Abstract
In the vertebrate retina, melatonin is synthesized by the photoreceptors with high levels of melatonin at night and lower levels during the day. Melatonin exerts its influence by interacting with a family of G-protein-coupled receptors that are negatively coupled with adenylyl cyclase. Melatonin receptors belonging to the subtypes MT1 and MT2 have been identified in the mammalian retina. MT1 and MT2 receptors are found in all layers of the neural retina and in the retinal pigmented epithelium. Melatonin in the eye is believed to be involved in the modulation of many important retinal functions; it can modulate the electroretinogram (ERG), and administration of exogenous melatonin increases light-induced photoreceptor degeneration. Melatonin may also have protective effects on retinal pigment epithelial cells, photoreceptors and ganglion cells. A series of studies have implicated melatonin in the pathogenesis of age-related macular degeneration, and melatonin administration may represent a useful approach to prevent and treat glaucoma. Melatonin is used by millions of people around the world to retard aging, improve sleep performance, mitigate jet lag symptoms, and treat depression. Administration of exogenous melatonin at night may also be beneficial for ocular health, but additional investigation is needed to establish its potential.
Introduction
Melatonin is a neurohormone that plays important roles in the temporal regulation of many aspects of physiology (review in: Wiechmann and Summers, 2008). Accumulating evidence indicates that melatonin plays important roles in retinal physiology and pathophysiology. However, the mechanisms by which melatonin can affect the physiology and pathophysiology of the retina are not well defined. This lack of data is partially due to the fact that the vast majority of mouse strains are genetically incapable of synthesizing melatonin (see Goto et al., 1989, Tosini and Menaker, 1998) and therefore this important animal model has not been used to dissect the action and the mechanisms by which melatonin can influence retinal functions. Our laboratories have recently developed transgenic mice on a melatonin-proficient background (C3H-f+/+) in which melatonin receptors have been genetically removed. These new models are providing important clues on the mechanisms by which melatonin affects retinal function. The aim of this review is to summarize the current literature on the role that melatonin plays in vertebrate retinal physiology.
Regulation of Melatonin Synthesis and Metabolism
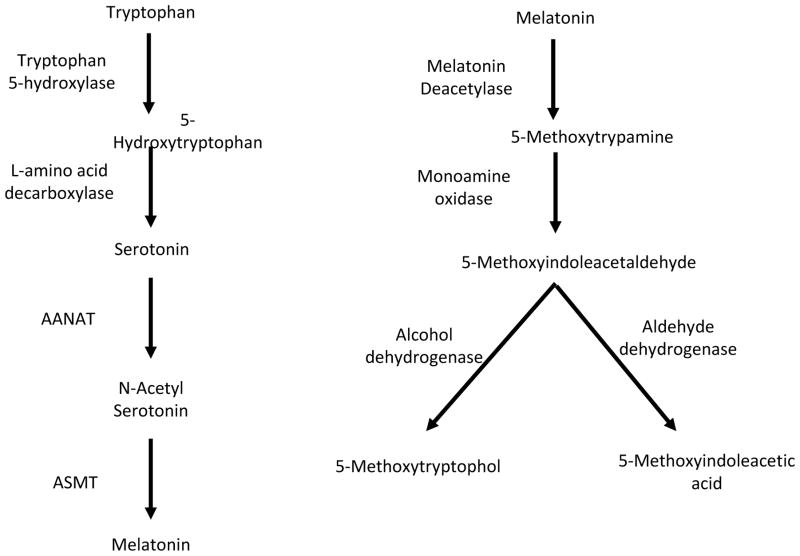
Melatonin is synthesized in the retina of many vertebrate species (from lamprey to mammals) via a well-defined biosynthetic pathway (Tosini and Meanker, 1996; Menaker et al., 1997). Melatonin synthesis starts with the uptake of the amino acid tryptophan from the blood. Tryptophan is converted to melatonin by a series of enzymatic reactions producing serotonin and N-acetylserotonin as important intermediates (Figure 1). In the retina, melatonin is almost exclusively produced by the photoreceptors cells (Cahill and Besharse, 1993; Liu et al., 2004) and under some pathological conditions by other retinal cell types (Sakamoto et al., 2004). In addition, it has been reported that melatonin can also be produced – in smaller amounts - by ganglion cells in the chicken retina (Garbarino-Pico et al., 2004). Once produced, melatonin is not stored but freely diffuses out of the cells. The amount of melatonin produced by the retina is small compared to that in the pineal gland, the primary source of circulating melatonin, and retinal melatonin is thought to act as a local neuromodulator within the eye. However, in a few instances (e.g., quails) retinal melatonin may contribute to the levels of the hormone in the blood (Underwood et al., 1984). In most vertebrate species, retinal melatonin synthesis and levels are high during the night and low during the day (reviewed in: Tosini et al., 2008); however, in a few species (i.e., trout and European sea bass) retinal melatonin levels are high during the daytime (Iigo et al., 1997; Besseau et al., 2006). In the vast majority of the species investigated thus far melatonin synthesis in the retina is under control of retinal circadian clocks since the retinae of fish, amphibians, reptiles, birds and mammals synthesize melatonin in the rhythmic fashion when they are maintained in vitro under constant darkness (reviewed in: Iuvone et al., 2005). In many species, the retinal clock that controls melatonin synthesis appears to be in photoreceptor cells. In Xenopus, chicken and rat, rhythmic melatonin synthesis persists in retinae in which the inner retina has been destroyed (Cahill and Besharse, 1993; Zawilska and Iuvone, 1992; Thomas et al., 1993; Sakamoto et al., 2006; Tosini et al., 2007). In addition, melatonin synthesis and clock gene expression are rhythmic in monolayer cultures of embryonic chick photoreceptors (Chaurasia et al., 2006a).
Figure 1.
Melatonin synthesis starts with up-take of the circulating amino acid tryptophan and the subsequent 5-hydroxylation by tryptophan hydroxylase. 5-Hydroxytryptophan is then converted to serotonin by the action of aromatic L-amino acid decarboxylase. Serotonin is acetylated by arylalkylamine N-acetyltransferase (AANAT) to N-acetylserotonin, which is subsequentely O-methylated and converted to melatonin by acetylserotonin methyltransferase (ASMT), which is also known as hydroxyindole-O-methyltransferase. The metabolism of retinal melatonin illustrated on the right has been demonstrated for Xenopus, reptiles, teleost fish, and chicken but not in mammals (see text).
The key regulatory step in melatonin synthesis is catalyzed by arylalkylamine N-acetyltransferase (AANAT), which converts serotonin to N-acetylserotonin. AANAT is subject to both transcriptional and posttranslational regulation (Iuvone et al., 2005, Figure 2). The control of the transcription of the Aanat gene in photoreceptors is under direct control of the circadian clock (Chen and Baler, 2000) and is independent from suprachiasmatic nuclei (SCN) of the hypothalamus, the master circadian clock. Aanat mRNA rhythmicity in retinal photoreceptors persists after the SCN has been lesioned (Sakamoto et al., 2000) and in cultured photoreceptor cells (Chaurasia et al., 2006a). The chicken and rat Aanat genes contain circadian E-box enhancer elements in their promoters that are directly activated by the Bmal1/Clock and Bmal1/NPAS2 heterodimers (Chen and Baler, 2000; Chong et al., 2000; Haque et al., 2010). Thus, Aanat is considered to be a clock-controlled gene. The Aanat promoter also contains cyclic AMP-response elements that contribute to the circadian expression of the gene (Baler et al., 1997; Baler et al., 1999; Haque et al., 2011)
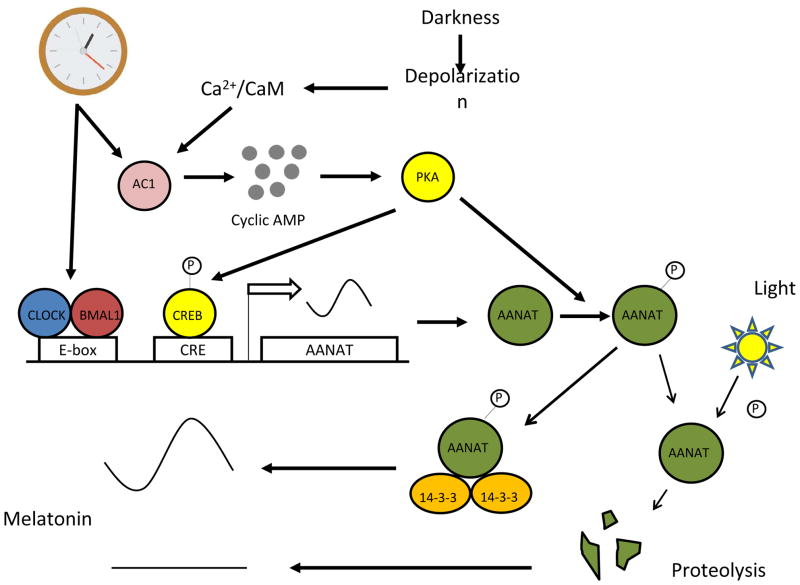
Figure 2.
Regulation of retinal melatonin levels by light and the circadian clock. At night in darkness cAMP levels are elevated, activating PKA, which induces Aanat gene transcription and phosphorylates AANAT protein. Phosphorylated AANAT (pAANAT) associates with 14-3-3 proteins, which activate and stabilize the enzyme resulting in increased conversion of serotonin to N-acetylserotonin, and ultimately to melatonin. Light exposure decreases cAMP levels resulting in dephosphorylation of AANAT and its subsequent degradation by proteasomal degradation. The circadian clock controls melatonin levels by directly regulating Aanat transcription and by gating the cAMP signaling cascade.
AANAT is also subject to posttranslational regulation (Fig. 2). Retinal AANAT is phosphorylated at night (Pozdeyev et al., 2006). Phosphorylation of AANAT promotes its binding to 14:3:3 proteins, which stabilizes and activates the enzyme (Ganguly et al., 2001; Obsil et al., 2001; Pozdeyev et al., 2006). This process is regulated by the retinal circadian clock by controlling the circadian expression of Adcy1, which encodes the type 1 Ca2+/calmodulin-stimulated adenylyl cyclase (AC1) (Fukuhara et al., 2004; Chaurasia et al., 2006b). The rhythm in AC1 in turn generates circadian rhythms of cyclic AMP and PKA-dependent phosphorylation of AANAT (Ivanova and Iuvone, 2003; Fukuhara et al., 2004; Chaurasia et al., 2006b).
Post-translational mechanisms ensure that melatonin levels are maintained at extremely low levels in presence of light. For example, AANAT activity is abolished in animals maintained in constant light (Nowak et al., 1989) and light exposure in the middle of the night induces a very rapid decrease in AANAT activity in the pineal gland and retina (Klein et al., 1997; Hamm et al., 1983). Light exposure rapidly decreases cAMP levels in photoreceptor cells (Orr et al., 1976; DeVries et al., 1978; Nir et al., 2002; Ivanova and Iuvone, 2003) and promotes the dephosphorylation of AANAT, its dissociation from 14–3–3, and its degradation by proteasomal proteolysis (Fukuhara et al., 2001; Iuvone et al., 2002; Pozdeyev et al., 2006). This effect of light appears to be partially a direct effect on photoreceptor cells, combined with an effect of dopamine. Dopamine is released from amacrine and interplexi form cells in response to light and acts on dopamine D4 receptors on the photoreceptor cells to further suppress cyclic AMP synthesis and Ca2+ levels and to inhibit melatonin biosynthesis (Cohen et al., 1992; Zawilska et al., 1994; Tosini and Dirden, 2000; Nir et al., 2002; Ivanova et al., 2008). Such tight control of retinal melatonin levels suggests that high melatonin levels during the light-phase may be deleterious for the photoreceptor cells (Wiechmann and O’Steen, 1992; Sugarawa et al., 1998).
Melatonin biosynthesis is also regulated by circadian control of tryptophan hydroxylase, which converts tryptophan to 5-hydroxytrptophan (5HTP). Tph mRNA, the transcript that encodes tryptophan hydroxylase, is expressed in a circadian fashion in the retinas of many species, including Xenopus laevis, chicken, and rat (Green et al., 1994, 1995; Chong et al., 1998; Liang et al., 2004), and tryptphan hydroxylase activity and AANAT activity show similar daily rhythms (Thomas and Iuvone, 1991; Valenciano et al., 1999; Iuvone et al., 1999). Tryptophan hydroxylase activity may be rate limiting for melatonin biosynthesis at night in darkness, as exogenous 5HTP enhances melatonin synthesis in Xenopus and chicken retinas (Cahill and Besharse, 1990; Iuvone et al., 1999). In contrast to AAANAT, tryptophan hydroxylase is much less sensitive to acute light exposure and AANAT appears to be rate limiting under these conditions (Iuvone et al., 1999).
Another interesting aspect of retinal melatonin regulation is its metabolism. In non-mammalian vertebrates, retinal melatonin is metabolized within the eye (Grace et al., 1991; Cahill and Besharse, 1989; Li et al., 1997) via a well-defined pathway that involves melatonin deacetylation (see Figure 1). Several attempts to detect this pathway in the mammalian retina have failed (Rogawski et al., 1979; Hsu 1982; Grace et al., 1991) and therefore it is not clear whether melatonin is metabolized in the retina of mammals.
While melatonin synthesis in the retina is well established in many mammalian and non-mammalian species, its synthesis in the retina of primates, including humans, has been questioned. Melatonin has been detected in human retina using a specific gas chromatography mass spectrometric assay (Leino, 1984) and AANAT mRNA is expressed in human and macaque retinas (Coon et al., 1996; Coon et al., 2002). Melatonin receptors are present in human retina (Reppert et al., 1995; Scher et al., 2002; Savaskan et al., 2002; Savaskan et al., 2007). However, ASMT transcripts and activity are barely detectable in human and macaque retinas (Rodriguez et al., 1994; Coon et al., 2002). These observations suggest that primate retinas may not contain the complete melatonin biosynthetic pathway and that the source of melatonin in the retina is the circulating pool of pineal gland-derived hormone. Thus, retinal melatonin receptors may be occupied by pineal melatonin. Alternatively, retinal melatonin receptors might by occupied by N-acetylserotonin, the AANAT product, but the affinity of human melatonin receptors for N-acetylserotonin is several orders of magnitude lower than for melatonin (Yuan et al., 1991). The role of AANAT in primate retina is uncertain. In addition to binding to melatonin receptors, N-acetylserotonin activates TrkB receptors in the retina (Jang et al., 2010) and may provide neuroprotection to photoreceptors and retinal neurons (review in: Tosini et al., 2012). It has also been proposed that AANAT may serve to detoxify reactive arylalkylamines in the retina to prevent them from reacting with retinaldehyde (Klein 2004).
Melatonin: Site of Action and Signaling
Melatonin exerts its influence by interacting with a family of G-protein-coupled receptors (GPCR) that are negatively coupled with adenylyl cyclase (Reppert, 1997, Jockers et al., 2008) although cAMP-independent transduction pathways are also involved (Dubocovich et al., 2010). Two subtypes of melatonin receptors have been identified in mammals, the MT1 and MT2 receptors, which are encoded by the MTNR1A and MTNR1B genes, respectively. Both subtypes are expressed in the retina (reviewed in: Wiechmann and Summers, 2008). In rats MT1 receptors are found in the inner nuclear layer (horizontal and amacrine cells), the inner plexiform layer, retinal ganglion cells (RGCs), and the retinal pigmented epithelium (RPE) (Fujieda et al., 1999). Dopaminergic neurons in the guinea pig express MT1 receptors (Fujieda et al., 2000), suggesting that melatonin can directly modulate the activity of these cells. In humans, melatonin receptors (MT1 and MT2) have been located on the rod photoreceptors and on GCs (Savaskan et al., 2002; Scher et al., 2002; Meyer et al., 2002; Savaskan et al., 2007). In the mouse, MT1 receptors have been localized to photoreceptors, inner retinal neurons and RGCs (Baba et al., 2009; Sengupta et al., 2011). The fact that melatonin receptors are expressed on the same cells responsible for its synthesis raises the intriguing hypothesis that melatonin may feedback on the photoreceptors to regulate its own levels.
The signaling pathways activated by MT1 and MT2 receptors are very similar for the two subtypes when expressed heterologously (Jockers et al., 2008). The widely observed co-expression of MT1 and MT2 and their potential to form heteromeric complexes in vitro are of particular interest in this context. MT1 and MT2 were indeed among the first GPCRs that have been shown to homo- and heteromerize in a constitutive manner when transfected in HEK 293 cell at physiological levels (Ayoub et al., 2002). Interestingly, the propensity of melatonin receptors to form homo- and heteromers is not identical. Whereas the propensity of MT1/MT2 heteromer and MT1 homomer formation is similar, that of MT2 homomer formation is 3 to 4-fold lower, suggesting that the MT2 receptor preferentially exists as heteromeric complexes with MT1 or as monomers (Ayoub et al., 2004). Hence, it is possible that in the retina - and more specifically in the photoreceptors - MT1 and MT2 form functional melatonin receptor heteromeric units that may activate different pathways from those activated by MT1 and MT2 monomers.
Finally it is worth noting that there is a very large body of evidence documenting melatonin as an antioxidant (Reiter et al., 2009). In the retina melatonin acts as antioxidant in retinal photoreceptors (Marchiafava and Longoni, 1999). It decreases lipid peroxidation of polyunsaturated fatty (Guajardo et al., 2003), reduces NO-induced lipid peroxidation in rat retinal homogenates (Siu et al., 1999) and may also reduce retinal oxidative damage from ischemia-reperfusion injury (Celebi et al., 2002).
Role of Melatonin in the Modulation of Retinal Functions
Melatonin may alter the sensitivity of photoreceptors and second-order neurons at night when photopic input is at its lowest level (Wiechmann et al., 1988). In the carp retina melatonin can modulate glutamatergic transmission from cones to cone-driven bipolar cells (Huang et al., 2005) and may potentiate responses of ON bipolar cells to rod signals (Ping et al., 2008). In Xenopus laevis, melatonin, acting through melatonin receptors on rod photoreceptor membranes, directly stimulates the responsiveness of rod photoreceptors to light (Wiechmann et al., 2003). This supports the hypothesis that melatonin acts both as an autocrine and a paracrine signal and binds to specific receptors in photoreceptors and other retinal cells to increase visual sensitivity. Administration of exogenous melatonin in X. laevis and in the carp increases the amplitude of the scotopic ERG (Wiechmann et al., 2003; Ping et al., 2008). In chickens and pigeons, administration of exogenous melatonin during the day reduces the amplitude of the b-wave (Lu et al., 1995), and constant administration of melatonin abolishes rhythmicity of a-wave and b-wave implicit times and b-wave amplitude (McGoogan and Cassone, 1999). In humans, oral administration of melatonin decreases the amplitude of the cone ERG (Gagne et al., 2009) and the amplitude of the cone and mixed rod-cone response was negatively correlated with the concentration of endogenous salivary melatonin (Rufiange et al., 2002). In mice, administration of exogenous melatonin (1 mg/kg) during the day increases the amplitudes of a- and b- waves and lowers the scotopic threshold response to levels observed at night under control conditions (Baba et al., 2009); removal of MT1 receptors abolishes these effects (Baba et al., 2009). In melatonin proficient mice (C3H-f+/+) there are daily rhythms in both the scotopic and photopic ERG responses; these are absent in MT1 knock-out (MT1−/−) mice (Baba et al., 2009; Sengupta et al., 2011). Therefore, it is clear that melatonin has influence over many different visual functions, although the precise mechanisms by which this hormone mediates these functions are likely to vary in a species-dependent manner.
Photoreceptor rod outer segments (ROS) are continuously renewed by the assembly of new membrane disks at the base of the ROS and by displacement of older disks at the top of the outer segment. These old disks are shed from the apical part of the ROS and phagocytized by the RPE. These two phenomena occur every day in a synchronized fashion shortly after the onset of light in the rod photoreceptors, and at the onset of night in the cones. Moreover, the daily rhythms of disk shedding and phagocytosis persist in constant darkness, indicating that they are under the control of circadian clocks located in the retina (LaVail, 1976; Teirstein et al., 1980; Terman et al., 1993; Grace et al., 1996).
Earlier studies suggested a role for melatonin in the regulation of disk shedding. Exogenous melatonin led to activation of disk shedding in Xenopus retina (Besharse and Dunis, 1983) and an increase in the frequency of large phagosomes in rat RPE cells (White and Fisher, 1989). However, a study in which the circadian regulation of disk shedding was compared between in melatonin proficient mice (C3H/f+/+) and melatonin-deficient mice (C57/BL6) questioned the contribution of melatonin in the regulation of disk shedding in mice (Grace et al., 1999). This study reported that disk shedding was rhythmic in both strains and was not affected by administration of exogenous melatonin, thus suggesting that circadian factors other than melatonin are important for the regulation of the circadian rhythm in disk shedding. Additional studies are needed to determine the mechanisms regulating circadian disk shedding in mammals.
Melatonin as a Key Regulator of Retinal Circadian Rhythms
Several studies have shown that melatonin and dopamine play opposing roles in the regulation of retinal adaptive physiology (reviewed in: Green and Besharse, 2004; Tosini et al., 2008). Dopamine functions as a humoral signal for light, producing light adaptive physiology. Melatonin, on the other hand, produces dark-adaptive effects. In many species, the synthesis and release of both melatonin and dopamine are under circadian control, with melatonin released at night and dopamine during the daytime. Melatonin inhibits the release of dopamine through an action on melatonin receptors (Dubocovich, 1983; Boatright et al., 1994; Ribelayga et al., 2004a), and dopamine inhibits the synthesis and release of melatonin from photoreceptor cells by acting on D2-like dopamine receptors (Zawilska and luvone, 1992; Nguyen-Legros et al., 1996; Tosini and Dirden, 2000). Thus, the melatonin secreting photoreceptors and dopamine secreting amacrine/interplexiform cells form a cellular feedback loop functioning to regulate circadian retinal physiology. The circadian rhythm of dopamine release and metabolism appears to be dependent on melatonin. Retinal dopamine content and metabolism are circadian in mice that synthesize melatonin, but not in mice that are genetically incapable of synthesizing melatonin (Nir et al., 2000; Doyle et al., 2002, Pozdeyev et al., 2008); and daily injections of melatonin induce circadian rhythms of dopamine in retinas of mice that are unable to synthesize the neurohormone (Doyle et al., 2002). The role of melatonin in controlling DA rhythmicity is not unique to mice. Previous work in Xenopus laevis has also indicated that DA and D2-like receptors are involved in the entrainment of circadian rhythm of retinal melatonin synthesis (Cahill and Besharse, 1991; Hasegawa and Cahill, 1999). DA and quinpirole, a D2R-like agonist, induce Per2 mRNA levels in Xenopus photoreceptors (Steenhard and Besharse, 2000; Besharse et al., 2004) suggesting that DA -- via D2R-like receptors -- and Per2 are involved in the entrainment of the circadian clock located in the photoreceptors of X. laevis and thus in the regulation of retinal melatonin synthesis. In fish, regulation of rhythmic dopamine release also depends on activation of melatonin receptors (Ribelayga et al., 2004b).
Melatonin may have a profound impact of the function of the molecular clockwork. For example, disruption of MT1 melatonin signaling has a profound impact on the regulation of clock genes and clock-controlled genes in many tissues. Von Gall et al. (2002) reported that rhythmic expression of Period 1 (gene and protein) in the pituitary gland depends on melatonin via MT1 signaling and that melatonin affects the amplitude and phase of the transcripts of others clock genes (e.g., Per1, and Cry1) in the mouse retina (Dinet and Korf, 2007; Dinet et al., 2007). Such results indicated that melatonin, at least in some tissues, is not only a clock output, but can also regulates the expression of canonical clock genes. In this context, is important to mention that circadian clocks are directly involved in the regulation of cellular metabolism (Bass and Takahashi, 2010) and, consequently, alteration of the clock in cells like the photoreceptors with a high metabolic rate may result in adverse outcomes.
Melatonin and Retinal Pathophysiology
Melatonin has been implicated in the modulation of intraocular pressure (IOP) (Samples et al., 1988; Osborne and Chidlow 1994; Pintor et al., 2001; Wiechmann and Wirsig-Wiechmann, 2001; Alarma-Estrany et al., 2008) and it has been suggested that melatonin or melatonin analogs may be useful in the treatment of glaucoma (Lundmark et al., 2007; Belforte et al., 2010). In rabbits, topical application of melatonin or 5-methoxycarbonylamino-N-acetyltryptamine (5-MCA-NAT, a melatonin analogue) leads to a reduction in IOP, whereas luzindole (a MT1 and MT2 receptor antagonist) abolishes the effect of both compounds, supporting a role for MT1 or MT2 in the regulation of IOP (Pintor et al., 2001). 5-MCA-NAT application also reduces IOP in glaucomatous monkey eyes (Serle et al., 2004). Additional studies have reported that many melatonin antagonists, such as prazozin, DH-97 and 4-P-PDOT, reverse the effect of 5-MCA-NAT in a dose-dependent manner (Pintor et al., 2003). A recent study demonstrated that 5-MCA-NAT acts via MT1 or MT2 to reduce IOP (Alarma-Estrany et al., 2009). In humans, administration of oral melatonin causes a small but significant decrease in the IOP of individuals kept in bright light to suppress endogenous melatonin (Samples et al., 1988) and in patients undergoing cataract surgery (Ismail and Mowafi, 2009).
A recent study further supports a role for melatonin in the modulation of IOP levels and the development of glaucoma that is consistent with the pattern of melatonin synthesis. IOP in MT1−/− mice was higher (about 2 mmHg) than in the wild type mice during the night, but not during the day (Alcantara-Contreras et al., 2011). MT1−/− mice also showed a significant decrease in the number of cells in the ganglion cell layer during aging compared to wild type mice (Baba et al., 2009; Alcantara-Contreras et al., 2011), suggesting that even a small increase in nocturnal IOP may have a significant effect of RGCs survival. The observation that administration of exogenous melatonin in WT mice reduced IOP at night but not during the day further suggests a role for melatonin in the modulation of nocturnal IOP. Interestingly, removal of MT2 receptors did not affect the daily rhythm in IOP. However, exogenous melatonin was ineffective at lowering IOP in MT2 knock-out mice, suggesting that MT2 receptors, as well as MT1 receptors, may be involved in the regulation of the IOP. The observation that melatonin receptors (MT1 and MT2) are present in the ciliary body (Osborne and Chidlow 1994; Wiechmann and Wirsig-Wiechmann, 2001; Alarma-Estrany et al., 2008) further suggests a role for melatonin receptors in the regulation of IOP.
Altogether these results indicate that melatonin and its analogues could be a promising resource in the management and treatment of glaucoma, but further studies are required to understand the mechanism(s) by which melatonin and its receptors regulate IOP and possibly protect ganglion cells.
Melatonin may also have protective effects on other retinal cell types, including retinal pigment epithelial cells and photoreceptors. Melatonin protects cultured RPE cells from oxidative stress and ischemia-induced cell death (Osborne et al., 1998; Liang et al., 2004; Fu et al., 2012) and delays photoreceptor degeneration in rds mutant mice (Liang et al., 2001). In addition, the age-related loss of photoreceptors cells is accelerated in MT1−/− mice compared to wild type controls (Baba et al., 2009; Alcantara-Contreras et al., 2011). A series of studies have implicated melatonin in the pathogenesis of age-related macular degeneration (AMD). Yi et al. (2005) reported that daily administration of melatonin (3mg) may protect the retina and delay the progression of AMD. Rosen et al. (2009) reported that production of melatonin is decreased in AMD patients with respect to age-matched controls, suggesting that a deficiency in melatonin may play a role in the occurrence of AMD. In pseudophakic patients with AMD, daytime levels of melatonin were significantly higher than in pseudophakic patients without ocular pathology (Schmid-Kubista et al., 2009), suggesting that the daily rhythm of melatonin may be disrupted in AMD patients. Elevated daytime levels of melatonin may have a detrimental effect since melatonin enhances light-induced retinal degeneration (see below). Expression of melatonin receptors is altered in retinas of Alzheimer’s disease patients with degenerating photoreceptor cells, with increased expression of MT1 receptors and decreased expression of MT2 receptors (Savaskan et al., 2002, 2007). A further indication of the possible role of melatonin in age-related pathologies can be found in the observation that retinal melatonin synthesis decreases during aging (Pulido and Clifford, 1986; Tosini et al., 2006) and that the responsiveness to the administration of exogenous melatonin steadily decreases during aging (Baba et al. 2012). The mechanisms by which melatonin influences photoreceptor viability during aging are unknown, but it is reasonable to speculate that melatonin can affect the circadian clocks in photoreceptors and RPE cells and, thereby, metabolism in these cells.
Circadian clocks prevent the occurrence of high melatonin levels in the presence of light. It may be critical that melatonin levels are low during the daytime, as melatonin potentiates light-induced oxidative damage in the retina. Albino rats injected with melatonin and exposed to bright light showed significantly greater photoreceptor cell death than vehicle-treated controls (Wiechmann and O’Steen, 1992). Administration of luzindole (a melatonin receptor antagonist) at night significantly reduced light-induced photoreceptor degeneration of rats exposed to bright light on the following day (Sugawara et al., 1998). In addition, there is a circadian rhythm of sensitivity to light-induced retinal degeneration (Organisciak et al., 2000; Vaughn et al., 2002), which peaks at night when melatonin levels are high. The mechanisms by which melatonin increases the susceptibility of the retina to light damage are still unknown.
Conclusions and Future Research Directions
Recent genome wide association studies (GWAS) have indicated that polymorphisms in genes encoding melatonin receptors or melatonin synthesizing enzymes are associated with the pathogenesis of type 2 diabetes, polycystic ovary syndrome, and autism spectrum disorders (Bonnefond et al., 2012; Li et al., 2011; Chaste et al., 2010). Melatonin may be involved in several retinal pathologies but, unfortunately, no population studies using GWAS have examined the association of polymorphisms in melatonin-related genes with ocular diseases. Little is known about the mechanisms whereby melatonin regulates retinal physiology or affects retinal cellular viability. We believe that the transgenic mice generated by our laboratories may provide useful tools to probe the mechanisms by which melatonin affects retinal physiology and pathology. Finally, it is important to note that melatonin and its analogues are currently used by millions of people around the world to retard aging, improve sleep performance, ameliorate jet-lag symptoms and treat depression. Administration of exogenous melatonin at night may also benefit ocular health, but further translational and clinical investigations are needed to establish its potential.
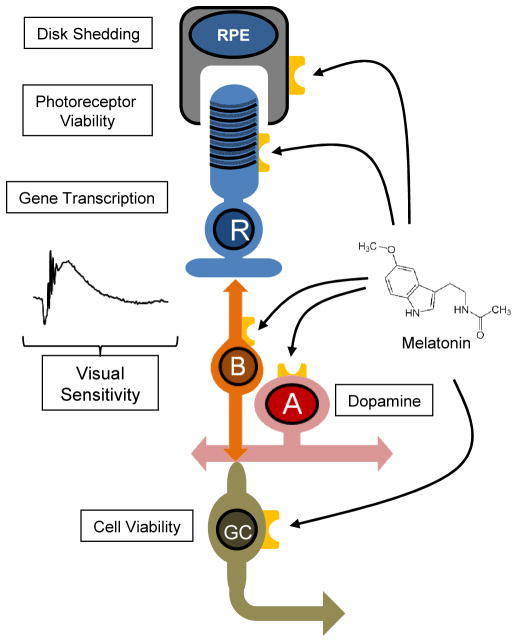
Figure 3.
Melatonin receptors are expressed in many retinal cell types. Activation of these receptors may modulate several retinal functions such as: disk shedding, retinal cell viability; visual sensitivity; and dopamine levels and metabolism. RPE=retinal pigmented epithelium; R= rod photoreceptors; B= bipolar cells; A= amacrine cells; GC= retinal ganglion cells.
Highlights.
Regulation of melatonin synthesis and metabolism.
Distribution of melatonin receptors in the vertebrate eye
Role of melatonin receptors in the regulation of retinal physiology
Role of melatonin in the development of retinal pathology
Use of melatonin to treat ocular diseases
Acknowledgments
Research in the authors’ laboratories is supported by grants from the National Institutes of Health [R01 NS43459, R21 EY028821, R01 EY022216 (GT); R01 EY004864 (PMI), P30 EY006360 (PMI)], and Research to Prevent Blindness, Inc. (RPB) (PMI). PMI is a recipient of Senior Scientific Investigator Award from RPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarma-Estrany P, Crooke A, Mediero A, Peláez T, Pintor J. Sympathetic nervous system modulates the ocular hypotensive action of MT2-melatonin receptors in normotensive rabbits. J Pineal Res. 2008;45:468–475. doi: 10.1111/j.1600-079X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Alarma-Estrany P, Crooke A, Pintor J. 5-MCA-NAT does not act through NQO2 to reduce intraocular pressure in New-Zealand white rabbit. J Pineal Res. 2009;47:201–219. doi: 10.1111/j.1600-079X.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- Alcantara-Contreras S, Baba K, Tosini G. Removal of Melatonin Receptor Type 1 Increases Intraocular Pressure and Retinal Ganglion Cells Death in the Mouse. Neuroscience Letters. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptors heterodimers with distint ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M, Martinez-Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Mazzoni F, Owino S, Contreras-Alcantara S, Strettoi E, Tosini G. Age-related changes in the daily rhythm of photoreceptor functioning and circuitry in a melatonin-proficient mouse strain. PLoS One. 2012;7:e37799. doi: 10.1371/journal.pone.0037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R, Covington S, Klein DC. The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. J Biol Chem. 1997;272:6979–6985. doi: 10.1074/jbc.272.11.6979. [DOI] [PubMed] [Google Scholar]
- Baler R, Covington S, Klein DC. Rat arylalkylamine N-acetyltransferase gene: upstream and intronic components of a bipartite promoter. Biol Cell. 1999;91:699–705. [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte NA, Moreno MC, de Zavalía N, Sande PH, Chianelli MS, Keller-Sarmiento MI, Rosenstein RE. Melatonin: a novel neuroprotectant for the treatment of glaucoma. J Pineal Res. 2010;48:353–64. doi: 10.1111/j.1600-079X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Zhuang M, Freeman K, Fogerty J. Regulation of photoreceptor Per1 and Per2 by light, dopamine and a circadian clock. Eur J Neurosci. 2004 Jul;20(1):167–74. doi: 10.1111/j.1460-9568.2004.03479.x. [DOI] [PubMed] [Google Scholar]
- Besseau L, Benyassi A, Møller M, Coon SL, Weller JL, Boeuf G, Klein DC, Falcón J. Melatonin pathway: breaking the ‘high-at-night’ rule in trout retina. Exp Eye Res. 2006;82:620–627. doi: 10.1016/j.exer.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis Neurosci. 1994;11:1013–1008. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Clément N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S, Hercberg S, Hadjadj S, Balkau B, Marre M, Lantieri O, Langenberg C, Bouatia-Naji N, Charpentier G, Vaxillaire M, Rocheleau G, Wareham NJ, Sladek R, McCarthy MI, Dina C, Barroso I, Jockers R, Froguel P Meta-Analysis of Glucose and Insulin-Related Traits Consortium (MAGIC) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Retinal melatonin is metabolized within the eye of Xenopus laevis. Proc Natl Acad Sci U S A. 1989;86:1098–1102. doi: 10.1073/pnas.86.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian regulation of melatonin in the retina of Xenopus laevis: limitation by serotonin availability. J Neurochem. 1990;54:716–719. doi: 10.1111/j.1471-4159.1990.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–71. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Celebi S, Dilsiz N, Yilmaz T, Kükner AS. Effects of melatonin, vitamin E and octreotide on lipid peroxidation during ischemia-reperfusion in the guinea pig retina. Eur J Ophthalmol. 2002;12:77–83. [PubMed] [Google Scholar]
- Chaste P, Clement N, Mercati O, Guillaume JL, Delorme R, Botros HG, Pagan C, Périvier S, Scheid I, Nygren G, Anckarsäter H, Rastam M, Ståhlberg O, Gillberg C, Serrano E, Lemière N, Launay JM, Mouren-Simeoni MC, Leboyer M, Gillberg C, Jockers R, Bourgeron T. Identification of pathway-biased and deleterious melatonin receptor mutants in autism spectrum disorders and in the general population. PLoS One. 2010;5:e11495. doi: 10.1371/journal.pone.0011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Pozdeyev N, Haque R, Visser A, Ivanova TN, Iuvone PM. Circadian clockwork machinery in neural retina: evidence for the presence of functional clock components in photoreceptor-enriched chick retinal cell cultures. Mol Vis. 2006a;12:215–223. [PubMed] [Google Scholar]
- Chaurasia SS, Haque R, Pozdeyev N, Jackson CR, Iuvone PM. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J Neurochem. 2006b;99:1142–1150. doi: 10.1111/j.1471-4159.2006.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Baler R. The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain Res Mol Brain Res. 2000;81:43–50. doi: 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem. 2000;275:32991–32998. doi: 10.1074/jbc.M005671200. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci US A. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon SL, Mazuruk K, Bernard M, Roseboom PH, Klein DC, Rodriguez IR. The human serotonin N-acetyltransferase (EC 2.3.1.87) gene (AANAT): structure, chromosomal localization, and tissue expression. Genomics. 1996;34:76–84. doi: 10.1006/geno.1996.0243. [DOI] [PubMed] [Google Scholar]
- Coon SL, Del Olmo E, Young WS, 3rd, Klein DC. Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87) J Clin Endocrinol Metab. 2002;87:4699–706. doi: 10.1210/jc.2002-020683. [DOI] [PubMed] [Google Scholar]
- DeVries GW, Cohen AI, Hall IA, Ferrendelli JA. Cyclic nucleotide levels in normal and biologically fractionated mouse retina: effects of light and dark adaptation. J Neurochem. 1978;31:1345–1351. doi: 10.1111/j.1471-4159.1978.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Dinet V, Korf HW. Impact of melatonin receptors on pCREB and clock-gene protein levels in the murine retina. Cell Tissue Res. 2007;330:29–34. doi: 10.1007/s00441-007-0468-5. [DOI] [PubMed] [Google Scholar]
- Dinet V, Ansari N, Torres-Farfan C, Korf HW. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonindeficient (C57BL) mice. J Pineal Res. 2007;42:83–91. doi: 10.1111/j.1600-079X.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tang M, Fan Y, Zou H, Sun X, Xu X. Anti-apoptotic effects of melatonin in retinal pigment epithelial cells. Front Biosci. 17:1461–1468. doi: 10.2741/3997. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Expression of mt1 melatonin receptor in rat retina: evidence for multiple cell targets for melatonin. Neuroscience. 1999;93:793–799. doi: 10.1016/s0306-4522(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GC-K, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neuroscience. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara C, Dirden JC, Tosini G. Photic regulation of melatonin in rat retina and the role of proteasomal proteolysis. Neuroreport. 2001;12:3833–3837. doi: 10.1097/00001756-200112040-00046. [DOI] [PubMed] [Google Scholar]
- Gagne AM, Danilenko KV, Rosolen SG, Herbert M. Impact of oral melatonin on the electroretinogram. J Circadian Rhythms. 2009;7:14. doi: 10.1186/1740-3391-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Gastel JA, Weller JL, Schwartz C, Jaffe H, Namboodiri MA, Coon SL, Hickman AB, Rollag M, Obsil T, Beauverger P, Ferry G, Boutin JA, Klein DC. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc Natl Acad Sci U S A. 2001;98:8083–8088. doi: 10.1073/pnas.141118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino-Pico E, Carpentieri AR, Contin MA, Sarmiento MI, Brocco MA, Panzetta P, Rosenstein RE, Caputto BL, Guido ME. Retinal ganglion cells are autonomous circadian oscillators synthesizing N-acetylserotonin during the day. J Biol Chem. 2004;279:51172–51181. doi: 10.1074/jbc.M309248200. [DOI] [PubMed] [Google Scholar]
- Chong NW, Cassone VM, Bernard M, Klein DC, Iuvone PM. Circadian expression of tryptophan hydroxylase mRNA in the chicken retina. Brain Res Mol Brain Res. 1998;61:243–250. doi: 10.1016/s0169-328x(98)00219-8. [DOI] [PubMed] [Google Scholar]
- Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Grace MS, Cahill GM, Besharse JC. Melatonin deacetylation: retinal vertebrate class distribution and Xenopus laevis tissue distribution. Brain Res. 1991;559:56–63. doi: 10.1016/0006-8993(91)90286-5. [DOI] [PubMed] [Google Scholar]
- Grace MS, Wang LM, Pickard GE, Besharse JC, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996;735:93–100. doi: 10.1016/0006-8993(96)00600-2. [DOI] [PubMed] [Google Scholar]
- Grace MS, Chiba A, Menaker M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci. 1999;16:909–918. doi: 10.1017/s0952523899165106. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Tryptophan hydroxylase expression is regulated by a circadian clock in Xenopus laevis retina. J Neurochem. 1994;62:2420–2428. doi: 10.1046/j.1471-4159.1994.62062420.x. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Guajardo MH, Terrasa AM, Catalá A. Protective effect of indoleamines on in vitro ascorbate-Fe2+ dependent lipid peroxidation of rod outer segment membranes of bovine retina. J Pineal Res. 2003;35:276–282. doi: 10.1034/j.1600-079x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J Neurochem. 1999;72:1812–20. doi: 10.1046/j.1471-4159.1999.0721812.x. [DOI] [PubMed] [Google Scholar]
- Hamm HE, Takahashi JS, Menaker M. Light-induced decrease of serotonin N-acetyltransferase activity and melatonin in the chicken pineal gland and retina. Brain Res. 1983;266:287–293. doi: 10.1016/0006-8993(83)90660-1. [DOI] [PubMed] [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Weller J, Klein D, Iuvone PM. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem. 2010;113:1296–1306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Chong NW, Ali F, Chaurasia SS, Sengupta T, Chun E, Howell JC, Klein DC, Iuvone PM. Melatonin synthesis in retina: cAMP-dependent transcriptional regulation of chicken arylalkylamine N-acetyltransferase by a CRE-like sequence and a TTATT repeat motif in the proximal promoter. J Neurochem. 2011;119:6–17. doi: 10.1111/j.1471-4159.2011.07397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LL. Brain aryl acylamidase. Int J Biochem. 1982;14:1037–1042. doi: 10.1016/0020-711x(82)90157-4. [DOI] [PubMed] [Google Scholar]
- Huang H, Lee SC, Yang XL. Modulation by melatonin of glutamatergic synaptic transmission in the carp retina. J Physiol. 2005;569:857–871. doi: 10.1113/jphysiol.2005.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo M, Sánchez-Vázquez FJ, Madrid JA, Zamora S, Tabata M. Unusual responses to light and darkness of ocular melatonin in European sea bass. Neuroreport. 1997;8:1631–1635. doi: 10.1097/00001756-199705060-00015. [DOI] [PubMed] [Google Scholar]
- Ismail SA, Mowafi HA. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth Analg. 2009;108:1146–1151. doi: 10.1213/ane.0b013e3181907ebe. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Chong NW, Bernard M, Brown AD, Thomas KB, Klein DC. Melatonin biosynthesis in chicken retina. Regulation of tryptophan hydroxylase and arylalkylamine N-acetyltransferase. Adv Exp Med Biol. 1999;460:31–41. [PubMed] [Google Scholar]
- Iuvone PM, Brown AD, Haque R, Weller J, Zawilska JB, Chaurasia SS, Ma M, Klein DC. Retinal melatonin production: role of proteasomal proteolysis in circadian and photic control of arylalkylamine N-acetyltransferase. Invest Ophthalmol Vis Sci. 2002;43:564–572. [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Haque R, Klein DC, Chaurasia SS. Circadian Clocks, Clock-Controlled Genes and Melatonin Biosynthesis in the Retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Alonso-Gomez AL, Iuvone PM. Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain Res. 2008;1207:111–119. doi: 10.1016/j.brainres.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K. N-Acetylserotonin Activates TrkB Receptor in a Circadian Rhythm. Proc Natl Acad Sci, U S A. 2010;107:3876–3881. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Bégay V, Falcón J, Cahill GM, Cassone VM, Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. [PubMed] [Google Scholar]
- Klein DC. The 2004 Aschoff/Pittendrigh lecture: Theory of the origin of the pineal gland--a tale of conflict and resolution. J Biol Rhythms. 2004;19:264–279. doi: 10.1177/0748730404267340. [DOI] [PubMed] [Google Scholar]
- La Vail MM. Rod outer segment disk shedding in the rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1073. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Leino M. 6-Methoxy-tetrahydro-beta-carboline and melatonin in the human retina. Exp Eye Res. 1984;38:325–30. doi: 10.1016/0014-4835(84)90170-2. [DOI] [PubMed] [Google Scholar]
- Li P, Pang SF, Tsang CW. Retinal 5-methoxytryptamine and 5-methoxyindole-3-acetic acid in the rat and quail: diurnal rhythms and interspecies differences. Biochem Biophys Res Commun. 1997;239:353–356. doi: 10.1006/bbrc.1997.7475. [DOI] [PubMed] [Google Scholar]
- Li C, Shi Y, You L, Wang L, Chen ZJ. Melatonin receptor 1A gene polymorphism associated with polycystic ovary syndrome. Gynecol Obstet Invest. 2011;72:130–134. doi: 10.1159/000323542. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Zaixin Y, Cideciyan AV, Jacobson SG, Bennett J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport. 2001;12:1011–1014. doi: 10.1097/00001756-200104170-00029. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Green L, Wang C, Alssadi R, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res. 2004;78:1069–75. doi: 10.1016/j.exer.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Liang J, Wessel JH, 3rd, Iuvone PM, Tosini G, Fukuhara C. Diurnal rhythms of tryptophan hydroxylase 1 and 2 mRNA expression in the rat retina. Neuroreport. 2004;15:1497–5100. doi: 10.1097/01.wnr.0000131007.59315.66. [DOI] [PubMed] [Google Scholar]
- Liu C, Fukuhara C, Wessel JH, Iuvone PM, Tosini G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315:197–201. doi: 10.1007/s00441-003-0822-1. [DOI] [PubMed] [Google Scholar]
- Lu J, Zoran MJ, Cassone VM. Daily and circadian variation in the electroretinogram of the domestic fowl: effects of melatonin. J Comp Physiol A. 1995;177:299–306. doi: 10.1007/BF00192419. [DOI] [PubMed] [Google Scholar]
- Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP, Rosenstein RE. Melatonin in the eye: implications for glaucoma. Exp Eye Res. 2007;84:1021–1030. doi: 10.1016/j.exer.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Marchiafava PL, Longoni B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res. 1999;26:184–189. doi: 10.1111/j.1600-079x.1999.tb00582.x. [DOI] [PubMed] [Google Scholar]
- McGoogan JM, Cassone VM. Circadian regulation of chick electroretinogram: effects of pinealectomy and exogenous melatonin. Am J Physiol. 1999;277:R1418–427. doi: 10.1152/ajpregu.1999.277.5.R1418. [DOI] [PubMed] [Google Scholar]
- Menaker M, Moreira LF, Tosini G. The Evolution of Vertebrate Circadian Organization. Brazilian Journal of Medical and Biological Research. 1997;30:305–313. doi: 10.1590/s0100-879x1997000300003. [DOI] [PubMed] [Google Scholar]
- Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, Wirz-Justice A, Savaskan E. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in dystrophic retinas of homozygous and heterozygous retinal degeneration slow (rds) mice. Brain Res. 2000;884:13–22. doi: 10.1016/s0006-8993(00)02855-9. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2273. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J, Chanut E, Versaux-Botteri C, Simon A, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem. 1996;67:2514–2520. doi: 10.1046/j.1471-4159.1996.67062514.x. [DOI] [PubMed] [Google Scholar]
- Nowak JZ, Zurawska E, Zawilska J. Melatonin and its generating system in vertebrate retina: circadian rhythm, effect of environmental lighting and interaction with dopamine. Neurochem Int. 1989;14:397–406. doi: 10.1016/0197-0186(89)90027-2. [DOI] [PubMed] [Google Scholar]
- Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F. Crystal structure of the 14-3-3zeta:serotonin N-acetyltransferase complex. A role for scaffolding in enzyme regulation. Cell. 2001;105:257–67. doi: 10.1016/s0092-8674(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- Osborne NN, Chidlow G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye. Exp Eye Res. 1994;59:3–9. doi: 10.1006/exer.1994.1076. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Nash MS, Wood JP. Melatonin counteracts ischemia-induced apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:2374–2383. [PubMed] [Google Scholar]
- Orr HT, Lowry OH, Cohen AI, Ferrendelli JA. Distribution of 3′:5′-cyclic AMP and 3′:5′-cyclic GMP in rabbit retina in vivo: selective effects of dark and light adaptation and ischemia. Proc Natl Acad Sci US A. 1976;73:4442–4445. doi: 10.1073/pnas.73.12.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Y, Huang H, Zhang XJ, Yang XL. Melatonin potentiates rod signals to ON type bipolar cells in fish retina. J Physiol. 2008;586:2683–2694. doi: 10.1113/jphysiol.2008.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J, Martin L, Pelaez T, Hoyle CH, Peral A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur J Pharmacol. 2001;416:251–254. doi: 10.1016/s0014-2999(01)00864-0. [DOI] [PubMed] [Google Scholar]
- Pintor J, Pelaez T, Hoyle CH, Peral A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: further evidence for an MT3 receptor. Br J Pharmacol. 2003;138:831–836. doi: 10.1038/sj.bjp.0705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev N, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, Du Y, Fu H, Weller J, Klein DC, Iuvone PM. Photic regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J Neurosci. 2006;26:9153–9161. doi: 10.1523/JNEUROSCI.1384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev NK, Tosini G, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:26691–26700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido O, Clifford J. Age-associated changes in the circadian rhythm of retinal N-acetylserotonin and melatonin in rats with pigmented eyes. Exp Gerontol. 1986;21:23–30. doi: 10.1016/0531-5565(86)90014-8. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 2009;44:175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci US A. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM. Melatonin receptors: molecular biology of a new family of G-protein-coupled receptors. J Biol Rhythms. 1997;12:528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004a;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol. 2004b;544:801–816. doi: 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez IR, Mazuruk K, Schoen TJ, Chader GJ. Structural analysis of the human hydroxyindole-O-methyltransferase gene. Presence of two distinct promoters. J Biol Chem. 1994;269:31969–31977. [PubMed] [Google Scholar]
- Rogawski MA, Roth RH, Aghajanian GK. Melatonin: deacetylation to 5-methoxytryptamine by liver but not brain aryl acylamidase. J Neurochem. 1979;32:1219–1226. doi: 10.1111/j.1471-4159.1979.tb11049.x. [DOI] [PubMed] [Google Scholar]
- Rosen R, Hu DN, Perez V, Tai K, Yu GP, Chen M, Tone P, McCormick SA, Walsh J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–1679. [PMC free article] [PubMed] [Google Scholar]
- Rufiange M, Dumont M, Lachappelle P. Correlating retinal function with melatonin secretion in subject with an early or late circadian phase. Invest Ophthalmol Vis Sci. 2002;43:2491–2499. [PubMed] [Google Scholar]
- Sakamoto K, Oishi K, Shiraishi M, Hamano S, Otsuka H, Miyake Y, Ishida N. Two circadian oscillatory mechanisms in the mammalian retina. Neuroreport. 2000;11:3995–3997. doi: 10.1097/00001756-200012180-00018. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Tosini G. Circadian Rhythms in the retina of rats with photoreceptor degeneration. J Neurochemistry. 2004;90:1019–1024. doi: 10.1111/j.1471-4159.2004.02571.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Liu C, Kasamatsu M, Iuvone PM, Tosini G. Intraocular injection of kainic acid does not abolish the circadian rhythm of arylalkylamine N-acetyltransferase mRNA in rat photoreceptors. Mol Vis. 2006;12:117–124. [PubMed] [Google Scholar]
- Samples JR, Krause G, Lewy AJ. Effect of melatonin on intraocular pressure. Curr Eye Res. 1988;7:649–653. doi: 10.3109/02713688809033192. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Wirz-Justice A, Olivieri G, Pache M, Kräuchi K, Brydon L, Jockers R, Müller-Spahn F, Meyer P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002;50:519–526. doi: 10.1177/002215540205000408. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, Flammer J, Eckert A, Müller-Spahn F, Meyer P. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- Scher J, Wankiewicz E, Brown GM, Fujieda H. MT1 melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sci. 2002;43:889–897. [PubMed] [Google Scholar]
- Schmid-Kubista KE, Glittenberg CG, Cezanne M, Holzmann K, Neumaier-Ammerer B, Binder S. Daytime levels of melatonin in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87:89–93. doi: 10.1111/j.1755-3768.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, Strettoi E, Iuvone PM, Tosini G. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6:e24483. doi: 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serle JB, Wang RF, Peterson WM, Plourde R, Yerxa BR. Effect of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, on intraocular pressure in glaucomatous monkey eyes. J Glaucoma. 2004;13:385–388. doi: 10.1097/01.ijg.0000133150.44686.0b. [DOI] [PubMed] [Google Scholar]
- Siu AW, Reiter RJ, To CH. Pineal indoleamines and vitamin E reduce nitric oxide-induced lipid peroxidation in rat retinal homogenates. J Pineal Res. 1999;27:122–128. doi: 10.1111/j.1600-079x.1999.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Steenhard BM, Besharse JC. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J Neurosci. 2000;20:8572–7. doi: 10.1523/JNEUROSCI.20-23-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Sieving PA, luvone PM, Bush RA. The melatonin antagonist luzindole protects retinal photoreceptors from light damage in the rat. Invest Ophthalmol Vis Sci. 1998;39:2458–2465. [PubMed] [Google Scholar]
- Teirstein PS, Goldman AI, O’Brien PJ. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest Ophthalmol Vis Sci. 1980;19:1268–73. [PubMed] [Google Scholar]
- Terman JS, Remé CE, Wirz-Justice A. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605:256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- Thomas KB, Iuvone PM. Circadian rhythm of tryptophan hydroxylase activity in chicken retina. Cell Mol Neurobiol. 1991;11:511–527. doi: 10.1007/BF00734813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993;601:303–307. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- Tosini G, Dirden JC. Dopamine Inhibits melatonin release in the mammalian retina: in vitro evidence. Neuroscience Lett. 2000;286:119–122. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- Tosini G, Chaurasia SS, Iuvone PM. Regulation of AANAT in the retina. Chronobiology International. 2006;23:381–391. doi: 10.1080/07420520500482066. [DOI] [PubMed] [Google Scholar]
- Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, Iuvone PM. The Circadian Clock System in Mammalian Retina. BioEssays. 2008;30:624–633. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Ye K, Iuvone PM. N-Acetylserotonin: Neuroprotection, Neurogenesis, and the Sleepy Brain. Neuroscientist. 2012 doi: 10.1177/1073858412446634. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H, Binkley S, Siopes T, Mosher K. Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica) Gen Comp Endocrinol. 1984;56:70–81. doi: 10.1016/0016-6480(84)90063-7. [DOI] [PubMed] [Google Scholar]
- Valenciano AI, Alonso-Gómez AL, Iuvone PM. Diurnal rhythms of tryptophan hydroxylase activity in Xenopus laevis retina: opposing phases in photoreceptors and inner retinal neurons. Neuroreport. 1999;10:2131–2135. doi: 10.1097/00001756-199907130-00025. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002;75:547–553. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Yang XL, Wu SM, Hollyfield JG. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 1988;453:377–380. doi: 10.1016/0006-8993(88)90182-5. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, O’Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 1992;33:1894–1902. [PubMed] [Google Scholar]
- Wiechmann AF, Wirsig-Wiechmann CR. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells. Exp Eye Res. 2001;73:617–623. doi: 10.1006/exer.2001.1073. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Vrieze MJ, Dighe R, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel(1c) melatonin receptor in transgenic Xenopus laevis retina. Invest Ophthalmol Vis Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–60. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- White MP, Fisher LJ. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res. 1989;29:167–179. doi: 10.1016/0042-6989(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Yi C, Pan X, Yan H, Guo M, Pierpaoli W. Effects of melatonin in age-related macular degeneration. Ann NY Acad Sci. 2005;1057:384–392. doi: 10.1196/annals.1356.029. [DOI] [PubMed] [Google Scholar]
- Yuan H, Lu Y, Pang SF. Binding characteristics and regional distribution of [125I]iodomelatonin binding sites in the brain of the human fetus. Neurosci Lett. 1991;130:229–232. doi: 10.1016/0304-3940(91)90403-g. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Iuvone PM. Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci Lett. 1992;135:71–74. doi: 10.1016/0304-3940(92)90138-w. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Derbiszewska T, Nowak JZ. Clozapine and other neuroleptic drugs antagonize the light-evoked suppression of melatonin biosynthesis in chick retina: involvement of the D4-like dopamine receptor. J Neural Transm Gen Sect. 1994;97:107–117. doi: 10.1007/BF01277947. [DOI] [PubMed] [Google Scholar]