Abstract
Objectives
Individuals with rheumatoid arthritis (RA) are at a greater risk for cardiovascular disease (CVD). Vitamin D deficiency is a potential risk factor for CVD and metabolic syndrome. Since patients with RA have a high prevalence of vitamin D deficiency, we investigated the association of vitamin D levels with cardiometabolic risk factors in a cohort of RA patients with no prior history of CVD.
Methods
Serum 25(OH)D levels were measured among RA patients enrolled in a cohort study of subclinical CVD. The cross-sectional associations of 25(OH)D level with traditional CVD risk factors, such as insulin resistance [estimated using the Homeostatic Model Assessment (HOMA)], adiopokines, markers of systemic inflammation and endothelial activation were explored, adjusting for pertinent sociodemographic, lifestyle, and RA characteristics.
Results
Among 179 RA patients, 73 (41%) had a 25(OH)D level <30ng/mL. Only 23 patients (13%) had a 25(OH)D level ≥45ng/mL. After adjusting for demographics and BMI, 25(OH)D remained significantly associated with HDL and inversely associated with HOMA-IR, fibrinogen, E-selectin, and s-ICAM. Significant associations with HDL, E-selectin, and s-ICAM were maintained after adjusting for DAS28 and autoantibody status. These associations were similar between groups subdivided by gender, ethnicity, body mass index, DAS28 level and autoantibody status.
Conclusions
These data suggest that vitamin D deficiency is common in RA and may be independently associated with several cardiometabolic intermediates in this population.
Rheumatoid arthritis (RA) is a systemic inflammatory arthritis associated with increased morbidity and mortality, primarily related to greater atherosclerotic burden. Compared to non-RA controls, patients with RA are at a 1.5–3 fold increased risk of cardiovascular events (1, 2). The risk factors contributing to this increased propensity towards atherosclerosis in RA are poorly understood and have been a focus of expanding research. RA specific risk factors, such as chronic systemic inflammation (3), altered lipid function (4) and change in body composition (5) have been identified as few of the possible mechanisms driving the increased cardiovascular risk. Thus, identification of these and other risk factors is critical as their modification may result in improved long term outcomes in the RA population.
Vitamin D deficiency has been associated with atherosclerosis and cardiometabolic risk factors, such as insulin resistance (6) hypertension (7) metabolic syndrome (8) and cardiovascular events (9, 10) in cross-sectional analyses of non-RA populations. In a meta-analysis of 28 studies, the highest levels of serum 25(OH)D were associated with a 43% reduction in cardiometabolic disorders, (ie CVD, DM and metabolic syndrome) [OR 0.57, 95% CI 0.48–0.68] (11). However, not all studies have confirmed these associations (12).
Vitamin D is a pleiotropic steroid molecule and exerts its physiological effects through the ubiquitous vitamin D receptor, a nuclear hormone receptor (13). Activated vitamin D is now implicated in regulating several genes with diverse biological functions (14, 15) which may explain its possible epidemiological associations with diverse chronic diseases, such as common cancers, cardiovascular disease (CVD) and autoimmune disorders (16, 17). Vitamin D also has known immunomodulatory effects (18) and its possible role in initiation and exacerbation of autoimmune diseases is an area of ongoing scientific study.
Low levels of vitamin D are noted to be common in RA, with several studies indicating greater than 50% prevalence of suboptimal vitamin D levels in this population (19, 20). Our data suggest that up to 60% of RA patients have suboptimal vitamin D levels in the mid-Atlantic region (21).
It is plausible that vitamin D deficiency in RA may pose an independent risk of cardiometabolic disease and thus may provide a modifiable target to potentially reduce cardiovascular disease burden in this population. However, any possible link between vitamin D deficiency and cardiometabolic risk factors in RA has not been evaluated. We thus investigated the relationship of vitamin D levels with cardiometabolic risk factors in a cohort of RA patients without established CVD and hypothesized that low vitamin D levels would be associated with greater cardiometabolic burden this population.
PATIENTS AND METHODS
Participants and Enrollment
Study subjects were participants in the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis (ESCAPE RA) Study, a cohort study investigating the prevalence, progression, and risk factors for subclinical CVD in RA patients described previously (22). Participants were 45 to 84 years of age at enrollment and met the American College of Rheumatology (formerly the American Rheumatism Association) 1987 classification criteria for RA (23). Exclusion criteria were known prior CVD defined as a prior history of self-reported physician-diagnosed myocardial infarction, heart failure, coronary artery revascularization, angioplasty, peripheral vascular disease or procedures (excluding varicose vein procedures), implanted pacemaker or defibrillator devices, and current atrial fibrillation. The study was approved by the Institutional Review Board of the Johns Hopkins Hospital, with all subjects providing written informed consent.
Assessments
Clinical data and laboratory assessments derive from the second ESCAPE RA study visit, which occurred a median of 20 months after the baseline visit. Among the 197 participants enrolled at baseline, 186 completed the second study visit (a 94% retention rate).
Sociodemographic, Lifestyle, and Body Composition Assessments
Age, gender, race/ethnicity, educational attainment, and current and past smoking were assessed from patient-self report. Physical activity was assessed with the 7-Day Physical Activity Recall Questionnaire (24) with the weekly total of physical activity for intentional exercise activities (moderate or brisk walking for exercise, and moderate or vigorous individual or team sports and conditioning activities) calculated for each participant. Diabetes was defined as a fasting serum glucose>126 mg/dL or use of anti-diabetic agents. Anthropometric measures (height, weight, body mass, index (BMI), and waist and hip circumferences) were assessed as previously described (22).
RA Disease Characteristics
Forty-four joints were examined for swelling, tenderness, deformity, and surgical replacement or fusion by a single trained assessor. RA disease duration was assessed by patient self-report from the date of diagnosis. RA disease activity was calculated using the Disease Activity Score for 28 joints with CRP (DAS28-CRP) (25). Current and past use of glucocorticoids, biologic and non-biologic disease modifying antirheumatic drugs (DMARDs) was queried by detailed examiner-administered questionnaires.
The 21- item Stanford Health Assessment Questionnaire (HAQ) (26) was used to assess disability related to common activities. Single view, anterior-posterior radiographs of the hands and feet were obtained and scored using the Sharp-van der Heijde (SvH) method (27) by a single, trained radiologist blinded to patient characteristics.
Cardiovascular Risk Factor Assessments
Diabetes was defined as a fasting serum glucose>126 mg/dL or use of anti-diabetic agents. Insulin resistance was estimated from fasting serum glucose and insulin using the Homeostatic Model Assessment 2 (28). Blood pressure was measured three times in the seated position using an automated device after a five minute period of rest in a quiet room. The average of the last two measurements was used in the analysis. Cuff size was determined based on arm circumference per standardized protocol.
Laboratory Assessments
Fasting serum and plasma samples were collected on the day of body composition analysis and stored at −70 degrees C. 25(OH)D levels were measured using the standardized Diasorin radioimmunoassay. Vitamin D deficiency was defined as 25(OH)D level < 30 ng/ml, per expert consensus (29). C-reactive protein (CRP), fibrinogen, homocysteine, E-selectin, s-ICAM-1, adiponectin, resistin, leptin, and PAI-1 were measured at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT) as previously described (30). Fasting lipids were measured at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, MN). LDL-cholesterol was estimated in plasma specimens having a triglyceride value <400 mg/dL using the formula of Friedewald (31). Rheumatoid factor (RF) was assessed by ELISA, with seropositivity defined ≥ 40 units. Anti-CCP antibody was assessed by ELISA, with seropositivity defined ≥ 60 units.
Statistical Analysis
The distributions of all variables were examined. Means and standard deviations were calculated for all normally distributed continuous variables and medians and interquartile ranges were calculated for continuous variables that were not normally distributed. For categorical variables, counts and percentages were calculated. The associations of patient characteristics were explored according to levels of 25(OH)D categorized as deficient (<30ng/mL), low sufficient (30–44.99 ng/mL), and high sufficient (≥45ng/mL) using ANOVA for normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed continuous variables, and the Chi-square or Fisher’s exact test (as appropriate) for dichotomous variables. Ordinary linear regression was used to model the association of sociodemographic, lifestyle, body composition, and RA disease and treatment characteristics with 25(OH)D level, with β coefficients and their associated 95% confidence intervals (CIs) and p-values calculated. Characteristics associated with 25(OH)D in multivariable modeling were retained as adjustment covariates in subsequent models including cardiometabolic risk factors. The Shapiro-Wilk test confirmed that assumptions of normality were met for all multivariable models in which non-transformed 25(OH)D was the dependent variable.
The associations of 25(OH)D with cardiometabolic risk factors were explored first by calculating Spearman correlation coefficients. Next, the changes in 25(OH)D level associated with each unit increase in cardiometabolic risk factors were calculated for each risk factor using ordinary linear regression, first in simple models with the cardiometabolic risk factor as the only covariate (with variables transformed to normality as required). Next, multivariable models were constructed adjusting for the confounding covariates identified from preliminary modeling (above). Non-linear associations of cardiometabolic risk factors with 25(OH)D level were explored by comparing adjusted mean risk factor levels across the extent of 25(OH)D categorized into quartiles, also using multivariable linear regression. Heterogeneity in the associations of cardiometabolic risk factors with 25(OH)D according to strata of demographic (gender, ethnicity), BMI (dichotomized at the median), seropositivity for RF or anti-CCP, and DAS28 (dichotomized at the median) were explored using ANCOVA.
All statistical calculations were performed using Intercooled Stata 10 (StataCorp, College Station, TX). In all tests, a two-tailed α of 0.05 was defined as the level of statistical significance.
RESULTS
Among the 186 ESCAPE RA participants who completed their second study visit, 179 had 25(OH)D levels measured. Of these, 73 (41%) were vitamin D deficient (25(OH)D<30 ng/mL) and only 23 (13%) had a 25(OH)D level ≥ 45 ng/mL. The mean 25(OH)D level was 34±10 ng/mL. Participant characteristics according to 25(OH)D status are summarized in Table 1. In unadjusted comparisons, compared to RA patients with 25(OH)D levels ≥ 45 ng/mL, those with deficient levels (25(OH)D<30 ng/ml) were significantly more likely to be non-Caucasian (91 vs. 79%, respectively; p=0.019) and had a higher mean BMI (by an average of 1.6 units; p=0.059). Age, sex, education, reported exercise, smoking did not differ by 25(OH)D status.
Table 1.
Participant Characteristics According to 25-OH Vitamin D Status
| Total (n = 179) | 25(OH)D < 30 (n = 73) |
25(OH)D 30 – 44.99 (n = 83) |
25(OH)D ≥ 45 (n = 23) |
p | |
|---|---|---|---|---|---|
| Age, years | 61 ± 8 | 61 ± 8 | 62 ± 9 | 61 ± 8 | 0.63 |
| Male, n (%) | 69 (39) | 23 (32) | 35 (42) | 11 (48) | 0.24 |
| Caucasian, n (%) | 157 (88) | 58 (79) | 78 (94) | 21 (91) | 0.019 |
| Any college, n (%) | 132 (74) | 56 (77) | 61 (74) | 15 (65) | 0.55 |
| Body mass index. kg/m2 | 28.6 ± 5.3 | 29.1 ± 5.5 | 28.6 ± 5.2 | 27.5 ± 5.0 | 0.059 |
| Waist circumference, cm | 95 (85 – 106) | 93 (86 – 108) | 95 (84 – 106) | 95 (82 – 103) | 0.78 |
| Habitual exercise, min/day | 34 (4 – 75) | 33 (5 – 69) | 30 (0 – 64) | 47 (9 – 120) | 0.24 |
| Diabetes, n (%) | 11 (6.2) | 4 (6) | 5 (6) | 2 (9) | 0.82 |
| Ever smoking, n (%) | 100 (56) | 42 (58) | 47 (57) | 11 (48) | 0.67 |
| Current smoking, n (%) | 21 (12) | 13 (18) | 5 (6) | 3 (13) | 0.75 |
| Hormone replacement; (women), n (%) | 16 (15) | 6 (12) | 7 (15) | 3 (25) | 0.54 |
| RA duration, years | 10.5 (6 – 18.5) | 10 (7 – 18) | 11 (6 – 20) | 11 (5 – 19) | 0.94 |
| RF or anti-CCP seropositivity, n (%) | 137 (77) | 60 (82) | 63 (76) | 14 (64) | 0.066 |
| Any shared epitope alleles, n (%) | 124 (70) | 50 (69) | 59 (72) | 15 (65) | 0.70 |
| DAS28-CRP | 3.2 ± 1.1 | 3.4 ± 1.1 | 3.1 ± 1.0 | 2.8 ± 0.9 | 0.015 |
| Swollen joints (0–42) | 3 (2 – 6) | 4 (2 – 9) | 3 (2 – 6) | 3 (0 – 6) | 0.026 |
| Tender joints (0–44) | 5 (2 – 13) | 6 (2 – 14) | 5 (2 – 12) | 2 (0 – 14) | 0.062 |
| HAQ (0 – 3) | 0.75 (0.13–1.50) | 0.75 (0.13–1.63) | 0.75 (0.13–1.25) | 0.25 (0–1.50) | 0.27 |
| Baseline total SvH score | 43 (15 – 116) | 55 (21 – 123) | 33 (13 – 85) | 38 (12 – 121) | 0.31 |
| Duration of morning stiffness, minutes | 15 (4 – 30) | 15 (3 – 45) | 15 (3 – 30) | 15 (5 – 45) | 0.66 |
| Pain (100mm VAS) | 21 (6 – 46) | 20 (9 – 43) | 22 (5 – 49) | 20 (4 – 50) | 0.68 |
| Current prednisone, n (%) | 67 (39) | 27 (39) | 30 (37) | 10 (43) | 0.86 |
| Cumulative prednisone, grams | 4.6 (0.2 – 11.3) | 4.0 (0.9 – 8.3) | 5.4 (0.1 – 10.6) | 4.3 (0 – 11.7) | 0.76 |
| Current non-biologic DMARDs, n (%) | 151 (87) | 61 (87) | 70 (86) | 20 (87) | 0.99 |
| Current biologic DMARDs, n (%) | 84 (48) | 33 (47) | 39 (48) | 12 (52) | 0.92 |
Values are depicted as mean ± SD or median (interquartile range) unless otherwise noted
RA=rheumatoid arthritis; RF=rheumatoid factor; CCP=cyclic citrullinated peptide; DAS=disease activity score; CRP=C-reactive protein; HAQ=health assessment questionnaire; SvH=Sharp van der Heidje Score; VAS=visual analogue scale; DMARD=disease modifying anti-rheumatic drug
Among RA characteristics, those with deficient 25(OH)D levels were more likely to be RF or anti-CCP seropositive compared to those with 25(OH)D levels ≥ 45 ng/mL (82 vs. 64%, respectively; p=0.066). Mean DAS28 was significantly higher among participants with deficient 25(OH)D compared to the group with a level ≥ 45 ng/mL (3.4 vs. 2.8 units, respectively; p=0.015), with differences reflected in both higher median swollen and tender joint counts among those with deficient 25(OH)D. Other RA disease and treatment characteristics were not associated with 25(OH)D level. In a multivariable model also adjusting for season of assessment, lower 25(OH)D levels were significantly associated with non-Caucasian ethnicity, higher BMI, RF or anti-CCP seropositivity, and DAS28 (data not shown). These covariates were retained in subsequent multivariable models.
Association of 25(OH)D with Cardiometabolic Risk Factors
The distributions of selected cardiometabolic risk factors are summarized in Table 2. Among these, increasing 25(OH)D demonstrated modest yet statistically significant biologic correlation with higher HDL-C, and inverse correlation with LDL-C, HOMA-IR, CRP, fibrinogen, E-selectin, and s-ICAM (Table 3). Trends to significance were observed in the positive correlation of 25(OH)D with adiponectin and the inverse correlation of 25(OH)D with leptin.
Table 2.
Cardiometabolic Risk Factors Among 179 RA Patients
| Total (n = 179) | |
|---|---|
| Systolic blood pressure, mm Hg | 131 (117 – 145) |
| Diastolic blood pressure, mm Hg | 78 (71 – 84) |
| LDL-C, mg/dL | 107 (85 – 125) |
| HDL-C, mg/dL | 53 (40 – 70) |
| Triglycerides, mg/dL | 100 (69 – 142) |
| Fasting glucose, mg/dL | 91 (85 – 98) |
| HOMA-IR | 0.80 (0.50 – 1.40) |
| CRP, mg/L | 2.8 (1.0 – 7.20) |
| Fibrinogen | 364 (296 – 439) |
| Homocysteine | 9.7 (8.0 – 12.1) |
| E-selectin | 49 (38 – 68) |
| s-ICAM | 296 (247 – 361) |
| Adiponectin, mg/L | 35 (21 – 46) |
| Resistin, ng/mL | 14 (11 – 18) |
| Leptin, ng/mL | 15 (7 – 30) |
| PAI-1, ng/mL | 16 (11 – 23) |
Values depicted are median (interquartile range)
RA=rheumatoid arthritis; LDL=low density lipoprotein; HDL-high density lipoprotein; HOMA=homeostatic model assessment; CRP=C-reactive protein; PAI=plasminogen activator inhibitor
Table 3.
Crude and Adjusted Associations of Cardiometabolic Risk Factors with Vitamin D Levels (ng/mL)
| Spearman Correlation | Linear Regression Models: Change in 25(OH)D per unit change in cardiometabolic risk factor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With 25(OH)D | Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | ||||||
| r | p | β | p | β | p | β | p | β | p | |
| Systolic blood pressure | −0.024 | ns | ||||||||
| Diastolic blood pressure | 0.006 | ns | ||||||||
| LDL-C | −0.167 | 0.028 | −0.050 | 0.049 | −0.040 | 0.13 | −0.038 | 0.14 | −0.046 | 0.066 |
| HDL-C | 0.157 | 0.037 | 0.094 | 0.021 | 0.136 | 0.002 | 0.110 | 0.018 | 0.097 | 0.035 |
| log triglycerides | −0.001 | ns | ||||||||
| Fasting glucose | −0.118 | ns | ||||||||
| log HOMA-IR | −0.202 | 0.007 | −3.10 | 0.006 | −3.25 | 0.003 | −2.62 | 0.029 | −1.75 | 0.17 |
| log CRP | −0.177 | 0.018 | −1.34 | 0.023 | −1.29 | 0.029 | −1.10 | 0.061 | −0.43 | 0.51 |
| log fibrinogen | −0.231 | 0.002 | −8.63 | 0.004 | −8.47 | 0.006 | −7.32 | 0.018 | −4.95 | 0.12 |
| log homocysteine | 0.028 | ns | ||||||||
| log E-selectin | −0.162 | 0.030 | −4.73 | 0.001 | −4.48 | 0.002 | −4.40 | 0.003 | −3.52 | 0.023 |
| log s-ICAM | −0.174 | 0.020 | −6.69 | 0.007 | −7.52 | 0.002 | −7.24 | 0.003 | −5.83 | 0.020 |
| log adiponectin | 0.143 | 0.061 | 2.63 | 0.042 | 2.85 | 0.041 | 2.41 | 0.089 | 2.20 | 0.11 |
| log resistin | 0.020 | ns | ||||||||
| log leptin | −0.127 | 0.092 | −1.03 | 0.18 | −0.66 | 0.44 | 1.49 | 0.21 | 1.31 | 0.26 |
| log PAI-1 | −0.050 | ns | ||||||||
Model 1: adjusted for gender, ethnicity, and season
Model 2: adjusted for Model 1 covariates + body mass index
Model 3: adjusted for Model 2 covariates + RF/anti-CCP seropositivity status and DAS28
After adjusting for gender, ethnicity, and season of assessment (Table 3, Adjusted Model 1), 25(OH)D remained significantly associated with HDL and adiponectin, and inversely associated with HOMA-IR, CRP, fibrinogen, E-selectin, and s-ICAM. The magnitude of the association of 25(OH)D with CRP and adiponectin was reduced, and statistical significance lost, after adjusting for BMI (Table 3, Adjusted Model 2). Subsequent adjustment for RF or anti-CCP seropositivity and DAS28 further reduced the magnitude of association of 25(OH)D with all of the cardiometabolic risk factors; however, statistically significant associations were maintained for HDL-C, E-selectin, and s-ICAM (Table 3, Adjusted Model 3).
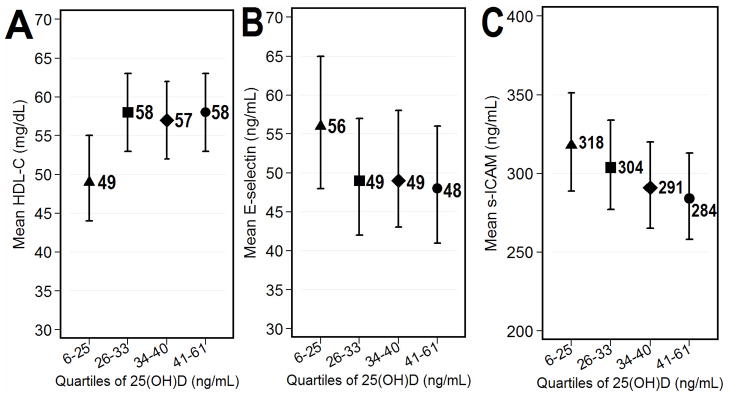
The associations of 25(OH)D level with HDL and E-selectin were not linear across the extent of 25(OH)D. The adjusted mean HDL was 9 mg/dL lower for participants in the lowest quartile of 25(OH)D (6 – 25 ng/mL) compared to those in the upper three quartiles (49 vs. 58 mg/dL, respectively; a difference of 16% (p=0.004)) (Figure 1, Panel A). However, the adjusted mean HDL was not significantly different between those in the upper three quartiles. The adjusted mean E-selectin was 7 ng/mL higher for participants in the lowest quartile of 25(OH)D compared to those in the upper three quartiles (56 vs. 49 ng/mL, respectively; a difference of 20% (p=0.072)) (Figure 1, Panel B). However, the adjusted mean E-selectin was not significantly different between those in the upper three quartiles. In contrast, the adjusted mean s-ICAM decreased linearly with higher 25(OH)D levels by an average of 11 ng/mL per quartile increase in 25(OH)D (p=0.020 for the linear trend across 25(OH)D; Figure 1, Panel C).
Figure 1.
Graphs depict adjusted means and 95% confidence intervals for cardiometabolic according to quartiles of 25(OH)D. Analyses were adjusted for gender, ethnicity, body mass index, season, rheumatoid factor or anti-CCP seropositivity, and DAS28. In panel A, the p-value for the comparison of the adjusted mean HDL-C between the lowest quartile of 25(OH)D and the combined upper three quartiles was 0.004. In panel B, the p-value for the comparison of the adjusted mean E-selectin between the lowest quartile of 25(OH)D and the combined upper three quartiles was 0.072. In panel C, the p-value for the linear trend in adjusted mean s-ICAM over the extent of 25(OH)D was 0.020.
Differences in the Association of 25(OH)D with Cardiometabolic Risk Factors According to Strata of Patient Characteristics
There was no evidence of significant heterogeneity in the association of 25(OH)D level with cardiometabolic risk factors between groups stratified by gender, ethnicity, BMI, or DAS28 level (data not shown).
DISCUSSION
In this study, we observed a significant cross-sectional association between lower vitamin D levels and several cardiometabolic risk factors in a cohort of RA patients. Specifically, 25(OH)D had a significant inverse association with E-selectin and s-ICAM and a positive association with HDL even after adjusting for several potential confounders, including RA disease activity. These findings are novel and this is the first report implicating vitamin D deficiency as a possible cardiometabolic risk factor in RA. The association of HDL and E-selectin levels with quartiles of 25(OH)D was noted to be non-linear whereas the relationship between s-ICAM and 25(OH)D was linear.
We also observed a high prevalence (41%) of vitamin D deficiency in this cohort, consistent with previous publications (19, 20). Also consistent with other reports (32, 33), vitamin D deficiency was higher among non-Caucasian and obese individuals among our cohort of RA patients. Significant associations of 25(OH)D levels with insulin resistance, LDL and adiponectin were attenuated after adjusting for demographic confounders, BMI and RA disease activity. Thus, the association of 25(OH)D levels with these metabolic risk factors could be partially explained by BMI and RA disease activity. Similar confounding was observed in a cohort of 181 SLE patients, where lower levels of vitamin D were significantly associated with higher diastolic BP, LDL, cholesterol, fibrinogen, self reported hypertension and diabetes. Most of these associations, however, became insignificant after adjustment for BMI (34).
Our findings in RA patients are consistent with previous reports of non-RA populations suggesting a relationship between low 25(OH)D concentration and prevalent cardiometabolic outcomes (35, 36). For example, analysis of NHANES III data showed that patients with the lowest 25(OH)D levels had a significantly higher prevalence of diabetes mellitus, hypertension and insulin resistance (7) compared to those with higher levels. Inverse association between vitamin D levels and cardiovascular events has also been reported in several cross-sectional (37–39) and prospective studies, including incident cardiovascular disease in the Framingham Offspring study (9, 40). However, other studies do not support this association and data from the few vitamin D supplementation studies and cardiovascular outcomes are inconclusive (41). Moreover, the long term safety of high dose vitamin D supplementation is an area of ongoing research.
In our study, we did not evaluate the association of 25(OH)D levels with cardiovascular events but explored its association with traditional risk factors that predispose to future CVD. Understanding these potential associations would be critical in our ability to modify these risks and possibly improve long term cardiovascular outcomes in RA.
There are several possible mechanisms governing the association between 25(OH)D and cardiometabolic risk factors. For one, pancreatic beta-cell function has been shown to be impaired at low levels of 25(OH)D (35). In addition, activated vitamin D regulates the renin-angiotensin sytem (42) and suppresses proliferation of vascular smooth muscles (43), potentially contributing to hypertension when vitamin D levels are suppressed. E-selectin and s-ICAM are vascular adhesion molecules that have been implicated in pathogenesis of atherosclerosis (44, 45). In vitro data suggest that activated vitamin D analogs modulate the expression of vascular adhesion molecules (46, 47), another mechanism through which vitamin D deficiency may potentiate atherogenesis. Our study is the first to report an inverse association between low levels of vitamin D and E-selectin/s-ICAM in RA.
Adiponectin is one of the several adipocytokines secreted by adipose tissue. Low adiponectin levels may be an independent risk factor for insulin resistance (48, 49) and atherosclerosis (50, 51). An association between vitamin D deficiency and low adiponectin levels was observed in individuals with abnormal glucose tolerance, independent of adiposity (52), suggesting that adiponectin may be a link between vitamin D and insulin resistance. We report a similar relation between 25(OH)D and adiponectin levels in RA; that is low levels of 25(OH)D correlated with low adiponectin levels. This association became insignificant in our cohort after controlling for BMI. However, these preliminary data point towards a possible relationship between adipokines and vitamin D in RA, which should be further explored.
Vitamin D deficiency in our cohort was associated with autoantibody seropositivity and increased RA disease activity as measured with DAS28-CRP, and its components of tender and swollen joint counts. This is consistent with previous publication(19) in which anti-CCP seropositivity was associated with an approximately 2-fold increased risk of vitamin D deficiency in a cohort of US veterans with RA. Similarly, inverse associations of 25(OH)D levels with DAS28-CRP and tender joint counts have also been shown in previous studies (53, 54).
Among study limitations, our observational data are hypothesis generating and these findings should be considered preliminary. Being cross-sectional, the temporality of association and causality cannot be established, as we are not able to determine whether vitamin D deficiency is a risk factor for metabolic syndrome in RA or whether the reverse may be true. Vitamin D deficiency is known to be associated with obesity, sedentary life style and may be a surrogate marker for overall poor health. We attempted to account for these established confounders by adjusting accordingly in our models; however, there remains the possibility for residual confounding due to measurement error or unaccounted for confounders.
In conclusion, we observed significant associations between low 25(OH)D levels and higher levels of several cardiometabolic risk factors, as well as increased disease activity, in a cohort of RA patients. These preliminary findings may have broad clinical implications in management of RA. However, further studies are required to validate these observations and to determine if vitamin D deficiency, via its association with cardiometabolic risk factors translates to clinical cardiovascular end points in patients with RA.
Significance and Innovation.
Our study is the first to explore the relationship between vitamin D levels and cardiometabolic risk factors in individuals with rheumatoid arthritis (RA).
Our data suggests that vitamin D deficiency in individuals with RA may be independently associated with several cardiometabolic risk factors.
These are hypothesis generating data, and suggest the need for further evaluation of possible role of vitamin D as a novel therapeutic target to reduce cardiovascular risk in RA.
Acknowledgments
This work is supported by Grant Numbers AR050026-01 (JMB) and 1K23AR054112-01 (JTG) from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases; a Clinical Investigator Fellowship Award from the Research and Education Foundation of the American College of Rheumatology (JTG); and the Johns Hopkins Bayview Medical Center General Clinical Research Center (Grant Number M01RR02719) as well as by The Johns Hopkins Arthritis Discovery Fund (UJH).
This work has no financial support or other benefits from commercial sources; there are no financial interests of any of the authors which could create a potential conflict of interest.
We would like to thank the Johns Hopkins Bayview Medical Center General Clinical Research Center and staff, the field center of the Baltimore MESA cohort, and the MESA Coordinating Center at the University of Washington, Seattle. We are indebted to the dedication and hard work of the ESCAPE RA Staff: Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, Shawn Franckowiak, and Brandy Miles and to the participants in the ESCAPE RA study who graciously agreed to take part in this research. Drs. Clifton Bingham III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Andrea Marx, Howard Hauptman, Achini Perera, Peter Holt, Alan Matsumoto, Megan Clowse, Gordon Lam and others generously recommended their patients for this study.
References
- 1.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol. 2003;30:36–40. [PubMed] [Google Scholar]
- 3.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–63. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 4.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–7. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giles JT, Allison M, Blumenthal RS, Post W, Gelber AC, Petri M, et al. Abdominal adiposity in rheumatoid arthritis: Association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum. 2010;62:3173–82. doi: 10.1002/art.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, et al. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139:329–34. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the united states: Data from the third national health and nutrition examination survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 8.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, et al. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas. 2010;65:225–36. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 13.Bikle DD. Vitamin D: Newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–84. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, et al. Direct transcriptional regulation of RelB by 1alpha,25-dihydroxyvitamin D3 and its analogs: Physiologic and therapeutic implications for dendritic cell function. J Biol Chem. 2003;278:49378–85. doi: 10.1074/jbc.M308448200. [DOI] [PubMed] [Google Scholar]
- 15.Sutton AL, MacDonald PN. Vitamin D: More than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–91. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 17.Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: Insights from epidemiological research. Toxicology. 2002;181–182:71–8. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 18.Maruotti N, Cantatore FP. Vitamin D and the immune system. J Rheumatol. 2010;37:491–5. doi: 10.3899/jrheum.090797. [DOI] [PubMed] [Google Scholar]
- 19.Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, et al. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38:53–9. doi: 10.3899/jrheum.100516. [DOI] [PubMed] [Google Scholar]
- 20.Rossini M, Maddali Bongi S, La Montagna G, Minisola G, Malavolta N, Bernini L, et al. Vitamin D deficiency in rheumatoid arthritis: Prevalence, determinants and associations with disease activity and disability. Arthritis Res Ther. 2010;12:R216. doi: 10.1186/ar3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haque UJ, Bartlett SJ. Relationships among vitamin D, disease activity, pain and disability in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:745–7. [PubMed] [Google Scholar]
- 22.Giles JT, Szklo M, Post W, Petri M, Blumenthal RS, Lam G, et al. Coronary arterial calcification in rheumatoid arthritis: Comparison with the multi-ethnic study of atherosclerosis. Arthritis Res Ther. 2009;11:R36. doi: 10.1186/ar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the stanford health assessment questionnaire functional disability index in patients with rheumatoid arthritis. J Rheumatol. 1988;15:1480–8. [PubMed] [Google Scholar]
- 27.van der Heijde D. How to read radiographs according to the Sharp/van der heijde method. J Rheumatol. 2000;27:261–3. [PubMed] [Google Scholar]
- 28.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 29.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 30.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the multi-ethnic study of atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–79. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: The framingham heart study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu PW, Rhew EY, Dyer AR, Dunlop DD, Langman CB, Price H, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61:1387–95. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–5. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 37.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: A community-based study. Int J Epidemiol. 1990;19:559–63. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 38.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–5. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 39.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 40.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapses SA, Manson JE. Vitamin D and prevention of cardiovascular disease and diabetes: Why the evidence falls short. JAMA. 2011;305:2565–6. doi: 10.1001/jama.2011.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13:954–9. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 44.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The atherosclerosis risk in communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 45.Rohde LE, Lee RT, Rivero J, Jamacochian M, Arroyo LH, Briggs W, et al. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:1765–70. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 46.Martinesi M, Bruni S, Stio M, Treves C. 1,25-dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int. 2006;30:365–75. doi: 10.1016/j.cellbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Martinesi M, Treves C, d’Albasio G, Bagnoli S, Bonanomi AG, Stio M. Vitamin D derivatives induce apoptosis and downregulate ICAM-1 levels in peripheral blood mononuclear cells of inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14:597–604. doi: 10.1002/ibd.20354. [DOI] [PubMed] [Google Scholar]
- 48.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 49.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447–52. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 50.Cote M, Cartier A, Reuwer AQ, Arsenault BJ, Lemieux I, Despres JP, et al. Adiponectin and risk of coronary heart disease in apparently healthy men and women (from the EPIC-norfolk prospective population study) Am J Cardiol. 2011;108:367–73. doi: 10.1016/j.amjcard.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 51.Persson J, Lindberg K, Gustafsson TP, Eriksson P, Paulsson-Berne G, Lundman P. Low plasma adiponectin concentration is associated with myocardial infarction in young individuals. J Intern Med. 2010;268:194–205. doi: 10.1111/j.1365-2796.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 52.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine. 2009;36:205–10. doi: 10.1007/s12020-009-9216-9. [DOI] [PubMed] [Google Scholar]
- 53.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56:2143–9. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 54.Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME, et al. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus southern europe. Clin Exp Rheumatol. 2006;24:702–4. [PubMed] [Google Scholar]