SUMMARY
Background
Very low birth weight neonates (≤1500 g, VLBWs) have a high rate of infection and distinct baseline immune function compared with more mature populations. In critically ill children and adults, sepsis increases subsequent infection risk. It is unknown whether sepsis modifies the risk of subsequent infection in VLBWs.
Methods
We conducted a retrospective cohort study of VLBWs ≤32 weeks gestation at birth cared for in 312 neonatal intensive care units in the United States from 1997–2011 (n=103,376). Early-onset sepsis (EOS, culture-positive only) and late-onset sepsis (LOS, culture-positive or clinical) cases were identified. Cox proportional hazards models were used to control for clinical variables between neonates with and without EOS to determine if EOS modified risk of LOS, necrotizing enterocolitis (NEC), or death.
Results
LOS occurred in 12,112/102,317 (11.8%) neonates without EOS and in 133/1059 (12.6%) of those with EOS. After adjustment for clinical variables, the risk of LOS was not different between neonates with or without a history of EOS (hazard ratio [HR]=0.92; 95% confidence interval [CI] 0.74, 1.16). EOS increased the risk of 120-day mortality (HR=1.78; 95% CI 1.49, 2.13).
Conclusions
In contrast to findings in children and adults, EOS was not associated with an increased risk of LOS in this cohort. Age-specific investigations are needed to determine if post-sepsis immunologic alterations are present.
Keywords: preterm, neonate, sepsis, immunoparalysis
1. Introduction
Infection is common in preterm neonates and is associated with significant short and long-term morbidities, increased health care costs, and mortality [1]. Previous studies in critically ill children and adults have described an increased risk of infection following an initial episode of sepsis or trauma [2]. The increased infection risk following sepsis is associated with sustained perturbations in immune system function, including reduced monocytic production of tumor necrosis factor alpha (TNF-α) following endotoxin exposure and/or reduced monocyte human leukocyte antigen (HLA)-DR surface expression. These post-sepsis immune alterations are collectively termed sepsis-induced “immunoparalysis” [3].
Distinct baseline immune system function in preterm neonates may change the frequency, type, duration, and clinical impact of infection-related immune alterations [4]. No study of immune function pre-/post-sepsis has been reported in preterm neonates. Thus, following infection, preterm neonates may manifest an equivalent or amplified state of immunoparalysis (with clinical infectious consequences similar to or more severe than those seen in older populations) or exhibit no immunoparalysis and/or no clinical consequence. Alternatively, an enhancement of immune function that results in a reduced risk of subsequent infection may occur. Previous reports of the effect of early infection on subsequent risk of infection in preterm neonates are mixed [5–7]. We hypothesized that clinically apparent immunoparalysis does not occur in the very low birth weight (≤1500 g, VLBW) preterm infant. We examined a large cohort of VLBW neonates to determine whether early-onset sepsis (EOS) modifies the risk for late-onset sepsis (LOS) in this population.
2. Methods
2.1. Patients
We examined data collected prospectively from clinicians’ daily progress notes on all neonates admitted from 1997–2011 in 312 neonatal intensive care units (NICUs) managed by the Pediatrix™ Medical Group. We excluded all neonates who died within the first day of life (DOL) without a blood culture drawn. We collected demographic information including maternal age, delivery method, receipt of prenatal care, administration of antenatal steroids or antibiotics, sex, race, gestational age (GA), birth weight, inborn or outborn status, and Apgar scores at 1 and 5 minutes. Medication records were collected and analyzed specifically for the length of antibiotic treatment with concurrent blood culture and use of inotropes on DOL 3. Laboratory values including complete blood counts with manual white blood cell differential count and C-reactive protein (CRP) levels were collected. Clinical factors including the presence or absence of mechanical ventilation, enteral feeding status, and highest fraction of inspired oxygen (FiO2) were collected for DOL 3.
2.2. Definitions
We defined EOS as a positive blood culture obtained on or before DOL 3, and LOS as a positive blood culture from DOL 4 to DOL 120. We chose 120 days as the cut-off for LOS cases because this permits capture of 99% of episodes of LOS and reduces the chances of confounding sepsis risk due to prolonged hospital stays [5]. When multiple positive blood cultures with the same genus and species were obtained within a 21-day period, they were treated as a common, single infection. Clinical LOS was defined as a negative blood culture with antibiotics started on the day of culture and continued for at least 5 days, and at least 1 of the following hematologic indices present within 24 hours of drawing the blood culture: immature to total neutrophil ratio >20%, CRP >1 mg/dL, or absolute neutrophil count <1500 cells/mm3.
Coagulase-negative Staphylococcus (CoNS) infections were divided into 3 categories: definite, probable, and possible as previously defined [8]. We defined a definite CoNS infection as 2 positive cultures drawn on the same day; probable CoNS infection as 2 positive cultures within a 4-day period, 3 positive cultures within a 7-day period, or 4 positive cultures within a 10-day period; and possible CoNS infection as a culture positive for CoNS that did not meet criteria for definite or probable CoNS sepsis. Only definite and probable CoNS infections were included in the analysis. Cultures growing known contaminants, including non-speciated streptococci, Bacillus sp., Corynebacterium sp., diphtheroids sp., gram-positive rods (not including Listeria sp.), Lactobacillus sp., Micrococcus sp., Stomatococcus sp., and Bacteroides sp., were considered negative. If multiple cultures were obtained on the same day, we considered only the positive culture.
We selected pharmacologic and clinical risk factors as surrogates for critical illness on DOL 3, including exposure to inotropes (epinephrine, dopamine, dobutamine, milrinone, vasopressin, norepinephrine, phenylephrine), need for mechanical ventilation (conventional ventilation or high-frequency ventilation), fraction of inspired oxygen, and enteral feeding status. We evaluated antibiotic therapy by counting the length of the first uninterrupted course of systemic antibiotics if initiated within the first 3 days of life. We considered necrotizing enterocolitis (NEC) to be present if a diagnosis of medical or surgical NEC was recorded between DOL 3 and DOL 120. Neonates with this diagnosis had 1 or more of the following clinical signs: bilious gastric aspirate or emesis, abdominal distention, or blood in stool without evidence of a rectal fissure; they also had 1 or more of the following radiographic findings: pneumatosis intestinalis, hepatobiliary gas, or pneumoperitoneum.
2.3. Statistical analysis
We used standard summary statistics to describe maternal and neonatal factors in patients with and without EOS. Median and interquartile ranges or frequency counts and percentages are reported for neonates with and without EOS and compared using Mann-Whitney and chi-square tests, respectively. The prevalence of 5 clinical outcomes was examined: 1) LOS, 2) LOS excluding CoNS, 3) clinical LOS, 4) NEC, and 5) mortality at 120 days. Associated hazard ratios (HR), 95% confidence intervals (CI), and p-values were based on Cox proportional hazards models. We censored for discharge or death when not included as an outcome up to 120 days of life. Covariates included in the model were selected from the following clinically relevant predictors using stepwise variable selection with addition and removal p-values of 0.2 and 0.25: surrogates of clinical illness on DOL 3 including nothing-by-mouth (NPO) status, need for inotropic support, mechanical ventilation and fraction of supplemental FiO2, duration of uninterrupted initial antibiotic therapy, and GA [5]. The same variables were initially considered for stepwise covariate selection for each model. Proportional hazards assumption was tested on the basis of Schoenfeld residuals and graphically using log-log plots. We conducted the analysis with STATA 12 (College Station, TX). This study was approved by the Duke University Institutional Review Board (IRB) and the Western IRB (for Pediatrix™ Medical Group sites).
3. Results
During the study period, Pediatrix™ Medical Group cared for 104,186 VLBW neonates with complete discharge data ≤32 weeks GA and ≤1500 grams birth weight. After excluding neonates who died within the first DOL without having had a blood culture drawn (n=810), our final cohort consisted of 103,376 neonates; 1059 of these had EOS (Figure 1). Mothers of neonates with EOS were older, were less frequently exposed to antenatal steroids, more frequently exposed to antibiotics, and more likely to deliver vaginally compared with mothers of neonates without EOS. Neonates with EOS were more likely to be black and were more likely to be outborn, have lower GA, lower birth weight, lower 5-minute Apgar scores, and have clinical characteristics of critical illness on DOL 3 (no enteral feeds, use of inotropes, need for mechanical ventilation, and FiO2 >50%) than neonates without EOS.
Figure 1.
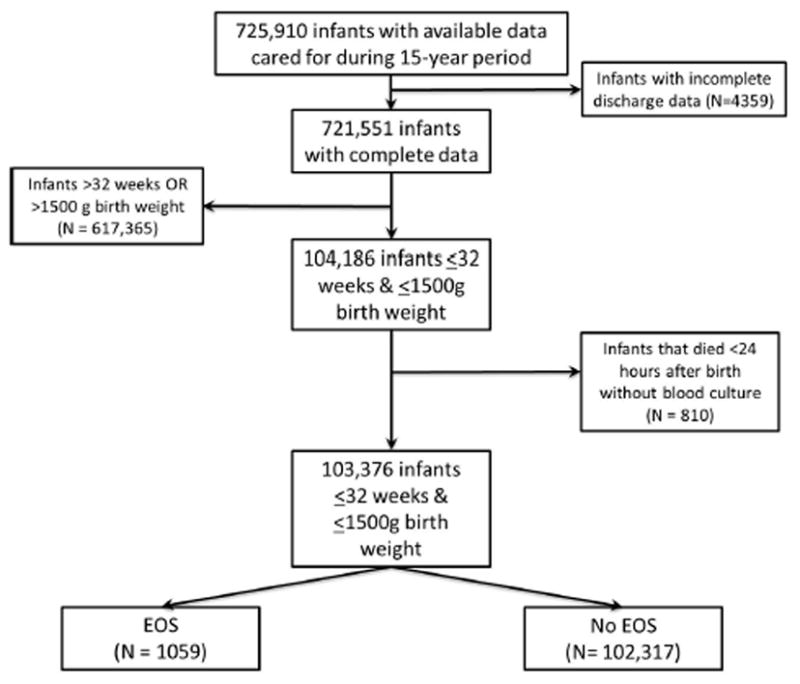
Study cohort.
Maternal variables were statistically different but not widely divergent between those infants with and without LOS, with the exception of frequency of vaginal delivery (Table 1). Infants who developed LOS were more likely to have reduced length of gestation, lower birth weight, and lower 5-minute Apgar scores with greater frequencies of black race, male sex, prolonged early antimicrobial treatment (antimicrobial treatment started at birth and continued for >5 days), delayed enteral feedings, and surrogates of critical illness on DOL 3 compared to infants without LOS.
Table 1.
Cohort demographics
| LOS (n=12,245) | No LOS (n=91,131) | p Value | |
|---|---|---|---|
| Maternal | |||
| Age (years), median (25th, 75th percentile) | 27 (22, 32) | 27 (22, 32) | <0.001 |
| Antenatal steroids, % | 74 | 73 | 0.67 |
| Antenatal antibiotics, % | 44 | 41 | <0.001 |
| Vaginal delivery, % | 32 | 28 | <0.001 |
| Prenatal care, % | 95 | 95 | 0.10 |
| Prolonged rupture of membranes, % | 21 | 22 | 0.01 |
|
| |||
| Neonatal | |||
| GA (weeks), median (25th, 75th percentile) | 26 (25, 28) | 28 (26, 30) | <0.001 |
| Birth weight (g), median (25th, 75th percentile) | 840 (675, 1065) | 1060 (835, 1305) | <0.001 |
| Race, % | <0.001 | ||
| White | 44 | 49 | |
| Black | 29 | 26 | |
| Hispanic | 23 | 20 | |
| Other, non-white | 4 | 5 | |
| Male, % | 54 | 51 | <0.001 |
| Inborn, % | 81 | 84 | <0.001 |
| Apgar 5 min (25th, 75th percentile) | 7 (6, 8) | 8 (7, 9) | <0.001 |
| No enteral feeds on DOL 3, % | 59 | 42 | <0.001 |
| Inotropes on DOL 3, % | 0.3 | 0.2 | <0.001 |
| Ventilator on DOL 3, % | 3 | 1 | <0.001 |
| FiO2 >50% on DOL 3, % | 0.7 | 0.4 | <0.001 |
| Early-onset sepsis (culture-positive), % | 1.1 | 1 | 0.47 |
| Prolonged early antimicrobial treatment*, % | 62 | 36 | <0.001 |
Antimicrobial treatment started at birth and continued for >5 days.
3.1. Relationship between EOS and LOS in VLBW neonates
In this cohort, LOS occurred in 12.6% of the EOS group (133/1059) and 11.8% of those without EOS (12,112/102,317). One hundred thirty-three (0.13%) VLBW neonates had both culture-positive EOS and LOS. We found that EOS was not associated with an increase in risk of developing LOS (Table 2). Separate analyses also showed EOS did not increase LOS risk when we specifically excluded cases of LOS caused by CoNS. Neonates with EOS also had an increase in all-cause mortality at 120 days. An analysis of the impact of EOS by pathogen class on our outcomes of interest is also included in Table 2. The duration of initial antimicrobial treatment (the length of the first uninterrupted course of systemic antibiotics initiated within the first 3 days of life started at birth) was examined as a continuous variable and was associated with a statistically significant decrease in the risk for LOS that is unlikely to be clinically significant (HR=0.997 [95% CI 0.996, 0.997]), p<0.001). Because culture-negative clinical sepsis is a common scenario in VLBW neonates [9], we examined the risk of LOS in neonates with any EOS (either clinical or culture-positive). We identified 13,144 infants with 13,244 cases of clinical EOS (excluding culture-positive EOS) in our cohort. The HR for LOS in neonates with clinical EOS was 0.92 (95% CI 0.86, 0.97; p=0.006) using a Cox proportional hazards model controlling for NPO status and mechanical ventilation on DOL 3, initial duration of antibiotics, and GA.
Table 2.
Effect of EOS on the risk of LOS, NEC, and 120-day mortality (OR [95% CI])
| LOS | LOS (no CoNS) | Clinical LOS | NEC | 120-day mortality | |
|---|---|---|---|---|---|
| EOS (n=1059) | 0.92 (0.74, 1.16) | 0.95 (0.74, 1.22) | 0.90 (0.70, 1.16) | 0.89 (0.70, 1.12) | 1.78 (1.49, 2.13)* |
| Gram- positive | 0.89 (0.61, 1.30) | 0.92 (0.61, 1.40) | 1.14 (0.79, 1.65) | 1.21 (0.86, 1.70) | 1.53 (1.11, 2.10) |
| Gram- negative | 0.97 (0.73, 1.31) | 0.97 (0.70, 1.34) | 0.80 (0.56, 1.13) | 0.73 (0.53, 1.02) | 2.08 (1.68, 2.59)* |
p<0.05 using Cox proportional hazards model after controlling for gestational age, duration of initial antibiotic therapy, and day of life 3 variables.
3.2. Impact of EOS on LOS pathogen type
Pathogens associated with EOS were predominantly gram-negative (667/1219 episodes, 55%), followed by gram-positive (459/1219, 38%), Candida sp. (58/1219, 5%), and unclassified organisms (35/1219, 2%). Three organisms accounted for 56% of EOS (Escherichia coli [375/1219, 31%], group B Streptococcus [204/1219, 17%], and Haemophilus influenzae [100/1219, 8%]). In this cohort, LOS was dominated by CoNS (29%), Staphylococcus aureus (16%), and Candida sp. (10%). Gram-negative organisms, led by Klebsiella sp. (7%), Escherichia coli (6%), and Enterobacter sp. (5%), collectively caused 25% of LOS. LOS pathogen class distribution was slightly altered by EOS status: gram-positive (54% vs. 63%, p=0.04), gram-negative (31% vs. 29%, p=0.48), CoNS (21% vs. 29%, p=0.06), or Candida sp. (19% vs. 10%, p=0.001) when compared to neonates without EOS.
4. Discussion
These data demonstrate a trend towards decreased risk of subsequent infection in preterm VLBW infants with culture-positive EOS and are in contrast to results in older patient populations. EOS also did not modify risk of clinical LOS, NEC, or LOS with specific exclusion of CoNS. When infants with any EOS (either clinical or culture-positive) were examined, a significant reduction in subsequent LOS risk was found. The larger number of infants with clinical EOS accounted for this statistical significance as the point estimate for LOS was 0.92 for both culture-positive and any EOS. Consistent with previous reports, we found mortality was greater for neonates with EOS (and specifically for gram-negative EOS) [10]. EOS (either gram-positive or gram-negative) increased the risk for the composite outcome of NEC or 120-day mortality. EOS slightly modified the pathogen distribution of LOS with a reduction in the frequency of gram-positive bacterial pathogens and an increase in the frequency of Candida sp. compared to those without EOS.
Increased risk of infection following sepsis, burn, or trauma in adults and older pediatric patients is a well-documented clinical phenomenon [11,12]. Frazier and Hall very thoroughly described the distinct immunologic changes associated with the physiologic phenomenon widely known as immunoparalysis, and described potential causal mechanisms including modified cytokine production (reduced TH1 cytokines [TNF-α, IFN-γ, IL-12] and increased TH2 cytokines [TGF-β, IL-4, IL-10]), decreased monocyte HLA-DR expression, lymphocyte apoptosis, T-regulatory cell-dominant adaptive immune response, glucocorticoid-mediated changes, and decreased pro-inflammatory gene transcription [3]. Many of these immune response patterns are present at baseline in preterm neonates [13–16].
Altered functional capacity of the innate and adaptive immune systems of the intrapartum fetus and newborn at baseline increases their risk of acquiring infection as compared with older patients [4,17]. The neonatal immune system must quickly adapt from the protected intra-uterine environment to tolerate acquisition of billions of commensal microorganisms and defend against potential pathogens [14]. Novel longitudinal studies of preterm and term neonatal-specific developmental immunology are emerging [18–21], and molecular methods have revealed a unique transcriptomic host response to sepsis in term neonates as compared with older pediatric patients [22]. However, there is a dearth of specific investigations into the functional adaptations of the neonatal immune system following infection and, if present, whether they are similar to the immunoparalysis described in older populations that leads to increased risk of subsequent infection. This knowledge gap in newborns—particularly pronounced in preterm neonates who experience a very high rate of infection—is contrasted by the many immunologic and epigenetic investigations following sepsis, trauma, or burns that have been undertaken in adult humans or animals [23].
In a large cohort of VLBW neonates with LOS, Stoll et al. did not identify EOS as an important risk factor for LOS development [5]. Recently, Strunk et al. reported that preterm neonates (n=838, <30 weeks gestational age) exposed to histologic chorioamnionitis (any: maternal, fetal, or both) had a decreased risk of LOS (HR 0.74 [95% CI 0.57, 0.95]) when compared to neonates with no exposure to chorioamnionitis, suggesting perinatal inflammation may paradoxically enhance the function of the preterm immune system and provide protection against LOS [7]. In contrast, Leviton et al. described an increased risk of LOS (odds ratio 2.2 [95% CI 1.4, 3.3]) in extremely low gestational age neonates (ELGAN, <28 weeks gestation) diagnosed with EOS [6]. Key differences in the ELGAN analysis as compared with our analysis included the definition of EOS (blood culture positive within the first week of life), examination only of survivors to 36 weeks postmenstrual age, and a significantly higher (over 20-fold) incidence of infants with both EOS and LOS (2.7% versus 0.13% in our cohort).
Our finding that VLBW neonates do not manifest an increased risk of subsequent infection following sepsis may reflect an absence of clinically relevant detrimental sepsis-associated immune alterations like those seen in older populations. Alternatively, the lack of subsequent infection risk in VLBWs may be due to survival of neonates with inherently more robust immune function or an immune-priming effect of infection. In line with the report by Strunk et al. that showed histologic chorioamnionitis exposure reduced the risk of LOS, additional precedents exist in both preclinical neonatal animal models and in neonatal humans for beneficial post-birth immune priming that leads to reduced subsequent infection-related mortality. Neonatal mice pretreated with low-dose specific Toll-like receptor (TLR) agonists experienced a significant survival advantage over saline pretreated animals when subsequently challenged with polymicrobial sepsis [24]. Multiple enhancements in innate immune function (altered cytokine production, reactive oxygen species production, neutrophil phagocytosis, improved bacterial clearance) were associated with the TLR-mediated immune priming survival improvements. Preterm humans given bacille Calmette-Guerin (BCG) vaccination (a TLR2/4/8/9 agonist [13]) at birth experienced a non-specific reduction in neonatal mortality over neonates who did not receive BCG [25]. These studies, in conjunction with our findings, show that much remains to be learned about the specific alterations in immune function that occur following infection during a critical period of immune system adaptation and development [26]. Importantly, these data show that neonates are different from children and adults and underscore the need for age-specific investigations of immune function.
We recognize the important limitations of this retrospective cohort study, which did not include pre-post sepsis assays of immune function that have been included in previous descriptions of immunoparalysis. We acknowledge that advances in neonatal care may have affected the incidence of LOS. Hospitalization rates for sepsis in the preterm infant have not changed significantly over the last 18 years [27]. To address this concern, we performed a specific analysis on the last 5 years of our cohort (2006–2011). We found no difference in the risk of LOS following EOS (HR=0.93 [95% CI 0.71, 1.22]). We accept that patient demographics, rates of infection, and pathogen types vary between NICUs and could potentially affect some of our demographic results. We recognize that we did not have specific information on the indications for initiating therapies we used as surrogates for critical illness or the duration of mechanical ventilation or central venous catheters. However, this is the largest published cohort of VLBW neonates specifically evaluated for clinical evidence of immunoparalysis and subsequent infection risk following EOS.
5. Conclusion
In contrast to older children and adults, preterm neonates who survive early sepsis do not exhibit an increased risk of subsequent sepsis. Age-specific serial investigations are needed to determine if post-sepsis immunologic alterations are present. Our findings have important implications for the ontogeny of the preterm immune system and highlight the need for future investigation into neonatal-specific immunologic responses to infection.
Acknowledgments
We would like to thank Assistant Professor Timothy T. Cornell (University of Michigan, Department of Pediatrics, Division of Critical Care Medicine) for his careful review of the manuscript and helpful comments.
Footnotes
No sponsoring agency played a role in the study design; collection, analysis, and interpretation of the data; writing of the report; or the decision to submit the manuscript for publication.
Conflict-of-interest statement
Dr. Benjamin receives support from the United States government for his work in pediatric neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01) and is principal investigator of the Pediatric Trials Network (government contract HHSN275201000002I); from the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org); and from industry for neonatal pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Smith receives support from NICHD- 1K23HD060040-01, DHHS-1R18AE000028-01, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Cohen-Wolkowiez receives support from the U.S. government for his work in pediatric clinical pharmacology (government contract HHSN267200700051C, PI: Benjamin); from the National Institute of Child Health and Human Development (1K23HD064814-01); and from the non-profit organization Thrasher Research Foundation. He is also a consultant for Pfizer and Janssen Pharmaceuticals. Drs. Lin, Hornik, Clark, Wynn, and Cotten have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landelle C, Lepape A, Français A, Tognet E, Thizy H, Voirin N, et al. Nosocomial infection after septic shock among intensive care unit patients. Infect Control Hosp Epidemiol. 2008;29:1054–65. doi: 10.1086/591859. [DOI] [PubMed] [Google Scholar]
- 3.Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55:647–68. xi. doi: 10.1016/j.pcl.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009;29:79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 6.Leviton A, Dammann O, Engelke S, Allred E, Kuban KC, O’Shea TM, et al. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatr. 2010;99:1795–800. doi: 10.1111/j.1651-2227.2010.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012;129:e134–41. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin DK, Jr, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052–6. doi: 10.1097/inf.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–7. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 11.MacLean LD, Meakins JL, Taguchi K, Duignan JP, Dhillon KS, Gordon J. Host resistance in sepsis and trauma. Ann Surg. 1975;182:207–17. doi: 10.1097/00000658-197509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–32. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3:90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 15.Birle A, Nebe CT, Gessler P. Age-related low expression of HLA-DR molecules on monocytes of term and preterm newborns with and without signs of infection. J Perinatol. 2003;23:294–9. doi: 10.1038/sj.jp.7210906. [DOI] [PubMed] [Google Scholar]
- 16.Genel F, Atlihan F, Ozsu E, Ozbek E. Monocyte HLA-DR expression as predictor of poor outcome in neonates with late onset neonatal sepsis. J Infect. 2010;60:224–8. doi: 10.1016/j.jinf.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–41. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 19.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–37. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, et al. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn JL, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, Anas N, et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med. 2011;17:1146–56. doi: 10.2119/molmed.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics. 2011;6:273–83. doi: 10.4161/epi.6.3.14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–52. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 26.Lisciandro JG, van den Biggelaar AH. Neonatal immune function and inflammatory illnesses in later life: lessons to be learnt from the developing world? Clin Exp Allergy. 2010;40:1719–31. doi: 10.1111/j.1365-2222.2010.03629.x. [DOI] [PubMed] [Google Scholar]
- 27.Lukacs SL, Schrag SJ. Clinical sepsis in neonates and young infants, United States, 1988–2006. J Pediatr. 2012;160:960–5. e1. doi: 10.1016/j.jpeds.2011.12.023. [DOI] [PubMed] [Google Scholar]


