Abstract
Background
Previous studies suggest that patients with chronic diseases, such as rheumatoid arthritis (RA), do not receive optimal preventive medical services including cancer screening tests. Few studies evaluated cancer screening in RA patients compared to non-RA.
Methods
Using data from a large US commercial insurance plan, we examined rates of screening tests for cervical, breast and colon cancer in patients with RA compared with non-RA. RA was defined as ≥ 2 diagnoses of RA and ≥ 1 prescription for a disease-modifying anti-rheumatic drug. Multivariable Cox models compared cancer screening test rates between RA and non-RA patients.
Results
Both RA (n=13,314) and non-RA (n=212,324) patients were screened, on average, once every 3 years for cervical cancer and once every two years for breast cancer during the follow-up (mean: 2.3 years). In the age-adjusted Cox regression model, women with RA were more likely to receive ≥ 1 Papanicolaou smear (HR 1.21, 95% CI 1.17–1.24), mammogram (HR 1.49, 95% CI 1.45–1.53), and colonoscopy (HR 1.69, 95% CI 1.61–1.77) compared to non-RA. Men with RA, were also more likely to receive a colonoscopy (HR 1.52, 95% CI 1.40–1.64) than non-RA patients. The results were robust in multivariable analyses adjusted for age, physician visit numbers, percent of visits made to primary care physicians and comorbidity index.
Conclusions
Individuals with RA did not appear to be at risk for receiving fewer cancer screening tests than non-RA patients. The majority of both RA and non-RA patients were regularly screened for cervical, breast, and colon cancer as recommended.
INTRODUCTION
Patients with rheumatoid arthritis (RA) are known to have a decreased life expectancy even with early and aggressive treatment, compared to the general population.[1–4] One of the main causes of deaths in RA patients is cancer.[2, 4] Screening and early detection of some common cancers can improve morbidity and mortality; thus, both the U.S. Preventive Services Task Force (USPSTF) and American Cancer Society recommend most adults be regularly screened for cervical, breast and colon cancer.[5–6] The USPSTF recommends screening for cervical cancer in women ages 21 to 65 years with Papanicolaou (Pap) smear every 3 years or, for women ages 30 to 65 years who want to lengthen the screening interval, screening with a combination of Pap smear and human papillomavirus (HPV) testing every 5 years. The USPSTF recommends biennial screening mammography for women aged 50 to 74 years for breast cancer and fecal occult blood (FOB) testing, sigmoidoscopy, or colonoscopy for adults aged 50 to 75 for colorectal cancer. The guidelines from the American Cancer Society are similar to these from the USPSTF except that mammogram is recommended annually for women aged 40 years and older.
Prior research has raised concerns that patients with chronic diseases, such as RA, do not receive optimal preventive medical services including cancer screening tests.[7–10] Data from a historical cohort of 1,335 adults with RA enrolled in a national fee-for-service insurance plan showed that RA patients received inadequate quality of care including health maintenance tests such as mammography and Pap smears.[8] Another study showed that 20% to 35% of older Americans on Medicare with arthritis never underwent mammography and colonoscopy over five years.[10] While it is important to recognize chronic disease such as RA as a potential barrier to preventive medical services and to raise awareness about the importance of cancer screening, most of the published studies did not compare cancer screening rates in patients with RA with a non-RA population.
A prior analysis from the Nurses’ Health Study suggested that cancer screening practices were similar among women with RA compared to women without RA.[11] It is unclear whether this study yielded different results because of the characteristics of study population, being all health care professionals. However, it is also possible that patients with RA see physicians more often and thus go through more medical exams including cancer screening tests than expected.
We studied a large cohort from a health care utilization database 1) to examine cancer screening rates in patients with RA compared with those without RA and 2) to assess the adherence to current adult cancer screening guidelines recommended by the USPTSF and American Cancer Society in both groups.[5–6]
METHODS
Data Source
We conducted a cohort study using the administrative claims data from a commercial US health plan which insures primarily working adults and their family members, and a small Medicare population for the period January 1, 2001 through June 30, 2008. This database contains longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensings on more than 28 million fully-insured subscribers, with medical and pharmacy coverage, to 14 Blue Cross/Blue Shield health plans across the United States. Personal identifiers were removed from the dataset before the analysis to protect subject confidentiality. Patient informed consent was, therefore, not required. The study protocol was approved by the Partners Healthcare Institutional Review Board.
Study Cohort
Adult subjects who had at least two visits coded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD 9-CM) code, 714.xx, for RA were identified for the RA cohort. Subjects entered the RA cohort at the first receipt of a disease-modifying anti-rheumatic drug (DMARD) prescription; thus, all persons in the RA cohort were required to have had two diagnoses and at least one filled prescription for a DMARD at the start of follow-up. A previous validation study showed that RA patients can be accurately identified using a combination of diagnosis codes and DMARD prescriptions in claims data.[12]
We identified two non-RA cohorts over age 18 for comparison. The first comparison cohort, described herein as the “non-RA cohort” consisted of adults who never had a diagnosis of RA during the study period. Follow-up for the non-RA cohort began at a randomly selected index date, and subjects were required to have at least 12 months of continuous health plan eligibility before the start of follow-up and at least 12 months of follow-up time after the index date. Subjects were then followed until the first of any of the following censoring events: development of solid tumors including breast, cervical, prostate, and colorectal cancer as well as HIV infection, loss of eligibility, admission to nursing home, end of study database, or death.
To compare cancer screening rates in RA patients with persons who have another chronic condition that requires regular visits to a physician, subjects with at least two visits coded for hypertension (ICD-9 401.X) were selected. Follow-up began at the date of 2nd hypertension diagnosis for this cohort. Subjects in this cohort were required to have similar eligibility requirements and were followed until similar censoring events.
Nursing home residents and subjects with claims for solid tumors, hematologic malignancies, myelodysplastic syndrome, human immunodeficiency virus (HIV) infection, or chemotherapy were excluded from all cohorts.
Cancer Screening Tests
Using diagnosis codes and procedure codes (see Appendix 1), several screening tests for cervical cancer, breast cancer and colon cancers were identified after the index date. Our outcome of interest includes the Pap smear test, mammogram, and conventional and virtual CT colonoscopy. Additional data on HPV DNA test, colposcopy, breast ultrasound and magnetic resonance imaging (MRI), breast biopsy, FOB test, barium enema, and flexible sigmoidoscopy were collected for secondary outcome defined as any screening or diagnostic test for cervical, breast and colon cancer.
Covariates
Variables potentially related to getting cancer screening tests were assessed using data from the 12 months before the index date. These variables included demographic factors (age and sex), comorbidities, and health care utilization factors (number of visits to any physicians, primary care physicians, rheumatologists, occurrence of acute care hospitalizations, and number of different prescription drugs). To quantify patients’ comorbidities, we calculated the Deyo-adapted Charlson Comorbidity Index based on ICD-9-CM.[13–14] The Comorbidity Index is a summative score, based on 19 major medical conditions including myocardial infarction, pulmonary, renal, hepatic disease, diabetes, cancer, HIV infection, etc. A score of 0 represents absence of comorbidity and a higher score indicates a greater number of comorbid conditions.
Statistical Analyses
We compared the baseline characteristics between the RA and non-RA cohorts. First, we estimated rates of three major cancer screening tests with 95% confidence interval (CI), calculated as the number of subjects who had at least one screening tests divided by the total person-time, in both RA and non-RA cohorts. Pap smear rates were calculated for all women aged 18 years or older, and then stratified by age. Mammogram rates were calculated for all women aged 40 years or older, and then stratified by age. For colonoscopy, either conventional or virtual, the rates were calculated with for all patients aged 50 years or older, and then stratified by age and gender. Rate ratios (RRs) with 95% CI were estimated by dividing the rate of the specific screening test among RA patients by that of non-RA.[15]
For the secondary outcomes defined as rates of any screening or diagnostic test specific to each cancer, similar analyses were carried out. To adjust for potential confounders, separate Cox proportional hazard models were used to compare the screening test rates for each cancer type among RA patients with those in non-RA cohort.[16] All the analyses were then repeated for the comparison between patients with RA and hypertension for secondary analyses. All analyses were done using SAS 9.1 Statistical Software (SAS Institute Inc., Cary, NC).
RESULTS
Cohort Selection
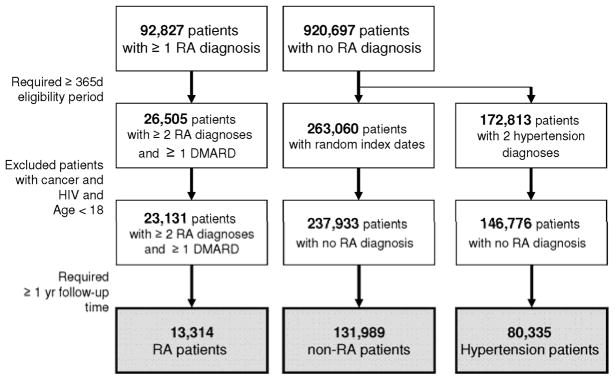
There were more than 1 million potentially eligible subjects in the study database. Initially, 92,827 subjects with at least one RA diagnosis after 365 days of enrollment and approximately 920,697 subjects with no RA diagnosis were identified. After requiring a second diagnosis of RA and at least one prescription for DMARD and applying the exclusion criteria, the 23,131 RA and 237,933 non-RA patients remained in the study cohort. Restricting to those with at least one year of follow-up time, our final study cohort included 13,314 RA patients and 131,989 non-RA patients. (See Figure 1) For the secondary analysis, 80,335 patients with hypertension were selected.
Figure 1. Selection of the study cohort.
RA: rheumatoid arthritis, DMARD: disease-modifying antirheumatic drug
The final study cohort included 13,314 RA patients and 131,989 non-RA patients. 80,335 patients with hypertension were selected for the secondary analysis.
Patient Characteristics
Table 1 presents baseline characteristics of our study cohorts: 13,314 RA patients, 131,989 non-RA patients and 80,335 hypertension patients. Mean age was 52 years for RA patients, 48 years for non-RA and 61 years for hypertension patients. The mean follow-up time was 2.54 years for RA, 2.37 years for non-RA and 2.33 years for hypertension patients. Substantial differences were observed between the cohorts and in particular between the RA and non-RA patients. Overall, more patients in the RA cohort were women, had comorbid conditions and visited a greater number of physicians, compared to non-RA cohort. Of the three groups, hypertension patients with hypertension were oldest and had the most comorbid conditions and hospitalizations.
Table 1.
Baseline characteristics of the study cohort in 12 months prior to the index date
| RA patients | Non-RA patients | Hypertension patients | |
|---|---|---|---|
| N | 13,314 | 131,989 | 80,335 |
| Follow-up period, mean/SD | 2.5 (1) | 2.4 (1) | 2.3 (1) |
|
| |||
| Demographic
| |||
| Age, mean/SD, years | 51.8 (12) | 47.8 (17) | 61.4 (15) |
| Women, n/% | 9,877 (74) | 89,698 (68) | 54,428 (68) |
|
| |||
| Comorbidities
| |||
| Comorbidity Index, mean/SD | 1.2 (1) | 0.2 (1) | 0.6 (1) |
| Chronic kidney disease, n/% | 87 (1) | 618 (0.5) | 1,187 (2) |
| Liver disease, n/% | 148 (1) | 624 (0.5) | 783 (1) |
| Hypertension, n/% | 3,687 (28) | 23,618 (18) | 72,886 (91) |
| Diabetes mellitus, n/% | 1,217 (9) | 7,968 (6) | 14,042 (18) |
| Inflammatory bowel disease, n/% | 179 (1) | 531 (0.4) | 403 (0.5) |
| Chronic obstructive pulmonary disease, n/% | 1,599 (12) | 8,561 (7) | 9,371 (12) |
| Heart failure, n/% | 264 (2) | 1,848(1) | 3,737 (5) |
|
| |||
| Health care utilization
| |||
| No. of total physician visits, mean/SD | 9.6 (8) | 3.6 (5) | 6.2 (6) |
| PCP visits | 3.9 (4) | 1.7 (3) | 3.2 (3) |
| % visit to PCPs | 42 | 51 | 58 |
| Rheumatologist visits | 2.0 (3) | n/a | n/a |
| % visit to rheumatologists | 24 | n/a | n/a |
| Hospitalization, n/% | 1,847 (14) | 10,633 (8) | 12,932 (16) |
| No. of prescription drug, mean/SD | 10.1 (6) | 3.7 (5) | 5.7 (6) |
RA: rheumatoid arthritis, SD: standard deviation, PCP: primary care physician, % visit to PCPs = Number of visits to PCPs/Number of total physician visits, % visit to rheumatologists = Number of visits to rheumatologists/Number of total physician visits
Screening Test Rates for Cervical, Breast and Colon Cancer
Among women aged 18–69 years, approximately 41–55% of RA patients, 40–49% non-RA patients and 36–70% hypertension patients had at least one Pap smear test per year. Among women aged 40 years and older, 55% of RA, 44% of non-RA and 47% hypertension patients had at least one mammogram per year. Among all subjects aged 50 years and older, 12% of RA patients, 9.5% of non-RA patients and 12% hypertension patients had at least one colonoscopy per year. Interestingly, fewer patients were getting a FOB test than a colonoscopy across all three groups. Overall, the rates of Pap smear, mammogram, FOB test and colonoscopy were lower in non-RA patients compared to patients with RA and hypertension. Table 2 presents crude rates of having at least one cancer screening per 1,000 person-years in all three cohorts. Assuming the test rates remain constant over time, we estimated that approximately 70% to 80% of all the women aged 18–69 years regardless of RA status would have at least one Pap smear every 3 years. For breast cancer, 60% of non RA and hypertension female patients and 70% of female patients with RA aged 40 years and above would have at least one mammogram every other year. For colon cancer, 70% of RA and non-RA and 61% of hypertension patients aged 50 years and older would undergo a colonoscopy every 10 years,
Table 2.
Crude rates (95% confidence intervals) per 1,000 person-years for having at least one cancer screening test in RA and non-RA patients.
| RA patients | Non-RA patients | Hypertension patients | |
|---|---|---|---|
|
Papanicolaou smear
| |||
| Women aged ≥ 18 | 376 (366–386) N=9,877 |
351 (348–354) N=89,698 |
225 (222–228) N=54,428 |
| Aged 18–29 | 547 (486–614) N=421 |
488 (477–490) N=12,305 |
688 (622–761) N=547 |
| Aged 30–69 | 406 (395–417) N=8,780 |
403 (399–407) N=66,286 |
357 (352–363) N=35,343 |
| Aged ≥ 70 | 70 (58–85) N=676 |
54 (51–57) N=11,107 |
55 (52–57) N=18,538 |
|
| |||
|
Mammogram
| |||
| Women aged ≥ 40 | 552 (538–566) N=8,338 |
438 (433–442) N=60,293 |
474 (469–479) N=51,449 |
|
| |||
|
Fecal occult blood test
| |||
| All subjects aged ≥ 50 | 114 (109–119) N=7,917 |
82 (81–84) N=56,061 |
84 (82–86) N=62,546 |
| Men | 75 (68–84) N=2,127 |
64 (62–67) N=17,096 |
80 (77–83) N=18,550 |
| Women | 128 (122–135) N=5,790 |
90 (88–93) N=38,965 |
86 (84–88) N=43,996 |
|
| |||
|
Colonoscopy
| |||
| All subjects aged ≥ 50 | 121 (114–127) N=7,917 |
95 (93–97) N=56,061 |
116 (114–118) N=62,546 |
| Men | 125 (115–136) N=2,127 |
103 (99–106) N=17,096 |
134 (130–137) N=18,550 |
| Women | 120(114–126) N=5790 |
92 (90–94) N=38,965 |
109 (107–112) N=43,996 |
Among women aged 18–39 years who had more than one Pap smear during the follow-up period, the average number of days between the first and second Pap smears was 437 for RA, 423 for non-RA and 439 for hypertension patients. Among women aged 40 years and older who had more than one mammogram during the follow-up period, the average number of days between the first and second mammograms were 414 for RA, 409 for non-RA and 406 for hypertension patients.
In the age-adjusted Cox regression model, women with RA were more likely to receive ≥ 1 Pap smear (HR 1.21, 95% CI 1.17–1.24), mammogram (HR 1.49, 95% CI 1.45–1.53), and colonoscopy (HR 1.69, 95% CI 1.61–1.77) compared to non-RA patients. Men with RA, were also more likely to receive a colonoscopy (HR 1.52, 95% CI 1.40–1.64) than non-RA patients. The results were robust in multivariable analyses adjusted for age, physician visit numbers, percent of visits made to primary care physicians and comorbidity index. On the other hand, there was no significant difference in having at least one Pap smear, mammogram or colonoscopy between patients with RA and hypertension in either age-adjusted or multivariable Cox regression models. (See Table 3)
Table 3.
Adjusted hazard ratios (HR) and 95 % confidence intervals (CI) for having at least one cancer screening test in patients with rheumatoid arthritis (RA) compared to those without.
| RA vs. non-RA patients | RA vs. hypertension patients | |||
|---|---|---|---|---|
|
| ||||
| Age-adjusted HR (95% CI) | Multivariable HR (95% CI) a | Age-adjusted HR (95% CI) | Multivariable HR (95% CI) a | |
| Papanicolaou smear | 1.21 (1.17–1.24) | 1.28 (1.24–1.32) | 0.98 (0.95–1.01) | 1.04 (1.00–1.07) |
| Mammogram | 1.49 (1.45–1.53) | 1.62 (1.57–1.67) | 0.94 (0.92–0.970 | 0.98 (0.95–1.01) |
| Colonoscopy | ||||
| Men | 1.52 (1.40–1.64) | 1.38 (1.26–1.51) | 0.99 (0.91–1.07) | 0.97 (0.89–1.05) |
| Women | 1.69 (1.61–1.77) | 1.55 (1.46–1.63) | 0.95 (0.90–1.00) | 0.87 (0.82–0.92) |
adjusted for age, physician visit numbers, percent of visits made to primary care physicians and comorbidity index
DISCUSSION
Our study shows that the majority of patients with RA, albeit not all, were regularly screened for cervical, breast and colon cancer as recommended and the rates of cancer screening tests were comparable to subjects without RA in the population and those with a chronic condition such as hypertension. These results are somewhat different from previous studies that suggested inadequate preventive health service utilization in patients with chronic disease including RA.[8–10, 17–19] However, it is important to note that some of the previously published studies did not compare RA cancer screening rates with a non-RA group.
In a previous study of 1,335 adults with RA enrolled in a U.S. national fee-for-service insurance plan,[8] RA patients received inadequate quality of care including health maintenance tests such as mammography and Pap smears. In addition, patients who had contacts with a primary care physician but no relevant specialist had better health maintenance service than those patients who did not have contact with either primary or specialist doctors. In a small single institution-based study [18], 68% of women under age 50 had at least one mammogram by the end of 2 years and 33% of women aged 50 years or older by one year. In women without a history of hysterectomy, the probability of having a Pap smear within 3 years was 77% in RA patients which is consistent with our results. The mean age of their study population was higher compared to our study population. These two studies did not have a comparison group, so their results could not provide whether the problem of inadequate (i.e., not 100%) health maintenance service is specific to RA or not.
A study using claims data from a random 5% sample of Medicare enrollees showed that RA patients were significantly less likely to receive evaluation for hyperlipidemia or screening for malignancy compared to patients with osteoarthritis.[10] Although this study included a large number of patients, randomly selected from the US Medicare database, all the patients were aged 65 years or above. Thus, their results as well as access to health care may not be generalizable to a broad range of patients.
Several characteristics of our study may explain the different results further. First, unlike many previous studies, this present study compared RA patients with non-RA patients. Similar findings were noted in a previous study of RA and non-RA patients in the Nurses’ Health Study, which showed that care to prevent acute myocardial infarction and cancer screening practices were similar among women with RA compared with women without RA, except aspirin use.[11] Furthermore, we made another comparison between RA patients and patients with a different chronic condition, hypertension, so that both groups would have a similar health care utilization pattern. As most preventive services and tests are provided by primary care physicians, we also collected data on the total number of visits to physicians and percent of visits made to rheumatologists as well as primary care physicians. As expected, RA patients had a higher number of visits to both primary care physicians and rheumatologists. The multivariable Cox models comparing RA patients with non-RA were adjusted for this difference as well as comorbidities.
Second, our study is based on data from a large commercial health plan that insures mainly working adults and their family and a small Medicare managed care population (approximately 10%) across the U.S. Preventive services including screening tests for cervical, breast and colon cancer are reimbursed by both the commercial health plan and Medicare. The availability of health insurance as well as access to the medical care in the study population may be still different compared to other study populations. Therefore, our results may not be generalizable to those without health insurance coverage for preventive services.
Third, there could be misclassification with the diagnoses of RA and hypertension as we mainly relied on claims data to identify them. However, both the ICD codes for RA and hypertension have been previously validated. [20–21] Fourth, although we excluded patients with a history of any malignancy and censored them if they were diagnosed with a cancer during the follow up, it is still possible that some of the tests might have been done for diagnosis rather than screening purposes.
The results of our study may have clinical implication for preventive care of RA patients. While RA patients in this study cohort receive similar screening tests for cancer at similar rates of the two non-RA cohorts we compared, it is not clear what the appropriate rates are for RA. Given the increased risk of some cancers in RA and concerns in the association between various types of RA treatment and malignancy,[22–24] it may be worth investigating the effectiveness of current cancer screening guidelines in patients with RA and in subgroups on specific treatments.
In conclusion, patients with RA did not appear to be at risk for receiving fewer cancer screening than non-RA patients. The majority of both RA and non-RA patients appeared to be screened on a regular basis suggested by the current recommendations, although the results may not be generalizable to those without medical insurance. Continuous efforts should be made to improve and maintain both patients’ and physicians’ awareness of importance of preventive health services in patients with chronic disease such as RA.
Acknowledgments
Kim is supported by the NIH grant K23 AR059677 and received research support from Takeda Pharmaceuticals North America and Pfizer.
Schneeweiss is principal investigator of the Brigham and Women’s Hospital DEcIDE Center on Comparative Effectiveness Research, funded by the Agency for Healthcare Research and Quality, and of the Harvard-Brigham Drug Safety and Risk Management Research Contract, funded by the US Food and Drug Administration. Schneeweiss has received consulting fees from WHISCON, LLC and Booz & Company and received research grants from Pfizer, Novartis and Boehringer Ingelheim.
Solomon is supported by the NIH grants K24 AR055989, P60 AR047782, R21 DE018750, and R01 AR056215. Solomon has received research support from Abbott Immunology, Amgen and Lilly and an educational grant from Bristol-Myers Squibb. He serves in an unpaid role on two Pfizer sponsored trials.
Footnotes
Disclosures
Myers and Liu have nothing to disclose for financial support or conflict of interest.
References
- 1.Gonzalez A, Kremers H, Crowson C, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 2.Mok C, Kwok C, Ho L, et al. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011;63:1182–9. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 3.Myllykangas-Luosujäirvi, Aho K, Isomäki H. Mortality in rheumatoid arthritis. Semin Arthritis Rheum. 1995;25:193–202. doi: 10.1016/s0049-0172(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 4.Sihvonen S, Korpela M, Laippala P, et al. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33:221–7. doi: 10.1080/03009740410005845. [DOI] [PubMed] [Google Scholar]
- 5.Smith R, Cokkinides V, Brooks D, et al. Cancer screening in the United States, 2011: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2011;61:8–30. doi: 10.3322/caac.20096. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Recommendations for Adults. [cited October 4, 2011]; Available from: http://www.uspreventiveservicestaskforce.org/adultrec.htm.
- 7.Redelmeier D, Tan S, Booth G. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–02. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 8.MacLean C, Louie R, Leake B, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284:984–92. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 9.Kiefe C, Funkhouser E, Fouad M, et al. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med. 1998;13:357–65. doi: 10.1046/j.1525-1497.1998.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis J, Arora T, Narongroeknawin P, et al. The delivery of evidence-based preventive care for older Americans with arthritis. Arthritis Res Ther. 2010;12:R144. doi: 10.1186/ar3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon D, Karlson E, Curhan G. Cardiovascular care and cancer screening in female nurses with and without rheumatoid arthritis. Arthritis Rheum. 2004;51:429–32. doi: 10.1002/art.20418. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Servi A, Polinski J, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Schneeweiss S, Seeger J, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–64. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 15.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 16.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 17.Bernatsky S, Cooper G, Mill C, et al. Cancer screening in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:45–9. [PubMed] [Google Scholar]
- 18.Kremers H, Bidaut-Russell M, Scott C, et al. Preventive medical services among patients with rheumatoid arthritis. J Rheumatol. 2003;30:1940–7. [PubMed] [Google Scholar]
- 19.Reddy S, Friedman S, Telford J, et al. Are patients with inflammatory bowel disease receiving optimal care? Am J Gastroenterol. 2005;100:1357–61. doi: 10.1111/j.1572-0241.2005.40849.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Servi A, Polinski J, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjerpe P, Merlo J, Ohlsson H, et al. Validity of registration of ICD codes and prescriptions in a research database in Swedish primary care: a cross-sectional study in Skaraborg primary care database. BMC Med Inform Decis Mak. 2010;10:23. doi: 10.1186/1472-6947-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Chang Y, Wang C, et al. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63:352–8. doi: 10.1002/art.30134. [DOI] [PubMed] [Google Scholar]
- 23.Mok C, Kwok C, Ho L, et al. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011;63:1182–9. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 24.Bongartz T, Sutton A, Sweeting M, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]