Summary
ALS is a devastating disease, progressing from detachment of motor nerve terminals to complete muscle paralysis and lethal respiratory failure within five years of diagnosis. Genetic studies have linked mutations in several genes to ALS, and mice bearing mutations in SOD1 recapitulate hallmark features of the disease. We have taken a novel approach to ALS by targeting and co-opting the normal retrograde signaling pathway that promotes attachment of nerve terminals to muscle. We crossed SOD1G93A mice with transgenic mice that express MuSK, a receptor tyrosine kinase required for retrograde signaling, and we used histological and behavioral assays to assess motor innervation and behavior. A threefold increase in MuSK expression delayed the onset and reduced the extent of muscle denervation, improving motor function for more than a month, without altering survival. These findings suggest that increasing MuSK activity by pharmacological means has the potential to improve motor function in ALS.
Introduction
The withdrawal of motor axons from muscle is the first sign of disease in familial and sporadic forms of ALS, portending a progressive and devastating loss of motor function that culminates in lethal muscle paralysis within five years of diagnosis (Fischer et al., 2004; Pasinelli and Brown, 2006; Schaefer et al., 2005). The mechanisms responsible for axon withdrawal are poorly understood, but the loss of neuromuscular synapses is sufficient to cause muscle paralysis and therefore central to the disease. Although the subsequent loss of motor neurons has received more attention, preventing or delaying motor neuron cell death without preserving neuromuscular synapses cannot stop disease progression. Moreover, studies designed to inhibit motor neuron cell death, either by blocking cell death pathways or providing broadly acting growth factors, have had only a modest impact on disease progression (Kostic et al., 1997; Pun et al., 2006; Sagot et al., 1995).
Dominant mutations in SOD1, TDP-43, FUS, and the recently discovered C9orf72 gene are responsible for familial forms of ALS and together represent ~17% of all cases of ALS (Chen-Plotkin et al., 2010; Kwiatkowski et al., 2009; Majounie et al., 2012; Pasinelli and Brown, 2006; Renton et al., 2011; Vance et al., 2009). The pathological hallmarks of the disease are well replicated in mice that overexpress mutant forms of SOD1 in motor neurons, providing an excellent model system for studying the pathology of ALS and identifying approaches to treat this devastating disease (Pasinelli and Brown, 2006). Although cell types other than motor neurons, including grey matter oligodendrocytes and microglia contribute to disease onset and progression (Ilieva et al., 2009; Kang et al., 2010), dominant mutations in SOD1 act largely in an autonomous manner within motor neurons, consistent with the idea that ALS is primarily, though not entirely, a disease of upper and lower motor neurons.
Skeletal muscles provide retrograde signals that promote the differentiation and stabilization of motor nerve terminals (Burden, 1998; Sanes and Lichtman, 2001). In the absence of these muscle-derived retrograde signals, developing motor axons grow aimlessly within muscle and fail to form synapses. The production of muscle-derived retrograde signals depends upon a synaptic receptor tyrosine kinase, termed MuSK, and Lrp4, a receptor for Agrin that forms a complex with MuSK (DeChiara et al., 1996; Kim et al., 2008; Weatherbee et al., 2006; Zhang et al., 2008). During normal development, Lrp4/MuSK signaling initiates neuromuscular synapse formation, whereas the subsequent stabilization of nascent synapses also requires neuronal Agrin, which binds to Lrp4 and strongly stimulates MuSK (Kim and Burden, 2008; Zhang et al., 2008; Zhang et al., 2011). In the absence of Agrin, neuromuscular synapses form, but motor axon terminals subsequently withdraw, leading to defective neuromuscular transmission and perinatal death (Gautam et al., 1996; Lin et al., 2001; Lin et al., 2005; Misgeld et al., 2005). Retrograde signaling also regulates the stability and maintenance of synapses in adult animals, as interfering with MuSK function in adult mice causes disassembly of neuromuscular synapses (Hesser et al., 2006; Kong et al., 2004). Because a failure to maintain neuromuscular synapses is central to all forms of ALS, we tested whether increasing retrograde signaling in SOD1 transgenic mice could stabilize neuromuscular synapses, delay axon withdrawal and ameliorate the symptoms of disease.
Results
Previously, we found that a modest (three-fold) increase in MuSK expression is sufficient to maintain neuromuscular synapses in agrin mutant mice, thereby preventing perinatal lethality and promoting postnatal survival (Kim and Burden, 2008). We therefore wondered whether increasing MuSK expression in a mouse model of ALS would stabilize neuromuscular synapses, delay motor axon withdrawal, and increase muscle function.
We crossed HSA::MuSK-L mice, which express three-fold more MuSK than wild-type mice, with SOD1 G93A mice, and we used histological assays to compare the rate and extent of denervation of SOD1G93A and MuSK-L; SOD1G93A mice. We stained whole mounts of the diaphragm muscle with antibodies against Synapsin to label nerve terminals and with α-bungarotoxin (α-BGT) to visualize acetylcholine receptors (AChRs) in muscle. In wild-type and MuSK-L transgenic mice, nerve terminals are apposed to AChRs, and the coincidence of Synapsin/AChR staining defines innervated synaptic sites (Figure 1). In SOD1G93A mice, axons from fast, fatigable motor neurons withdraw early in disease (Pun et al., 2006). Axons from slow, non-fatigable motor neurons are lost more slowly and compensate by sprouting and temporarily reoccupying denervated synaptic sites on fast myofibers (Pun et al., 2006). We first measured the number of synaptic sites that lacked Synapsin staining and were therefore completely denervated. In SOD1G93A mice, denervation of the diaphragm muscle became evident at P120, and the extent of denervation increased gradually over the next 20 days, reaching a maximum of ~50% at P140 (Figure 1), similar to the time course and extent of denervation observed in other muscles (Schaefer et al., 2005). In contrast, in SOD1G93A mice over-expressing MuSK, denervation of the diaphragm muscle began ten days later, and the extent of denervation remained less than 10% through P150 (Figure 1). By P160, the extent of denervation increased and approached the level found in SOD1G93A mice (Figures 1, S1). These findings indicate that MuSK over-expression protected synapses in SOD1G93A mice from denervation by delaying the onset and decreasing the extent of denervation for over 40 days.
Figure 1. MuSK over-expression delays the onset and reduces the extent of denervation in SOD1G93A mice.
a, Denervation becomes evident at P120 in SOD1G93A mice and at P130 in SOD1G93A mice over-expressing MuSK. The extent of denervation remains lower in mice over-expressing MuSK through P150. b, At P140, only 8% of synapses are denervated in SOD1G93A mice over-expressing MuSK, whereas 52% of synapses are denervated in SOD1G93A mice. c, Images of synapses from P140 mice stained with α-BGT to label AChRs and antibodies to Neurofilament and Synapsin to label motor axons and nerve terminals. Staining for Synapsin and AChRs overlap at innervated synapses, whereas Synapsin staining is absent from denervated synapses, which retain a high density of AChRs.
The remaining synapses included normally and partially innervated synapses. At partially innervated synapses, Synapsin staining incompletely overlapped the AChR-rich postsynaptic membrane, leaving patches of the postsynaptic membrane devoid of innervation (Figure 2). To determine whether MuSK expression preserved normal innervation, we measured the number of synaptic sites that were normally innervated. Figure 2 shows that the number of normally innervated synapses fell dramatically after P110 in SOD1G93A mice, reaching a plateau of 20% at P140. MuSK over-expression in SOD1G93A mice preserved normal innervation: the fall in normal innervation was delayed, and >60% of synapses remained normally innervated as late as P150. These findings show that MuSK over-expression preserves normally innervated synapses in SOD1G93A mice for over 40 days. MuSK over-expression may preserve innervation by delaying denervation, increasing reinnervation or both.
Figure 2. MuSK over-expression preserves full innervation in SOD1G93A mice.
a, In SOD1G93A mice, the number of normally innervated synapses falls to 20% by P140. In SOD1G93A mice over-expressing MuSK, nearly 80% of synapses remain normally innervated at P140. b, Normally innervated, partially innervated and denervated synapses are distinguished by differences in overlap of staining for Synapsin and AChRs.
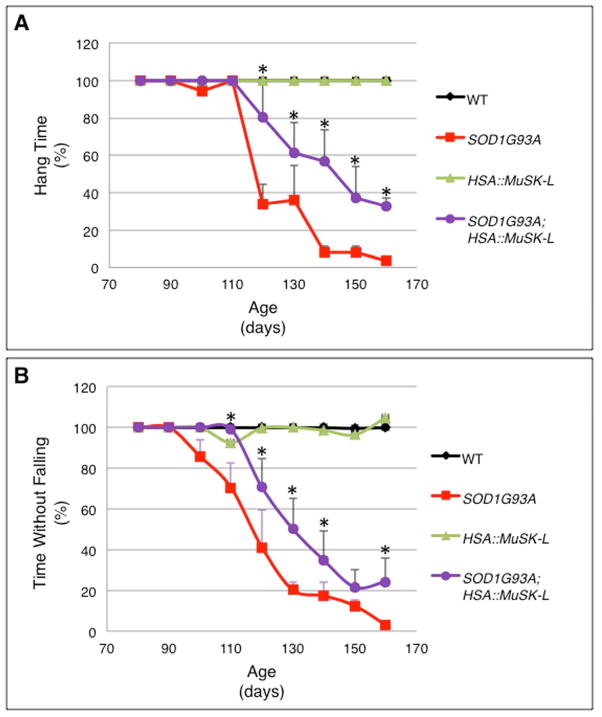
To determine whether the delay in onset and decrease in extent of denervation were accompanied by improved motor function, we used a Rota Rod and an inverted grid hanging test to measure the motor performance of SOD1G93A and MuSK-L; SOD1G93A mice. Figure 3 shows that MuSK-L; SOD1G93A mice out-performed SOD1G93A mice in both tests of motor function. MuSK-L; SOD1G93A mice clung to the inverted grid longer than SOD1G93A mice, beginning at P120 and continuing through P160, the last time point that we measured. Although the motor function of MuSK-L; SOD1G93A mice declined over time, SOD1G93A mice over-expressing MuSK out-performed SOD1G93A mice by 2–3 fold at P120 and P130 and by 5–9 fold from P140 through P160 (Figure 3a). Likewise, MuSK-L; SOD1G93A mice out-performed SOD1G93A mice on the Rota Rod from P100 through P160 (Figure 3b). Moreover, these differences in motor behavior were evident simply by monitoring the mobility of mice in their housing (Supplementary video 1). Nonetheless, the benefit from MuSK over-expression is not enduring, as the eventual withdrawal of nerve terminals and decrease in motor function leads to death at a time similar to SOD1G93A mice (Figure 4a).
Figure 3. Grip strength and Rota Rod performance are enhanced in SOD1G93A mice over-expressing MuSK.
a, The ability of SOD1G93A mice to cling to the wire grid declines rapidly after P110. MuSK over-expression increases the time that mice cling to the wire grid. b, SOD1G93A mice fall from the Rota Rod beginning at P100 whereas mice over-expressing MuSK begin to fall at P120. SOD1G93A mice over-expressing MuSK remain on the rotating bar longer than SOD1G93A mice at all subsequent times. We analyzed 25–30 mice at P80–P120, 35 mice at P130, 40 mice at P140, 32 mice at P150 and 32 at P160. Significantly different values (p<0.05) for SOD1G93A and SOD1G93A; HSA::MuSK-L mice are indicated (*).
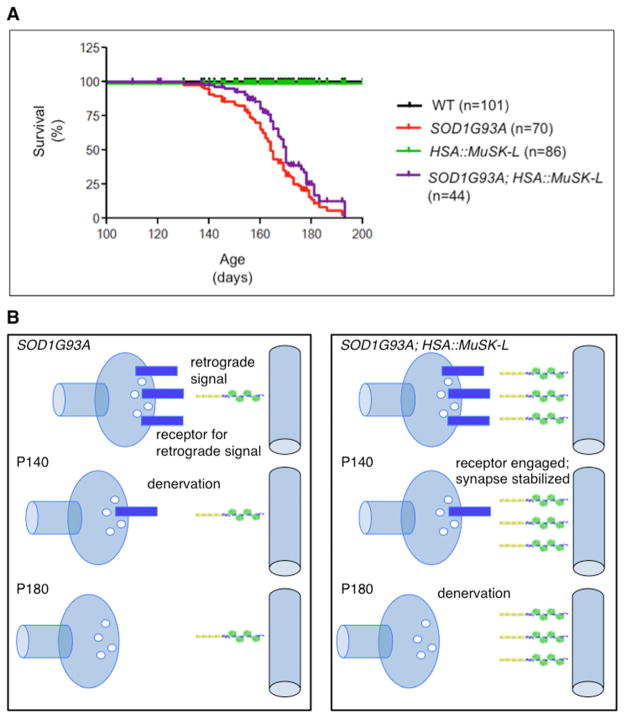
Figure 4. MuSK over-expression does not increase longevity of SOD1G93A mice.
a, The mean survival of SOD1G93A; HSA::MuSK-L mice was 164.82 ± 1.83 days, which is not significantly longer (p>0.05) than SOD1G93A mice (160.1 ± 2.09 days). b, Model for stabilizing motor nerve terminals. In SOD1G93A mice, synapses become denervated as motor neurons lose expression of receptors for muscle retrograde signals, which promote differentiation and muscle-attachment of nerve terminals. In SOD1G93A mice over-expressing MuSK, retrograde signaling is enhanced, preserving nerve terminal differentiation and muscle-attachment at times when receptor expression is reduced but not absent.
Discussion
We show that a modest increase in MuSK expression is sufficient to maintain neuromuscular synapses in SOD1G93A mice, delaying muscle denervation and improving muscle function for over 30 days. These findings indicate that the loss of motor nerve terminals can be delayed by co-opting a retrograde signaling pathway that normally functions to stimulate the differentiation and stabilization of these terminals. As such, our findings suggest a novel therapeutic approach to slow the steady decline in muscle strength and motor function in ALS. Moreover, because motor axon withdrawal is an early, characteristic and critical feature of disease in all forms of ALS, we expect that increasing MuSK activity might provide benefit in both familial and sporadic forms of ALS.
The benefit from MuSK over-expression is not permanent. The eventual withdrawal of nerve terminals and loss of motor function indicate that motor neurons ultimately become sufficiently compromised that motor terminals can no longer be stabilized by increasing MuSK signaling from muscle, suggesting that the terminals eventually lose their ability to respond to critical MuSK-dependent muscle-derived signals. Our recent studies demonstrate that Lrp4 is a critical muscle-derived retrograde signal that acts bi-directionally to coordinate presynaptic and postsynaptic differentiation (Yumoto et al., Nature, DOI 10.1038/nature11348). A failure to transport the Lrp4 receptor, or components that act downstream from this receptor, within motor axons may ultimately render motor neurons unresponsive to retrograde signaling (Figure 4b). Identification of the Lrp4 receptor might provide additional targets that can be manipulated to strengthen and prolong the response of compromised motor neurons. Alternatively, the eventual withdrawal of nerve terminals could be caused by the death of motor neurons, a late event in ALS that becomes apparent in SOD1G93A mice one to two months after denervation has reached a plateau (Fischer et al., 2004). If so, combining other therapies, aimed to promote motor neuron survival, with an increase in MuSK activity may lengthen the duration of benefit.
Like other receptor tyrosine kinases, tyrosine phosphorylation and activation of MuSK depend upon MuSK dimerization (Stiegler et al., 2009), which is stimulated by binding of Agrin to Lrp4 (Zhang et al., 2011). MuSK over-expression is sufficient to promote MuSK dimerization and increase MuSK kinase activity (Watty et al., 2000). It will be necessary, however, to find alternatives to MuSK over-expression to activate MuSK in vivo. Soluble forms of neuronal Agrin or Lrp4 may stimulate MuSK in vivo, or a screen for small molecule activators of MuSK may identify new agonists. Human single chain variable region antibodies (ScFv) to MuSK stimulate MuSK tyrosine phosphorylation and AChR clustering in cultured myotubes (Xie et al., 1997). The mechanisms by which these agonist antibodies stimulate MuSK are poorly understood, but these antibodies may provide an alternative means to activate MuSK in vivo, an approach that we are currently investigating.
Experimental Procedures
Animals
Mice overexpressing human SOD1 (B6.Cg-Tg(SOD1-G93A)1Gur/J) were purchased from Jackson Laboratory (Bar Harbor, ME) and crossed with HSA::MuSK-L transgenic mice, which were also maintained on a C57BL/6J background (24). SOD1G93A mice were genotyped by PCR (5′-CATCAGCCCTAATCCATCTGA-3′ and 5′-CGCGACTAACAATCAAAGTGA-3′). Primers for IL-2 were used as an internal control (5′-CTAGGCCACAGAATTGAAAGATCT-3′ and 5′-GTAGGTGGAAATTCTAGCATCATCC-3′).
HSA::MuSK-L transgenic mice express 3-fold more MuSK than wild-type mice (Kim and Burden, 2008). Genotyping was performed by PCR (5′-GAAGCAACCTTTCCTTCCTGAG-3′ and 5′-ATTTTCCCTGAGAGCATTGTCC-3′). All experiments were approved by the Animal Care and Use Committee at NYU School of Medicine.
Immunohistochemistry
Diaphragm muscles from adult mice were dissected and fixed for 1.5 h at room temperature in 1% formaldehyde in phosphate buffered saline (PBS). Muscles were washed three times for 15 min in PBS, incubated for 15 min with 0.1M glycine in PBS and rinsed in PBS and 0.5% Triton X-100 (PBT). Muscles were incubated for 1 h in PBT containing 4% normal goat serum (PBTG), and overlaying connective tissue was diligently removed. Axons and nerve terminals were labeled with rabbit polyclonal antibodies against Neurofilament (NF, 1:3000; Synaptic Systems, Goettingen, Germany) and Synapsin (Syn, 1:2000; Synaptic Systems, Goettingen, Germany) overnight at 4° C in PBTG. After three 1 h washes in PBT, muscles were incubated at 4° C overnight with Alexa-488 goat anti-rabbit IgG (1:500; Invitrogen) and Alexa-594-conjugated-α-BGT (1:1000 in PBTG; Invitrogen, San Diego, CA) to label AChRs. Muscles were washed three times with PBS over 1 h, post fixed (1% formaldehyde in PBS) for 10 min, rinsed in PBS and mounted in Vectashield under a glass coverslip (Vector Labs, Burlingame, CA).
Diaphragm muscles from P90 to P160 wild-type (WT), HSA::MuSK-L, SOD1G93A and SOD1G93A; HSA::MuSK-L mice were stained with Alexa-594-conjugated-α-BGT and antibodies to NF and Syn. Confocal images of diaphragm muscles were captured on a Zeiss 510 confocal laser scanning microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) using a 40X PlanApo objective. Images were compiled into a reconstructed image, and the number of normally innervated, partially innervated and fully denervated synapses was quantified. For each experiment, at least 100 synaptic sites were counted for each genotype, and experiments were performed at least three times.
Survival
To measure longevity, we followed the survival of 300 animals up to 10 months: WT, n=101; HSA::MuSK-L, n=86; SOD1G93A, n=70; and SOD1G93A; HSA::MuSK-L, n=44. Kaplan Meier survival curves were generated with GraphPad Prism software.
Behavioral tests
Motor function was assessed once per week on a Rota Rod (EZ-Rod 3.05; Accuscan Instruments, Inc., Columbus, Ohio). In each experiment. we measured the running performance of at least 6 animals of each genotype from P80 to P160. Mice were placed on a Rota Rod (3.0 cm rotating cylinder) rotating at 1 rpm, and the speed of rotation was gradually increased from 1 to 12 rpm over the course of 40 sec and then maintained at 12 rpm for a maximum of five min. We recorded the time that mice remained on the Rota Rod. Wild-type and HSA::MuSK-L mice routinely ran for the full five min, yielding an assigned value of 100%; the values for other mice were expressed relative to wild-type mice.
Motor fatigue was assessed using an inverted grid hanging test (Kaja et al., 2007). Individual mice were placed in the center of a wire grid, which was mounted 80cm above a laboratory bench. After gently inverting the grid, we maintained the grid in an inverted position for a maximum of 60 sec and recorded the time that mice remained attached to the grid. Wild-type mice routinely remained attached to the grid for the duration and were assigned a value of 100%; the values for other mice were expressed relative to wild-type mice.
Statistical analysis
All data are expressed as group means ± SEM. For the Kaplan-Meier survival analysis, the log-rank test was used, and survival curves were considered significantly different at P <0.05. When appropriate, the one-way ANOVA followed by a Newman-Keuls Multiple Comparison’s post-hoc analysis were used to test for differences between samples, and data were considered significantly different at P <0.05.
hSOD1 copy number assessment
To learn whether SOD1G93A copy number changed during the course of these experiments, we measured copy number using a real time PCR assay. Genomic DNA was extracted from tails using a Qiagen DNA extraction kit (QIAGEN, Valencia, CA). Brilliant® II SYBR Green QPCR Master Mix reagent (Stratagene, Santa Clara, CA) was used for the real-time amplification of DNA, ranging from 0.15 to 20 ng. Following heating at 50° C for 2 min and 95° C for 10 min, DNA was amplified by 40 cycles of 95° C for 15 sec and 60° C for 1 min., as suggested by the Jackson Laboratories (Bar Harbor, ME). We found that the copy number did not change during the course of these experiments (Figure S2).
Supplementary Material
Highlights.
An increase in MuSK expression delays the onset of muscle denervation in ALS mice.
MuSK expression reduces the extent of denervation in ALS mice.
MuSK expression improves motor function and behavior in ALS mice.
MuSK agonists have the potential to improve motor function in ALS.
Acknowledgments
We thank Martin Raff, Dan Littman and Ruth Lehmann for their comments on the manuscript, Andrea Gomez for drawing the graphical abstract and Mary Jean Sunshine and personnel in the transgenic mouse core, who are supported by a NYU Cancer Institute Center Support Grant (NIH/NCI 5P30CA16087-31). This work was supported by a grant from the Robert Packard Center for ALS Research and a postdoctoral fellowship to M.P.G. from the Ministerio de Educacion y Ciencia of the Spanish Government. This study is dedicated to the memory of Tony Judt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burden SJ. The formation of neuromuscular synapses. Genes Dev. 1998;12:133–148. doi: 10.1101/gad.12.2.133. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nature reviews Neurology. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Experimental neurology. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Hesser BA, Henschel O, Witzemann V. Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Molecular and cellular neurosciences. 2006;31:470–480. doi: 10.1016/j.mcn.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. The Journal of cell biology. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, van de Ven RC, van Dijk JG, Verschuuren JJ, Arahata K, Frants RR, Ferrari MD, van den Maagdenberg AM, Plomp JJ. Severely impaired neuromuscular synaptic transmission causes muscle weakness in the Cacna1a-mutant mouse rolling Nagoya. The European journal of neuroscience. 2007;25:2009–2020. doi: 10.1111/j.1460-9568.2007.05438.x. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nature neuroscience. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XC, Barzaghi P, Ruegg MA. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO reports. 2004;5:183–188. doi: 10.1038/sj.embor.7400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Chio A, Restagno G, Nicolaou N, Simon-Sanchez J, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet neurology. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature reviews Neuroscience. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nature neuroscience. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot Y, Dubois-Dauphin M, Tan SA, de Bilbao F, Aebischer P, Martinou JC, Kato AC. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:7727–7733. doi: 10.1523/JNEUROSCI.15-11-07727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature reviews Neuroscience. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. The Journal of comparative neurology. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- Stiegler AL, Burden SJ, Hubbard SR. Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. Journal of molecular biology. 2009;393:1–9. doi: 10.1016/j.jmb.2009.07.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watty A, Neubauer G, Dreger M, Zimmer M, Wilm M, Burden SJ. The in vitro and in vivo phosphotyrosine map of activated MuSK. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4585–4590. doi: 10.1073/pnas.080061997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- Xie MH, Yuan J, Adams C, Gurney A. Direct demonstration of MuSK involvement in acetylcholine receptor clustering through identification of agonist ScFv. Nature biotechnology. 1997;15:768–771. doi: 10.1038/nbt0897-768. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Coldefy AS, Hubbard SR, Burden SJ. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK) The Journal of biological chemistry. 2011;286:40624–40630. doi: 10.1074/jbc.M111.279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.