switch condition, partly supporting the preparation account. However, the data also support increased recruitment of task-set updating when switches are infrequent, a result that is consistent with the decreased baseline task-set activation proposed by the activation account.
Conclusions
The results of the present experiment provide new insights on the effects of probability on task switching by the simultaneous manipulation of switch probability and the factors of cue informativeness and predictability. Nonetheless, because the present study is the first to examine specifically switch-probability effects with behavioral data, cue-locked and response-locked ERP data, it will be important to confirm the current conclusions in future studies. Furthermore, although not possible with the current data, it will be of interest to determine the precise intracranial generators that contribute to the early and late frontal and parietal stay-pure and switch-stay positivities, the neural correlates of, respectively, task-set updating and task-set reconfiguration processes.
Despite these limiting factors, the results confirm that switch probability influences the timing and demand for the processes involved in task-set updating and reconfiguration. It has been demonstrated that switch-probability effects are best explained by a model that we will call “the extended activation account.” This new account posits that baseline task-set activation increases along with switch probability, which necessitates increased task-set updating on stay trials in order to inhibit the alternative task. Conversely, low levels of baseline task-set activation during infrequent switching necessitate increased task-set updating on switch trials to both activate the relevant and inhibit the alternative task set. The extended activation account also posits pre-cue preparation in the form of task-set updating on stay trials and task-set reconfiguration on predictable switch trials when switches occur frequently. Importantly, the extended activation account complements previous task-switching models (e.g., Monsell, 2003) by providing an explanation of switch probability effects on the behavioral and ERP data.
Highlights.
Study tested different accounts that propose to explain switch probability effects.
Frequent task switching increases general and decreases specific switch RT costs.
Frequent task switching is associated with increased baseline task-set activation.
Pre-cue advance preparation appears for frequent but not infrequent switching.
Switch probability effects can be explained by an extended activation account.
Acknowledgments
We wish to thank Charles L. Brown III for computer programming and help in all technical matters. We thank Brenda Malcolm and Efrat Schori for their invaluable assistance in participant recruitment and neuropsychological evaluation. Special thanks go to Michael Bersick for data collection and analyses. We also thank the reviewers for their helpful suggestions. This research was supported by grant AG005213 from the NIA and by the New York State Department of Mental Hygiene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
By contrast with the ANOVA that did not consider Predictability, the Cue Informativeness by Switch Probability interaction was only marginally significant [F(1,15) = 3.0, p = .1 η2 = .17]. However, when Switch Probability by Predictability ANOVAs were performed separately for trials following informative and uninformative cues, only the latter returned a significant effect of Switch Probability (F < 1; F(1,15) = 8.9, p < .01, η2 = .37, respectively), mirroring the result reported in the analysis that did not consider Predictability.
The authors thank Durk Talsma for this suggestion.
References
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: exploring the dynamic control of tasks. In: Umiltà C, Moscovitch M, editors. Attention and Performance XV. Cambridge: MIT press; 1994. pp. 421–452. [Google Scholar]
- Arrington CM, Logan GD, Schneider DW. Separating cue encoding from target processing in the explicit task-cuing procedure: are there "true" task switch effects? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:484–502. doi: 10.1037/0278-7393.33.3.484. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Perianez JA, Knight RT. Think differently: a brain orienting response to task novelty. Neuroreport. 2002;13:1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- Dreisbach G, Haider H. Preparatory adjustment of cognitive control in the task switching paradigm. Psychonomic Bulletin & Review. 2006;13(2):334–338. doi: 10.3758/bf03193853. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mecklinger A, John O. Age differences in task switching and response monitoring: evidence from ERPs. Biological Psychology. 2007;75:52–67. doi: 10.1016/j.biopsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Electrophysiological correlates of error correction. Psychophysiology. 2005;42:72–82. doi: 10.1111/j.1469-8986.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Cycowicz YM, Horton C. Development of and change in cognitive control: A comparison of children, young adults, and older adults. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:91–102. doi: 10.3758/CABN.9.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Johnson JR. Age-Related Changes in Executive Function: An Event-Related Potential (ERP) Investigation of Task-Switching. Aging, Neuropsychology, and Cognition. 2008;15:95–128. doi: 10.1080/13825580701533769. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Bryck RL, Jonides J, Albin RL, Badre D. The mind’s, eye, looking inward? In search of executive control in internal attention shifting. Psychophysiology. 2003;40:572–585. doi: 10.1111/1469-8986.00059. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the Use of Information: Strategic Control of Activation of Responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. NeuroImage. 2010;51:421–431. doi: 10.1016/j.neuroimage.2010.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard J, Wan CK. LISREL approaches to interaction effects in multiple regression. Thousand Oaks. US: Sage Publications; 1996. [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F. The spatial and temporal dynamocs of anticipatory preparation and response inhibition in task-switching. NeuroImage. 2010a;51:432–449. doi: 10.1016/j.neuroimage.2010.01.090. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Michie PT, Karayanidis F. Sequence effects in cued task switching modulate response preparedness and repetition priming processes. Psychophysiology. 2010b;47:365–386. doi: 10.1111/j.1469-8986.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Barnhardt J, Zhu J. The contribution of executive processes to deceptive responding. Neuropsychologia. 2004;42:878–901. doi: 10.1016/j.neuropsychologia.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Barnhardt J, Zhu J. The deceptive response: effects of response conflict and strategic monitoring on the late positive component and episodic memory-related brain activity. Biological Psychology. 2003;64:217–253. doi: 10.1016/j.biopsycho.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Jost K, Mayr U, Roesler F. Is task switching nothing but cue priming? Evidence from ERPs. Cognitive, Affectice, and Behavioral Neuroscience. 2008;8:74–84. doi: 10.3758/cabn.8.1.74. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Coltheart M, Michie PT, Murphy K. Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology. 2003;40:329–348. doi: 10.1111/1469-8986.00037. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Jamadar S, Ruge H, Phillips N, Heathcote A, Forstmann BU. Advance preparation in task-switching: converging evidence from behavioral, brain activation, and model-based approaches. Frontiers in Psychology. 2010;1 doi: 10.3389/fpsyg.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Mansfield EI, Galloway K, Smith J, Provost A, Heathcote A. Anticipatory reconfiguration elicited by fully and partially informative cues that validly predict a switch in task. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:202–215. doi: 10.3758/CABN.9.2.202. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Provost A, Brown S, Paton B, Heathcote A. Switch-specific and general preparation map onto different ERP components in a task-switching paradigm. Psychophysiology. 2011;48:559–568. doi: 10.1111/j.1469-8986.2010.01115.x. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, Hetrick WP. Event-related potential correlates of task switching and switch costs. Psychophysiology. 2005;42:56–71. doi: 10.1111/j.1469-8986.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A. Age differences in attentional control: an event-related potential approach. Psychophysiology. 2005;42:407–416. doi: 10.1111/j.1469-8986.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Lavric A, Mizon GA, Monsell S. Neurophysiological signature of effective anticipatory task-set control: A task-switching investigation. European Journal of Neuroscience. 2008;28:1016–1029. doi: 10.1111/j.1460-9568.2008.06372.x. [DOI] [PubMed] [Google Scholar]
- Manzi A, Nessler D, Czernochowski D, Friedman D. The development of anticipatory cognitive control processes in task-switching: An ERP study in children, adolescents, and young adults. Psychophysiology. 2011;48:1258–1275. doi: 10.1111/j.1469-8986.2011.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: the role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Mayr U, Keele SW. Changing internal constraints on action: the role of backward inhibition. Journal of Experimental Psychology: General. 2000;129:4–26. doi: 10.1037//0096-3445.129.1.4. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of Processing Mode Prior to Task Performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1423–1442. [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. The Journals of Gerontology. 2001;56B:P88–P102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Monsell S, Mizon GA. Can the task-cuing paradigm measure an endogenous task-set reconfiguration process? Journal of Experimental Psychology: Human Perception and Performance. 2006;32:493–516. doi: 10.1037/0096-1523.32.3.493. [DOI] [PubMed] [Google Scholar]
- Monsell S, Sumner P, Waters H. Task-set reconfiguration with predictable and unpredictable task switches. Memory & Cognition. 2003;31:327–342. doi: 10.3758/bf03194391. [DOI] [PubMed] [Google Scholar]
- Nicholson R, Karayanidis F, Bumak E, Poboka D, Michie PT. ERPs dissociate the effects of switching task sets and task cues. Brain Research. 2006a;1095:107–123. doi: 10.1016/j.brainres.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Nicholson R, Karayanidis F, Davies A, Michie PT. Components of task-set reconfiguration: Differential effects of "switch to" and "switch away" cues. Brain Research. 2006b;1121:160–176. doi: 10.1016/j.brainres.2006.08.101. [DOI] [PubMed] [Google Scholar]
- Nicholson R, Karayanidis F, Poboka D, Heathcote A, Michie PT. Electrophysiological correlates of anticipatory task-switching processes. Psychophysiology. 2005;42:540–554. doi: 10.1111/j.1469-8986.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- Pashler H. The psychology of attention. Cambridge, MA: MIT press; 1999. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rubin O, Meiran N. On the Origins of the Task Mixing Cost in the Cueing Task-Switching Paradigm. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1477–1491. doi: 10.1037/0278-7393.31.6.1477. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. Journal of Cognitive Neuroscience. 2002;14:1139–1150. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Annual Review of Neuroscience. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM. Attention. In: Atkinson, R.J.H RA, Lindzey G, Luce RD, editors. Stevens’ handbook of experimental psychology. New York: John Wiley & Sons; 1998. pp. 739–811. [Google Scholar]
- Swainson R, Jackson SR, Jackson GM. Using advance information in dynamic cognitive control: an ERP study of task-switching. Brain Research. 2006;1105:61–72. doi: 10.1016/j.brainres.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Talsma D, Sikkens J, Theeuwes J. Stay Tuned: What is Special about not Shifting Attention? PLoS one. 2011;6(3):e16829. doi: 10.1371/journal.pone.0016829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers S, West R. Neural correlates of cue retrieval, task set reconfiguration, and rule mapping in the explicit cue task switching paradigm. Psychophysiology. 2008;45:588–601. doi: 10.1111/j.1469-8986.2008.00658.x. [DOI] [PubMed] [Google Scholar]
- West R. The effects of aging on controlled attention and conflict processing in the Stroop task. Journal of Cognitive Neuroscience. 2004;16:103–113. doi: 10.1162/089892904322755593. [DOI] [PubMed] [Google Scholar]
- West R, Langley MM, Bailey K. Signaling a switch: Neural correlates of task switching guided by task cues and transition cues. Psychophysiology. 2011;48:612–623. doi: 10.1111/j.1469-8986.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Murray MM, Javitt DC, Foxe JJ. Distinct Neurophysiological Mechanisms Mediate Mixing Costs and Switch Costs. Journal of Cognitive Neuroscience. 2008;21:105–118. doi: 10.1162/jocn.2009.21009. [DOI] [PubMed] [Google Scholar]