Abstract
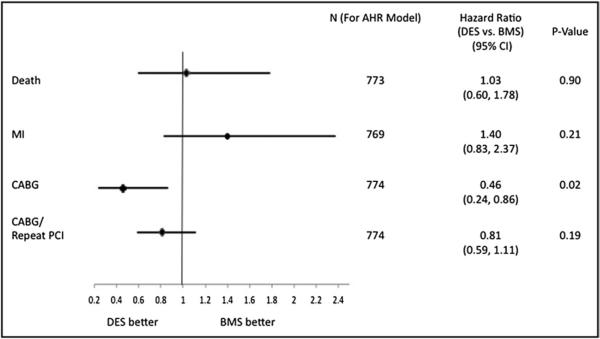
We compared the effectiveness of drug-eluting stents (DESs) to bare-metal stents (BMSs) in ostial lesions from an unrestricted patient cohort with 3-year follow-up. DESs have proved more effective at decreasing repeat revascularization rates compared to BMSs in patients with uncomplicated coronary artery disease. Whether DESs provide similar benefits in ostial lesions is not clearly defined. We analyzed data from 775 patients in the National, Heart, Lung, and Blood Institute Dynamic Registry undergoing stenting of ostial lesions with DESs or BMSs. Patients were followed for 3 years for the occurrence of myocardial infarction (MI), repeat revascularization (coronary bypass surgery/repeat percutaneous coronary intervention), and death. In total 439 patients had 464 ostial lesions treated with BMSs and 336 patients had 351 ostial lesions treated with DESs. Adjusted DES versus BMS 3-year hazard ratios were 1.03 (95% confidence interval 0.60 to 1.78, p = 0.90) for death, 1.40 (0.83 to 2.37, p = 0.21) for MI, and 0.81 (0.59 to 1.11, p = 0.19) for repeat revascularization. In patients undergoing percutaneous coronary intervention for aorto-ostial disease (n = 200), death and repeat revascularization did not differ between stent types, but DES-treated patients had more MI during follow-up. For coronary ostial disease (n = 574), 3-year observed rates of death or MI did not differ; however, repeat revascularization was more common in the BMS group. In conclusion, use of DESs for ostial lesions was associated with no difference in the hazard of death, MI, or overall rates of repeat revascularization compared to BMS use.
Drug-eluting stents (DESs) have proved more effective than bare-metal stents (BMSs) in decreasing the need for repeat revascularization.1–3 Complex lesions, however, have generally been excluded from initial randomized comparisons. As a result, the effectiveness of DESs compared with BMSs in complex coronary lesions including ostial lesions is less clear. Ostial lesions present a unique challenge given the higher prevalence of calcification, turbulent blood flow patterns, rigidity, elastic recoil, and ability to achieve correct stent placement compared to nonostial lesions.4–6 Further more, aorto-ostial lesions, representing aortic wall disease, are a unique subset of ostial lesions where the pathology of ostial lesion is different. Previous studies comparing DESs to BMSs in ostial lesions are limited in the number of patients studied, location of lesions, and duration of follow-up.7–17 The purpose of this report is to describe 3-year outcomes after unrestricted use of DESs versus BMSs in ostial coronary lesions from the National, Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry.
Methods
This dynamic registry is a multicenter NHLBI-sponsored prospective observational study of consecutive patients undergoing percutaneous coronary intervention (PCI) at selected centers in North America. It is composed of 5 “waves” of patient enrollment, each enrolling <2,000 patients since 1997, with the intent to study changes in PCI technology over time. Waves 1 to 3 enrolled patients when only BMSs were available. Waves 4 (2004) and 5 (2006) enrolled patients during the DES era. To decrease election bias, BMS-treated patients were selected only from waves 1 to 3.
Trained research coordinators collected demographic, clinical, angiographic, and procedural data pertaining to the index PCI procedure and vital status, repeat hospitalization, and medication use information during follow-up using standardized report forms. Hospital charts and coronary angiograms were reviewed to assess inpatient outcomes. Follow-up data were collected at 1 month, 6 months, and annually thereafter by direct patient contact. Patients enrolled in waves 1 and 3 were followed for 1 year and follow-up for patients in waves 2, 4, and 5 was extended. Routine follow-up angiography was not performed and staged PCI was not considered repeat PCI. Lesion-specific data were collected to determine target vessel revascularization rates.
Death was included as all-cause mortality. Other end points evaluated were myocardial infarction (MI) and any repeat revascularization (PCI or any coronary artery bypass grafting after index PCI). MI was defined as the presence of ≥2 of the following findings: typical chest pain lasting 20 minutes and not relieved by nitroglycerin; serial electrocardiograms showing changes from baseline in ST and T waves and/or Q waves in <2 contiguous leads; increase in creatine kinase to <2 times upper limit of normal with a creatine kinase-MB index of <5%; and increase in troponin to <2 times upper limit of normal. Ostial lesions were defined as aorto-ostial lesions (right coronary artery, left main coronary artery, saphenous vein graft, or arterial graft ostial lesions) or coronary ostial lesions within the coronary tree of <50% stenosis severity by visual assessment.
Continuous variables were compared by Student's t or Wilcoxon nonparametric tests and categorical variables by chi-square or Fisher's exact tests. Three-year cumulative event rates were estimated with the Kaplan-Meier method, and un-adjusted survival curves were compared using log-rank statistic. Cox proportional hazards model was used to estimate 3-year hazard ratios (HRs) for clinical events in relation to stent type. Covariate adjustment was performed with demographic, clinical, and lesion/procedural characteristics entered into outcome-specific models including an indicator variable for stent type. For each outcome potential confounders were adjusted for in a forward stepwise manner to determine the final model, and variables with a p value <0.10 were included in age-adjusted final models. Proportionality assumptions for Cox models were met. Patients not developing an event of interest by 3 years for those enrolled in waves 2, 4, and 5 were censored at 3 years and the same was done at 1 year for patients enrolled in waves 1 and 3. A 2-sided p value <0.05 was considered significant for all statistical analyses. We performed a subgroup analysis evaluating clinical event rates in patients treated with aorto-ostial and coronary ostial lesions.
Results
Baseline characteristics comparing differences between BMS- and DES-treated patients are presented in Table 1. The 2 groups were of similar age and had a similar proportion of woman patients. DES-treated patients were more likely to have a history of diabetes, hypertension, hypercholesterolemia, and previous PCI. BMS-treated patients were more likely to present with acute coronary syndromes and cardiogenic shock and less likely to receive periprocedural thienopyridine therapy.
Table 1.
Baseline characteristics of patients with ostial lesions treated with bare-metal stent versus drug-eluting stent
| Variable | BMS (n = 439) | DES (n = 336) | p Value |
|---|---|---|---|
| Mean age (years) | 65.5 | 65.4 | 0.86 |
| Women | 39.2% | 35.7% | 0.32 |
| Body mass index (kg/m2) | 28.3 | 29.7 | <0.001 |
| Diabetes mellitus | 30.9% | 39.7% | 0.01 |
| Insulin therapy | 10.4% | 16.4% | 0.01 |
| Hypertension | 69.6% | 84.0% | <0.001 |
| Hy perch ole sterole mia | 65.7% | 83.6% | <0.001 |
| Smoker | 0.79 | ||
| Current | 18.8% | 18.8% | |
| Former | 43.3% | 45.5% | |
| Previous myocardial infarction | 37.5% | 27.5% | 0.004 |
| Previous angioplasty | 29.1% | 40.2% | <0.001 |
| Previous coronary bypass | 28.3% | 32.0% | 0.54 |
| Co-morbidities | |||
| Cerebrovascular | 8.3% | 11.4% | 0.15 |
| Renal insufficiency | 7.2% | 9.3% | 0.28 |
| Peripheral arterial disease | 12.9% | 11.1% | 0.43 |
| Ejection fraction, mean (%) | 51.1 | 51.9 | 0.27 |
| Number of coronary arteries diseased | 0.06 | ||
| 1 | 32.3% | 23.5% | |
| 2 | 30.3% | 34.5% | |
| 3 | 37.1% | 41.7% | |
| Mean left main coronary artery stenosis >50% | 12.1% | 22.0% | <0.001 |
| Reason for revascularization | |||
| Myocardial infarction | 20.1% | 18.5% | 0.57 |
| Unstable angina | 50.0% | 35.4% | <0.001 |
| Stable angina | 21.0% | 27.4% | 0.04 |
| Cardiogenic shock | 3.9% | 0.6% | 0.003 |
| Periprocedural medications | |||
| Thienopyridines | 61.3% | 86.6% | <0.001 |
| Heparin | 96.6% | 65.8% | <0.001 |
| Low-molecular-weight heparin (waves 2–5) | 2.9% | 3.3% | 0.79 |
| Glycoprotein IIb/IIIa inhibitor | 41.5% | 36.3% | 0.15 |
| Discharge medications | |||
| Aspirin | 91.1% | 98.5% | <0.001 |
| Angioten sin-converting enzyme inhibitor | 38.8% | 51.2% | <0.001 |
| β Blocker | 64.7% | 84.1% | <0.001 |
| Calcium channel blocker | 26.9% | 16.8% | <0.001 |
| Statins | 56.3% | 83.8% | <0.001 |
| Thienopyridines | 89.2% | 99.1% | <0.001 |
| Mean number of lesions | 3.7 | 3.9 | 0.20 |
| Mean lesions attempted | 1.8 | 1.5 | 0.001 |
| Procedural success | 96.1% | 98.2% | 0.09 |
| Mean stents/patient | 1.85 | 1.83 | 0.82 |
| Insurance status | 0.075 | ||
| Medicare | 50.3% | 42.4% | |
| Public | 11.8% | 12.2% | |
| Private | 36.0% | 41.5% | |
| Serf | 1.8% | 3.9% | |
In total 439 patients had 464 ostial lesions attempted with BMSs compared to 351 ostial lesions attempted with 336 DESs (Table 2). At the lesion level, the location of lesions and reference vessel diameter were similar between the 2 groups. Lesions treated with DESs were significantly longer, treated with more stents/lesion, and more likely to be class C compared to BMS-treated lesions. BMS-treated lesions were more likely to be thrombotic, which is consistent with higher rates of acute coronary syndromes in BMS-treated patients. Overall angiographic and procedural success rates were high and similar for the DES and BMS groups.
Table 2.
Lesion characteristics and outcomes of bare-metal versus drug-eluting stent-treated patients undergoing stenting for ostial lesions
| Variable | BMS (n = 464) | DES (n = 351) | p Value |
|---|---|---|---|
| Location | 0.43 | ||
| Left main coronary artery | 5.6% | 7.7% | |
| Left anterior descending coronary artery | 36.9% | 34.8% | |
| Left circumflex coronary artery | 21.2% | 25.1% | |
| Right coronary artery | 24.2% | 22.2% | |
| Bypass graft | 12.1% | 10.3% | |
| Mean reference vessel diameter (mm) | 3.2 | 3.2 | 0.72 |
| Mean lesion length (mm) | 11.7 | 15.5 | <0.001 |
| Complex lesion types | |||
| Total occlusion | 8.4% | 6.8% | 0.41 |
| Thrombus present | 12.8% | 7.8% | 0.02 |
| Calcified lesion | 36.4% | 40.5% | 0.24 |
| Ulcerated lesion | 10.5% | 11.4% | 0.70 |
| American College of Cardiology/American Heart Association classification | <0.001 | ||
| A | 4.6% | 1.7% | |
| B1 | 17.3% | 22.6% | |
| B2 | 52.7% | 37.4% | |
| C | 25.4% | 38.3% | |
| Sirolimus-eluting stent | 65.8% | ||
| Paclitaxel-eluting stent | 34.2% | ||
| Procedural complications | |||
| Major dissection | 5.2% | 2.3% | 0.03 |
| Perforation | 0.4% | 0.0% | 0.22 |
| Embolization | 1.3% | 1.4% | 0.87 |
| Side branch occlusion | 1.9% | 2.3% | 0.74 |
| Angiographic success | 97.8% | 98.0% | 0.87 |
| Stents/lesion (mean) | 1.17 | 1.28 | 0.006 |
Rate of in-hospital death (BMS 2.5% vs DES 0.6%, p = 0.04) was higher in the BMS group. Rates of MI (BMS 4.8% vs DES 4.5%, p = 0.83), coronary artery bypass grafting (BMS 1.1% vs DES 0.0%, p = 0.05), stroke (BMS 0.9% vs DES 0.3%, p = 0.29), and bleeding requiring transfusion (BMS 3.9% vs DES 2.1%, p = 0.15) were similar between groups. DES-treated patients were more likely to receive dual antiplatelet therapy, statins, β blockers, and angiotensin-converting enzyme inhibitors on discharge, possibly reflecting different practice patterns during BMS and DES recruitment phases.
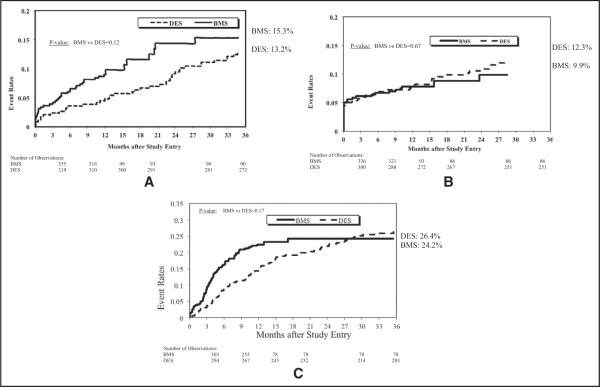
At 3-year follow-up, observed rates of death, MI, and repeat revascularization did not differ (Table 3 and Figure 1). In patients undergoing repeat procedures, repeat PCI did not dif fer; however, coronary artery bypass grafting was significantly higher in the BMS group. After adjustment, there was no difference in rate of death, MI, and repeat revascularization (Figure 2). Overall 3-year target vessel revascularization rates from available data were not different for the DES versus BMS groups.
Table 3.
Observed three-year event rates
| Variable | BMS (n = 439) | DES (n = 336) | p Value |
|---|---|---|---|
| All-cause death | 15.30% | 13.20% | 0.12 |
| Myocardial infarction | 9.90% | 12.30% | 0.67 |
| Repeat percutaneous coronary intervention | 16.30% | 23.80% | 0.68 |
| Coronary artery bypass grafting | 10.40% | 5.30% | 0.002 |
| Coronary artery bypass grafting/repeat percutaneous coronary intervention (repeat revascularization) | 24.20% | 26.40% | 0.17 |
| Target vessel revascularization | 9.30% (137)* | 7.80% (314) | 0.6 |
For the bare-metal stent group, follow-up beyond 1 year was available only for wave 2.
Figure 1.
Kaplan–Meier plots of 3-year cumulative incidence of (A) death, (B) myocardial infarction, and (C) coronary artery bypass grafting/repeat percutaneous coronary intervention in patients undergoing percutaneous coronary intervention for ostial lesions using bare-metal versus drug-eluting stents.
Figure 2.
Adjusted hazard rates/relative risk of patients undergoing percutaneous coronary intervention for ostial lesions. AHR = adjusted hazard ratio; CABG = coronary artery bypass grafting.
Of 775 patients in the study cohort, 200 (26%) underwent PCI for aorto-ostial lesions (right coronary artery, left main coronary artery, and vein grafts). For the DES versus BMS comparison in patients with aorto-ostial disease, 3-year observed rates of death (BMS 21.8% vs DES 15.9%, p = 0.40; adjusted HR 2.6, 95% confidence interval [CI] 0.8 to 8.1, p = 0.10) and repeat revascularization (BMS 17.4% vs DES 32.1%, p = 0.33; adjusted HR 1.6, 95% CI 0.8 to 3.1, p = 0.15) did not differ; however, DES-treated patients had more MI on follow-up (BMS 3.9% vs DES 16.2%, HR 5.4, 95% CI 1.3, 22.6, p = 0.02). DES-treated patients had a trend toward a higher rate of repeat PCI compared to BMS-treated patients (BMS 9.9% vs DES 28.3%, p = 0.06; adjusted HR 2.1, 95% CI 1.0, 4.7, p = 0.06).
In patients undergoing PCI for coronary ostial lesions, 3-year cumulative rates of death (BMS 13.3% vs DES 12.0%, p = 0.18) and MI (BMS 11.8% vs DES 10.9%, p = 0.43) did not differ; however, repeat revascularization was more common in the BMS group (BMS 26.4% vs DES 23.3%, p = 0.02; adjusted HR 0.7, 95% CI 0.5 to 1.0, p = 0.04).
Discussion
Previous studies comparing DESs to BMSs for off-label indications have reported that up to 20% of patients in the “off-label” group undergo PCI for ostial lesions. As a result, a separate analysis evaluating stent use in this lesion category is warranted. In this prospective observational cohort study of patients undergoing PCI for ostial lesions with DESs versus BMSs, we noted no difference in rates of death, MI, or repeat revascularization by 3 years according to stent type. Observed and adjusted coronary artery bypass grafting rates were significantly higher in BMS-treated patients. Previous randomized and observational studies have consistently shown lower target vessel revascularization rates with DESs compared to BMSs in other lesion subsets, a benefit not evident in our analysis.1–3,18
Our data are derived from the NHLBI Dynamic Registry. BMS data were acquired from patients undergoing stenting before DES introduction to lessen selection bias because currently the types of patients treated with DESs versus BMSs differ markedly in several respects. Despite this approach, residual baseline differences remained between the DES versus BMS groups. When differences were present, some high-risk features were higher in the BMS group and others in the DES group.
As a whole, patients enrolled in this study more commonly had features associated with poor clinical outcomes than patients in published randomized DES versus BMS trials.1–3 For example, patients in our study were older and had higher rates of diabetes, previous MI, previous coronary artery bypass grafting, low ejection fraction, peripheral vascular disease, cerebrovascular disease, renal failure, multi-vessel and left main coronary artery disease compared to patients studied in randomized DES versus BMS trials. Patients in this study had higher annual mortality rates compared to patients enrolled in randomized stent trials and observational studies.7,8,10,14 The greater prevalence of morbid conditions and higher mortality noted in our study cohort likely reflects unrestricted use of DESs and consecutive enrollment of patients in each of the 5 waves. A proportion of the aorto-ostial subgroup included patients with previous coronary artery bypass grafting (ostial saphenous vein graft lesions), making this is a higher-risk cohort compared to previous analyses.
We did not have lesion level data available on follow-up, and as a result target lesion revascularization rates could not be calculated. The inability to distinguish between target lesion revascularization and nontarget lesion revascularization rates has the propensity to underestimate the benefit of DES in regard to rate of repeat target lesion revascularization procedures because disease progression in nonstented regions cannot be determined.
We noted a significantly higher rate of coronary artery bypass grafting in the BMS group and a trend toward a higher rate of repeat PCI in the DES group. The most likely explanation is that patients presenting for repeat procedures in the BMS era were more commonly referred for coronary artery bypass grafting as the preferred revascularization strategy in the setting of stent failure. Strategies available for treatment of stent restenosis, i.e., repeat balloon angioplasty or additional stenting with BMS, had limited success. The sharp decrease in rates of restenosis noted with DES treatment increased the likelihood to treat target lesion revascularization or nontarget lesion revascularization lesions with repeat DES before referring a patient for surgery.19–21
Our subgroup analysis included an evaluation of patients according to location of ostial lesions, specifically aortoostial and coronary ostial lesions. Aorto-ostial lesions differ from coronary ostial lesions by histopathologic characteristics such as more fibrous cellularity, calcification, and sclerosis.6,22,23 Long-term stent recoil may play a more important role in the aorto-ostial lesion type compared to ostial lesions located within the coronary tree.6
In patients undergoing PCI for aorto-ostial lesions, death and target vessel revascularization were similar, whereas MI was more frequent in the DES group. Our finding of a higher rate of MI during follow-up in the DES group in patients undergoing PCI for aorto-ostial lesions differs from previous reports. Park et al8 reported no difference in MI (BMS 0.5% vs DES 1.2%, p = 0.6) in 356 patients undergoing PCI with DESs versus BMSs for aorto-ostial disease. However, that study excluded patients with high-risk features. Furthermore, very few patients were treated for saphenous vein graft lesions. No obvious explanation for this finding is apparent from our analysis and it is likely that, given the small numbers of patients in this subgroup analysis, the difference may be due to chance alone.
There was no difference in rate of death or MI between the DES and BMS groups for coronary ostial lesions, a finding consistent with randomized stent trials. However, unlike aorto-ostial lesions, we did detect a decrease in target vessel revascularization in patients undergoing PCI for coronary ostial lesions. Aorto-ostial lesions represent aortic wall disease, the progression of which is possibly not altered by drugs selected to attenuate neointimal hyperplasia. This mechanism, combined with the generally larger reference vessel diameter of aorto-ostial lesions, could explain the lack of DES benefit in this lesion subset.24
Results from the present study should be interpreted within the context of the overall design. This was an observational study, and patients were not randomized to receive DESs versus BMSs. As a result, significant baseline patient characteristics and procedural differences were present before adjustment and residual confounding may be present after adjustment, as is expected in any observational study. Patients did not undergo routine angiographic follow-up and we were unable to determine rates of target lesion revascularization or in-stent restenosis. Lesion progression in nonstented segments may underestimate the benefit of DESs. Information on stent thrombosis was not available in all waves of the dataset. However, previous analyses from the same dataset and other registries have shown stent thrombosis rates to be ≤1%, even when off-label indications are included.18,25,26
References
- 1.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 2.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 3.Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Münzel T, Popma JJ, Fitzgerald PJ, Bonan R, Kuntz RE. ENDEAVOR II Investigators. Randomized, double-blind, multicenter study of the ENDEAVOR zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angio-graphic results of the ENDEAVOR II trial. Circulation. 2006;114:798–806. doi: 10.1161/CIRCULATIONAHA.105.591206. [DOI] [PubMed] [Google Scholar]
- 4.Kereiakes DJ. Percutaneous transcatheter therapy of aorto-ostial stenoses. Cathet Cardiovasc Diagn. 1996;38:292–300. doi: 10.1002/(SICI)1097-0304(199607)38:3<292::AID-CCD18>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Topol EJ, Ellis SG, Fishman J, Leimgruber P, Myler RK, Stertzer SH, O'Neill WW, Douglas JS, Roubin GS, King SB. Multicenter study of percutaneous transluminal angioplasty for right coronary artery ostial stenosis. J Am Coll Cardiol. 1987;9:1214–1218. doi: 10.1016/s0735-1097(87)80458-8. [DOI] [PubMed] [Google Scholar]
- 6.Tsunoda T, Nakamura M, Wada M, Ito N, Kitagawa Y, Shiba M, Yajima S, Iijima R, Nakajima R, Yamamoto M, Takagi T, Yoshitama T, Anzai H, Nishida T, Yamaguchi T. Chronic stent recoil plays an important role in restenosis of the right coronary ostium. Coron Artery Dis. 2004;15:39–44. doi: 10.1097/00019501-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto H, Ishikawa T, Mutoh M, Imai K, Mochizuki S. Angiographic and clinical outcomes after sirolimus-eluting stent implantation to de novo ostial lesion of the right coronary artery: a retrospective study. Circ J. 2008;72:880–885. doi: 10.1253/circj.72.880. [DOI] [PubMed] [Google Scholar]
- 8.Park DW, Hong MK, Suh IW, Hwang ES, Lee SW, Jeong YH, Kim YH, Lee CW, Kim JJ, Park SW, Park SJ. Results and predictors of angiographic restenosis and long-term adverse cardiac events after drug-eluting stent implantation for aorto-ostial coronary artery disease. Am J Cardiol. 2007;99:760–765. doi: 10.1016/j.amjcard.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Nassar H, Gotsman I, Gerganski P, Moseri M, Lotan C, Gotsman M. Cutting balloon angioplasty and stent implantation for aorto-ostial lesions: clinical outcome and 1-year follow-up. Clin Cardiol. 2009;32:183–186. doi: 10.1002/clc.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iakovou I, Ge L, Michev I, Sangiorgi GM, Montorfano M, Airoldi F, Chieffo A, Stankovic G, Vitrella G, Carlino M, Corvaja N, Briguori C, Colombo A. Clinical and angiographic outcome after sirolimus-eluting stent implantation in aorto-ostial lesions. J Am Coll Cardiol. 2004;44:967–971. doi: 10.1016/j.jacc.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 11.Freeman M, Clark DJ, Andrianopoulos N, Duffy SJ, Lim HS, Brennan A, Charter K, Shaw J, Horrigan M, Ajani AE, Sebastian M, Reid CM, Farouque HM. Melbourne Interventional Group. Outcomes after percutaneous coronary intervention of ostial lesions in the era of drug-eluting stents. Catheter Cardiovasc Interv. 2009;73:763–768. doi: 10.1002/ccd.21941. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Li JJ, Chen JL, Qiao SB, Xu B, Yang YJ, Gao RL, Qin XW, Yuan JQ, Yao M, Wu YJ, Liu HB, Dai J, You SJ. Drug-eluting stents for the treatment of ostial coronary lesions: comparison of sirolimus-eluting stent with paclitaxel-eluting stent. Coron Artery Dis. 2008;19:507–511. doi: 10.1097/MCA.0b013e32830936d4. [DOI] [PubMed] [Google Scholar]
- 13.Capranzano P, Sanfilippo A, Tagliareni F, Capodanno D, Monaco S, Sardella G, Giordano A, Sangiorgi GM, Tamburino C. Long-term outcomes after drug-eluting stent for the treatment of ostial left anterior descending coronary artery lesions. Am Heart J. 2010;160:973–978. doi: 10.1016/j.ahj.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Barlis P, Kaplan S, Dimopoulos K, Ferrante G, Di Mario C. Comparison of bare-metal and sirolimus- or paclitaxel-eluting stents for aortoostial coronary disease. Cardiology. 2008;111:270–276. doi: 10.1159/000128602. [DOI] [PubMed] [Google Scholar]
- 15.Kishi K, Kimura T, Morimoto T, Namura M, Muramatsu T, Nishikawa H, Hiasa Y, Isshiki T, Nobuoshi M, Mitsudo K. j-Cpher Registr Investigators. Sirolimus-eluting stent implantation for ostial left anterior descending coronar arter lesions: three-ear outcome from the j-Cpher Registr. Circ Cardiovasc Interv. 2011;4:362–370. doi: 10.1161/CIRCINTERVENTIONS.111.961904. [DOI] [PubMed] [Google Scholar]
- 16.Seung KB, Kim H, Park DW, Lee BK, Lee CW, Hong MK, Kim PJ, Chung WS, Tahk SJ, Park SW, Park SJ. Effectiveness of sirolimuseluting stent implantation for the treatment of ostial left anterior descending arter stenosis with intravascular ultrasound guidance. J Am Coll Cardiol. 2005;46:787–792. doi: 10.1016/j.jacc.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Tsagalou E, Stankovic G, Iakovou I, Melzi G, Cosgrave J, Ge L, Michev I, Chieffo A, Airoldi F, Carlino M, Montorfano M. Colombo A. Earl outcome of treatment of ostial de novo left anterior descending coronar arter lesions with drug-eluting stents. Am J Cardiol. 2006;97:187–191. doi: 10.1016/j.amjcard.2005.07.131. [DOI] [PubMed] [Google Scholar]
- 18.Abbott JD, Voss MR, Nakamura M, Cohen HA, Selzer F, Kip KE, Vlachos HA, Wilensk RL, Williams DO. Unrestricted use of drug-eluting stents compared with bare-metal stents in routine clinical practice: findings from the National Heart, Lung, and Blood Institute Dnamic Registr. J Am Coll Cardiol. 2007;50:2029–2036. doi: 10.1016/j.jacc.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Lee BK, Park DW, Park KH, Choi BR, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Comparison with conventional therapies of repeated sirolimus-eluting stent implantation for the treatment of drug-eluting coronar stent restenosis. Am J Cardiol. 2006;98:1451–1454. doi: 10.1016/j.amjcard.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Mishkel GJ, Moore AL, Markwell S, Shelton MC, Shelton ME. Long-term outcomes after management of restenosis or thrombosis of drug-eluting stents. J Am Coll Cardiol. 2007;49:181–184. doi: 10.1016/j.jacc.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 21.Mehilli J, Brne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, Massberg S, Laugwitz KL, Schömig A, Kastrati A. ISAR-DESIRE 2 Investigators. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronar restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronar Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis 2) stud. J Am Coll Cardiol. 2010;55:2710–2716. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Rissanen V. Occurrence of coronar ostial stenosis in a necrops series of mocardial infarction, sudden death, and violent death. Br Heart J. 1975;37:182–191. doi: 10.1136/hrt.37.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popma JJ, Dick RJ, Haudenschild CC, Topol EJ, Ellis SG. Atherectom of right coronar ostial stenoses: initial and long-term results, technical features and histologic findings. Am J Cardiol. 1991;67:431–433. doi: 10.1016/0002-9149(91)90057-r. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg DH, Mishra S, Javaid A, Slottow TL, Buch AN, Ro P, Okabe T, Smith KA, Torguson R, Xue Z, Pichard AD, Satler LF, Kent KM, Suddath WO, Waksman R. Comparison of effectiveness of bare metal stents versus drug-eluting stents in large (> or = 3.5 mm) coronar arteries. Am J Cardiol. 2007;99:599–602. doi: 10.1016/j.amjcard.2006.09.105. [DOI] [PubMed] [Google Scholar]
- 25.Lemos PA, Hoe A, Goedhart D, Arampatzis CA, Saia F, van der Giessen WJ, McFadden E, Sianos G, Smits PC, Hofma SH, de Feter PJ, van Domburg RT, Serrus PW. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamcin-Eluting Stent Evaluated at Rotterdam Cardiolog Hospital (RESEARCH) stud. Circulation. 2004;109:1366–1370. doi: 10.1161/01.CIR.0000121358.26097.06. [DOI] [PubMed] [Google Scholar]
- 26.Williams DO, Abbott JD, Kip KE. DEScover Investigators. Outcomes of 6906 patients undergoing percutaneous coronar intervention in the era of drug-eluting stents: report of the DEScover Registr. Circulation. 2006;114:2154–2162. doi: 10.1161/CIRCULATIONAHA.106.667915. [DOI] [PubMed] [Google Scholar]