Abstract
The discovery of NF-κB signaling pathways has greatly enhanced our understanding of inflammatory and immune responses. In the canonical NF-κB pathway, the proteasomal degradation of IκBα, an inhibitory protein of NF-κB, is widely accepted to be a key regulatory step. However, contradictory findings have been reported as to whether the immunoproteasome plays an obligatory role in the degradation of IκBα and activation of the canonical NF-κB pathway. Such results were obtained mainly using traditional gene deletion strategies. Here, we have revisited the involvement of the immunoproteasome in the canonical NF-κB pathway using small molecule inhibitors of the immunoproteasome, namely UK-101 and LKS01 targeting β1i and β5i, respectively. H23 and Panc-1 cancer cells were pretreated with UK-101, LKS01 or epoxomicin (a prototypic inhibitor targeting both the constitutive proteasome and immunoproteasome). We then examined whether these pretreatments lead to any defect in activating the canonical NF-κB pathway following TNFα exposure by monitoring the phosphorylation and degradation of IκBα, nuclear translocation of NF-κB proteins and DNA binding and transcriptional activity of NF-κB. Our results consistently indicated that there is no defect in activating the canonical NF-κB pathway following selective inhibition of the immunoproteasome catalytic subunits β1i, β5i or both using UK-101 and LKS01, in contrast to epoxomicin. In summary, our current results using chemical genetic approaches strongly support that the catalytic activity of the immunoproteasome subunits β1i and β5i is not required for canonical NF-κB activation in lung and pancreatic adenocarcinoma cell line models.
INTRODUCTION
The NF-κB (Nuclear Factor-κB/Rel) family of transcription factors plays a crucial role in controlling genes involved in numerous biological processes such as immune and inflammatory responses, cell growth, differentiation and apoptosis.1, 2 Since the first identification of NF-κB over two decades ago, dysregulation of NF-κB has been implicated in an ever-expanding list of diseases including inflammation, autoimmune diseases and cancer.1 In mammalian cells, the NF-κB transcription factors can exist as heterodimers or homodimers of NF-κB family members c-Rel, p65 (RelA), RelB, p50 (NF-κB1) and p52 (NF-κB2). Among various forms of NF-κB dimers, the complex of p50/p65 is considered to be mainly responsible for NF-κB activity in most cell types. In typical unstimulated cells, the NF-κB complex is associated with its inhibitory protein IκB and remains inactive in the cytosol.3, 4 In response to a variety of stimuli including exposure to tumor necrosis factor-α (TNFα), IκB is phosphorylated, ubiquitinated and subsequently degraded by the proteasome, allowing the free NF-κB complex to enter the nucleus.5 It has been widely accepted that proteasomal degradation of IκB is a key initial step regulating the canonical NF-κB pathway. However, whether the immunoproteasome plays an essential role in IκB degradation and canonical NF-κB activation has been a controversial question for over a decade.6–10
The ubiquitin-proteasome pathway plays an important role in the degradation of many regulatory proteins involved in a wide array of cellular processes.11–13 Central to these processes is the degradation of targeted proteins by the proteasome, a multiprotease complex. In mammalian cells, there are two major forms of proteasomes: the constitutive proteasome and its alternative form, the immunoproteasome. Both forms of proteasomes contain a 20S core, a barrel-shaped multisubunit complex with 4-stacked rings composed of two outer α-rings and two inner β-rings. Each of the two β-rings contains three distinct catalytic subunits, forming six Ntn (N-terminal nucleophile) hydrolase-type catalytic centers in the inner chamber. The major differences between the constitutive proteasome and immunoproteasome lie in their catalytic subunit compositions; the constitutive proteasome contains β1 (Y), β2 (Z) and β5 (X) subunits while the immunoproteasome contains their inducible counterparts, β1i (LMP2), β2i (MECL1) and β5i (LMP7).14, 15 In contrast to the constitutive proteasome, which is expressed in most tissues and cell types, the immunoproteasome is mainly expressed in cells of hematopoietic origin and can be induced in non-hematopoietic cells following exposure to certain inflammatory cytokines. Upregulation of the immunoproteasome in non-hematopoietic cells has been implicated in a number of disease conditions including inflammation, neurodegenerative diseases and cancer.14, 16 In addition, an increasing number of studies suggest that the immunoproteasome has important cellular functions beyond immune responses.16–18
With regard to NF-κB activation, earlier investigations suggested that the immunoproteasome plays a crucial role in activating the canonical NF-κB pathway via degradation of IκBα.6, 8 In their studies, Hayashi and Faustman reported that IκB degradation and NF-κB activation were defective in non-obese diabetic mice with altered β1i expression and in β1i-knockout mice.6, 8 In contrast, other investigators reported that there were no apparent defects in NF-κB activation in non-obese diabetic mice or in the T2 cell line lacking β1i and β5i.7, 10 It was further suggested that these contradictory findings may be related to differences in gender, mouse strain, cell type and/or disease condition.9 We cannot rule out the possibility that the observed phenotype is related to indirect, compensatory responses, given that most of these findings were obtained using traditional gene deletion strategies. Overall, it is still unclear whether the immunoproteasome is involved in NF-κB activation.
As an approach to circumvent concerns associated with traditional gene deletion strategies, biologically active small molecules have been increasingly used to investigate functions of proteins. This small molecule-based approach, so-called “chemical genetics”,19–21 is complementary to conventional genetic approaches and utilizes selective binding of small molecule probes to a protein target and subsequent functional modulation.22–25 This small molecule approach may be particularly relevant for investigating the functions of the proteasome, given that the knockdown of a single proteasome subunit can influence the entire proteasome assembly process due to its scaffold function. For example, it has been shown that genetic knockdown of a single proteasome subunit can lead to either a complete failure in proteasome assembly or formation of hybrid/mixed proteasomes with differing proteolytic activity and biological functions.26, 27
Here, we investigated whether the immunoproteasome plays a crucial role in NF-κB activation using a small molecule-based approach. Specifically, we have employed subunit-specific proteasome inhibitors developed in our laboratory, namely UK-101 (targeting β1i) and LKS01 (targeting β5i) as well as epoxomicin (a broadly acting proteasome inhibitor primarily targeting β5, β1i and β5i). Using these small molecule inhibitors, we were able to block the chymotrypsin-like activity of the immunoproteasome via targeted inhibition of β1i, β5i or both subunits and to determine the biological consequences in TNF-α-induced NF-κB activation in lung and pancreatic adenocarcinoma cell line models. Our results indicate that the chymotrypsin-like activity of the immunoproteasome is not required for canonical NF-κB activation in lung and pancreatic adenocarcinoma cell line models.
RESULTS
UK-101 and LKS01 can selectively target immunoproteasome subunits β1i and β5i without influencing constitutive proteasome subunits β1 and β5
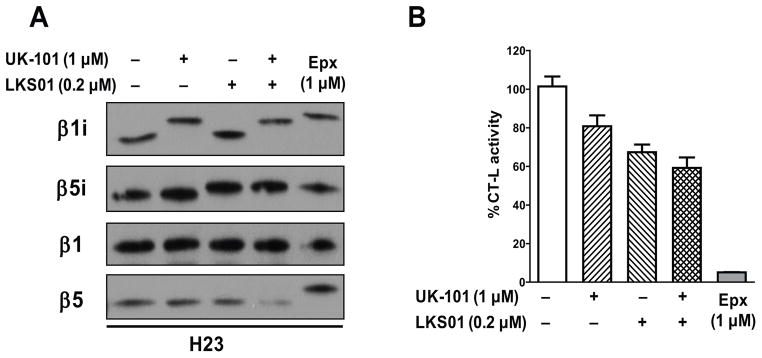
The epoxyketone pharmacophores of UK-101 and LKS01 are shown to covalently modify the N-terminal threonine nucleophile of proteasome catalytic subunits, forming a 6-membered morpholino ring.28 Previously, we reported that UK-101 and LKS01 selectively interact with β1i and β5i, respectively.29–31 Here, we have further confirmed this selectivity by detecting a mobility shift caused by covalent modification of proteasome catalytic subunits. As shown in Fig. 1A, treatment of H23 cells with UK-101 and LKS01 led to covalent modification of β1i and β5i, respectively, but not other subunits. Similarly, co-treatment of UK-101 and LKS01 led to selective modifications of both β1i and β5i. In contrast, epoxomicin, a natural product containing the epoxyketone pharmacophore known to target both the constitutive proteasome and immunoproteasome, covalently modified the catalytic subunits of β1i, β5i and β5. Selective inactivation of these catalytic subunits by UK-101 and LKS01 was also observed in Panc-1 cells (Suppl. Fig. 1). All three compounds tested (i.e. UK-101, LKS01, epoxomicin) contain the epoxyketone pharmacophore and covalently modify only the catalytically active subunits.28, 29 Thus, our results indicate that H23 and Panc-1 cells express functionally active immunoproteasome complexes containing β1i and β5i subunits.
Fig. 1.
UK-101 and LKS01 selectively target the immunoproteasome subunits β1i and β5i without influencing the constitutive proteasome subunits β1 and β5. (A) Treatment with UK-101 (1 μM) or LKS01 (0.2 μM) selectively inhibits the β1i and β5i subunits, respectively, as shown by the increased apparent molecular sizes resulting from covalent modifications of β1i or β5i subunits without inhibiting the β1 or β5 subunits. In contrast, epoxomicin, an inhibitor targeting both the constitutive proteasome and immunoproteasome, covalently modifies β1i and β5i as well as β5. (B) Treatment with UK-101, LKS01 or both (inhibiting β1i, β5i or both) leads to only a partial inhibition of the chymotrypsin-like (CT-L) activity, while but treatment with epoxomicin (inhibiting β5, β1i and β5i) results in nearly complete inhibition of the CT-L activity.
The CT-L activity of proteasomes is attributed mainly to the β5, β1i and β5i subunits. Thus, we determined the effect of UK-101, LKS01 or co-treatment with UK-101 and LKS01 on the CT-L activity. Using extracts prepared from H23 cells, we observed that UK-101, LKS01 or co-treatment with UK-101 and LKS01 results in only a partial inhibition of the CT-L activity (Fig. 1B). These findings further support the ability of UK-101 and LKS01 to selectively inhibit the targeted subunits. In contrast, treatment with epoxomicin which targets all subunits responsible for CT-L activity resulted in nearly complete inhibition of the CT-L activity.
Inhibition of immunoproteasome subunits possessing CT-L activity does not block TNFα-induced phosphorylation and degradation of IκBα
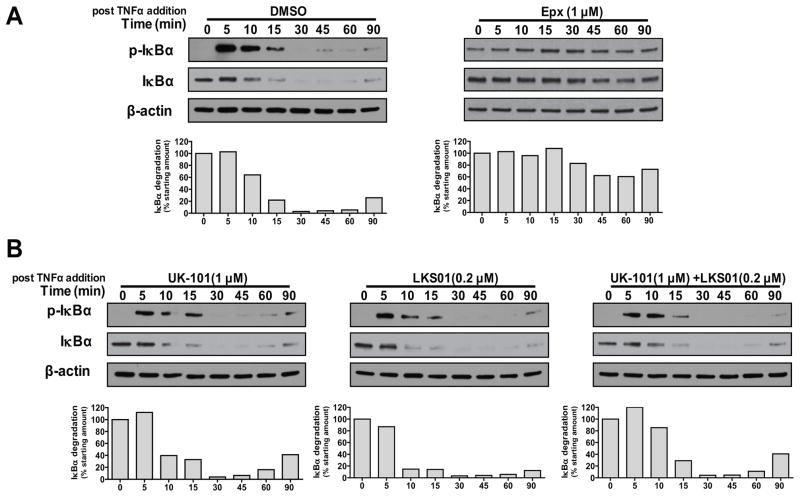
Phosphorylation and degradation of IκBα is a key initial step for the canonical NF-κB activation. Thus, we investigated the impact of selective immunoproteasome inhibition on the phosphorylation and degradation of IκBα using UK-101 and LKS01. As shown in Fig. 2A (left panel), TNFα induced the rapid phosphorylation and degradation of IκBα in H23 cells. The total and phosphorylated IκBα protein was almost undetectable after 30 min, and IκBα started reappearing around 90 min following TNFα addition, indicating a feedback regulation of IκBα synthesis. Epoxomicin pretreatment almost completely blocked TNFα-induced degradation of IκBα in H23 cells (Fig. 2A, right panel). It was noted that epoxomicin pretreatment alone (even prior to TNFα addition) led to accumulation of the phosphorylated IκBα, which may be related to the effect of epoxomicin on IκBα homeostasis or on the upstream molecule IκB kinase (IKK) as reported for bortezomib, another broadly acting proteasome inhibitor.32 Despite the initially increased level of the phosphorylated IκBα, epoxomicin treatment effectively prevented TNFα-induced degradation of IκBα. In contrast, the β1i-targeting inhibitor UK-101 did not block TNFα-induced phosphorylation and degradation of IκBα (Fig. 2B, left panel), indicating that β1i is not involved in this process. Similar to UK-101, the β5i-targeting inhibitor LKS01 had no effect on TNFα-induced IκBα degradation (Fig. 2B, middle panel). Given that β1i or β5i alone is only partially responsible of the CT-L activity of the immunoproteasome,29 we pretreated cells with both compounds prior to the addition of TNFα. Similar to the results obtained with UK-101 or LKS01 alone, simultaneous inhibition of both β1i and β5i did not prevent TNFα-induced IκBα degradation (Fig. 2B, right panel). Similar results were obtained using Panc-1 cells (Suppl. Fig. 2). Taken together, our results indicate that selective inhibition of β1i and β5i has no effect on the phosphorylation and degradation of IκBα in H23 and Panc-1 cells.
Fig. 2.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not block TNFα-stimulated phosphorylation and degradation of IκBα. (A) In H23 cells pretreated with vehicle (DMSO), TNFα (15 ng/ml) was able to stimulate phosphorylation and subsequent degradation of IκBα when monitored by immunoblotting over 90 min. However, the expoxomicin pretreatment almost completely abolished TNFα-stimulated degradation of IκBα. (B) Pretreatment with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination, did not have a negative impact on TNFα-stimulated phosphorylation and degradation of IκBα. Densitometric analyses were performed to quantify the intensities of IκBα and β-actin bands, and the calculated extent of IκBα degradation is shown in bar graphs.
UK-101, LKS01 or both do not prevent TNFα-stimulated nuclear translocation of the NF-κB complex, p65/p50
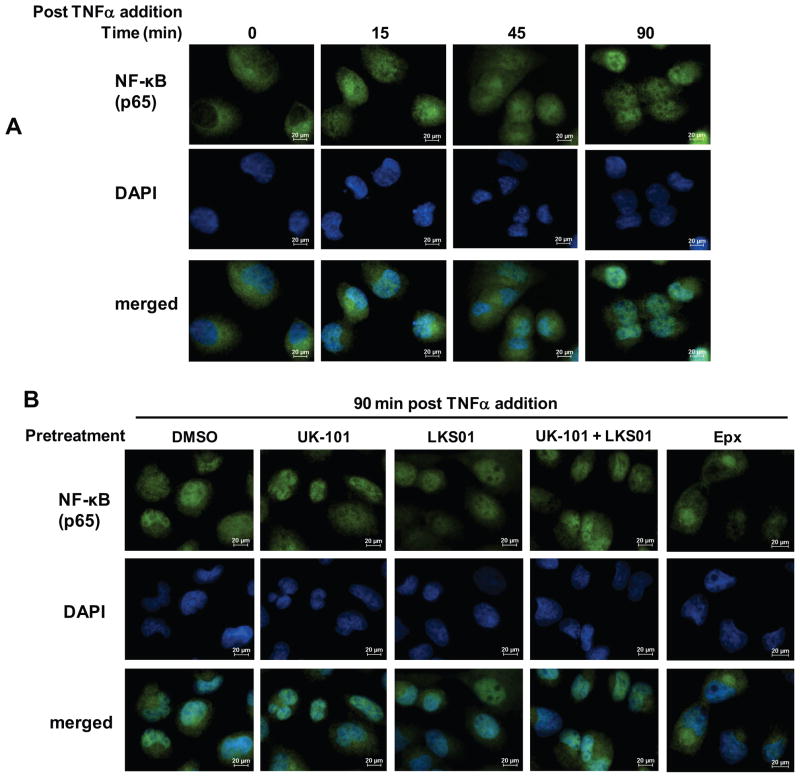
Although it is widely accepted that proteasomes regulate NF-κB activation through degradation of IκBα, we wanted to further investigate the effects of inhibiting immunoproteasome catalytic subunits on NF-κB pathways downstream of IκBα degradation. Thus, we investigated the effects of UK-101 and LKS01 on NF-κB nuclear translocation using immunofluorescence imaging. As expected, TNFα induced nuclear translocation of the NF-κB protein, p65 in a time-dependent manner. At 90 min following TNFα addition, p65 is almost completely translocated to the nucleus (Fig. 3A and Suppl. Fig. 3 for Panc-1). In line with our results on IκBα degradation (Fig. 2), pretreatment with UK-101 or LKS01, either individually or in combination, did not block nuclear translocation of p65 (Fig. 3B). In contrast, the epoxomicin pretreatment prevented the p65 subunit from entering the nucleus (Fig. 3B).
Fig. 3.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not block TNFα-stimulated translocation of NF-κB to the nucleus. (A) TNFα-stimulated translocation of NF-κB (p65) to the nucleus was monitored at the indicated times following TNFα (15 ng/ml) addition. (B) Addition of TNFα (15 ng/ml, 90 min) led to nuclear translocation of NF-κB (p65) in H23 cells pretreated with UK-101 (1 μM), LKS01 (0.2 μM) (either individually or in combination) or vehicle (DMSO). In contrast, cells pretreated with epoxomicin (1 μM) did not show clear translocation of NF-κB (p65) to the nucleus following the addition of TNFα.
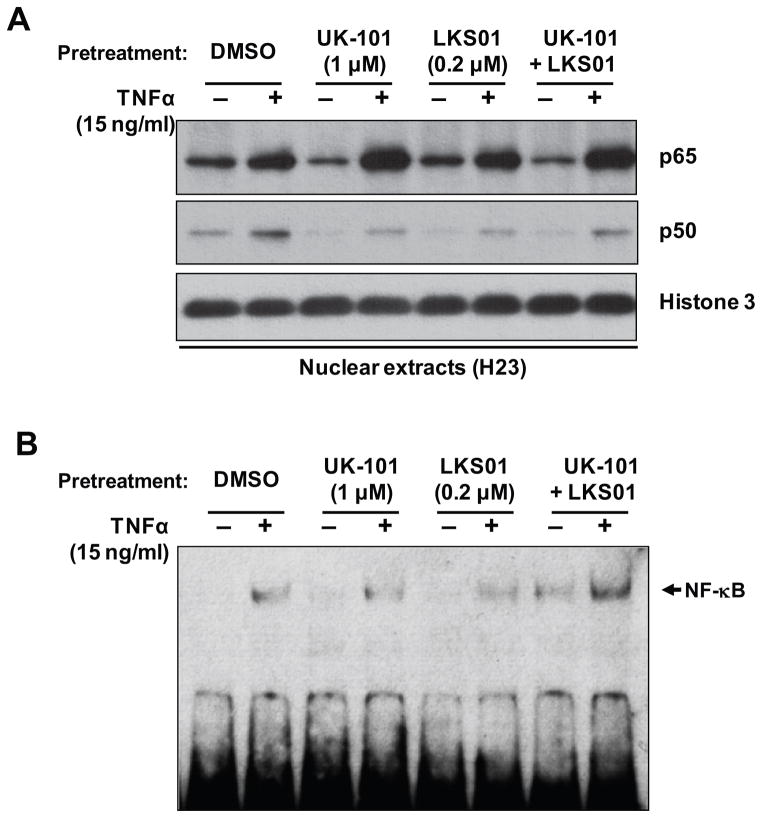
In order to further verify the lack of inhibitory effect of UK-101 or LKS01 treatment on NF-κB nuclear translocation, we determined the levels of the p65 and p50 subunits in nuclear extracts by immunoblotting analyses. Consistent with the results from immunofluorescence staining (Fig. 3), pretreatment with UK-101 or LKS01, either individually or in combination, did not prevent nuclear translocation of the p65 and p50 subunits following TNFα treatment (Fig. 4A). It appeared that nuclear translocation of p50 subunit was slightly decreased by the pretreatment with UK-101 or LKS01. However, there was no apparent change in nuclear translocation of p65 subunit (Fig. 4A). We next investigated whether accumulation of NF-κB in the nucleus results in sustained DNA binding of NF-κB. Specifically, we performed an EMSA using the same nuclear extracts and an NF-κB binding probe. The results showed that TNFα treatment was able to promote the DNA binding of NF-κB in H23 cells pretreated with UK-101, LKS01 or both (Fig. 4B).
Fig. 4.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not block NF-κB DNA binding activity. (A) Immunoblotting results show that the stimulatory effect of TNFα (15 ng/ml, 90 min) on the nuclear translocation of NF-κB subunits p65 and p50 is not attenuated by pretreatment with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination, in H23 cells. (B) EMSA of nuclear extracts prepared from H23 cells showing that the TNFα-stimulated DNA binding activity of NF-κB is not diminished by pretreatment with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination, in H23 cells. The arrow marks the band that represents specific NF-κB binding.
UK-101, LKS01 or both do not block the transcriptional activity of NF-κB
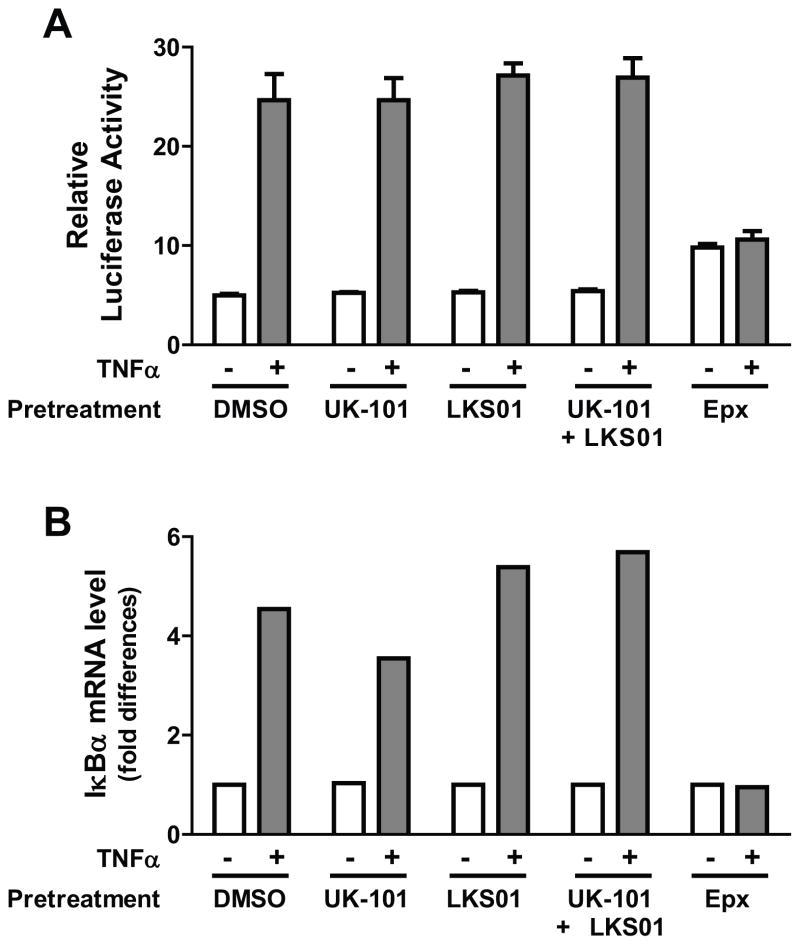
To further examine the impact of immunoproteasome-selective inhibitors on transcriptional activity of the NF-κB complex, a transient reporter assay was performed. H23 cells were transfected with a luciferase reporter gene harboring consensus NF-κB response elements and treated with UK-101, LKS01, a combination of UK-101 and LKS01, epoxomicin or vehicle for 1.5 hr prior to the addition of TNFα (15 ng/ml, 3 h). The results showed that the luciferase activity was markedly increased by TNFα addition and that pretreatment with UK-101, LKS01 or both did not alter the NF-κB-mediated induction of luciferase activity following the addition of TNFα (Fig. 5A). In contrast, the TNFα-stimulated induction of luciferase activity was completely blocked by epoxomicin (Fig. 5A). The results from the NF-κB reporter assay were further validated by performing quantitative RT-PCR for the cellular levels of the IκBα transcript, which is rapidly synthesized upon NF-kB activation via an NF-κB feedback control. As shown in Fig. 5B, the expression levels of IκBα mRNA were consistently stimulated by TNFα in cells pretreated with UK-101, LKS01 or vehicle, but not in cells pretreated with epoxomicin. These results indicate that neither β1i nor β5i is crucial for NF-κB activation.
Fig. 5.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not block NF-κB-mediated transcriptional activation induced by TNFα. (A) Results using an NF-κB reporter plasmid show that the stimulatory effect of TNFα on NF-κB transcriptional activity is maintained in H23 cells pretreated with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination. In contrast, pretreatment with epoxomicin (1 μM) led to a slightly increased reporter gene expression and no further TNFα-stimulated NF-κB transcriptional activity. (B) Quantitative RT-PCR results show that the stimulatory effect of TNFα on the IκBα transcript, a downstream target of NF-κB, is maintained in H23 cells pretreated with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination. In contrast, pretreatment with epoxomicin (1 μM) led to no changes in IκBα transcript levels by TNFα.
DISCUSSION
The NF-κB pathway plays an important role in regulating transcription of a large number of genes involved in inflammation, cell growth and survival.1 While it is widely accepted that the proteasome is a key component in NF-κB activation, it has been controversial which proteasome subtype (the constitutive proteasome or the immunoproteasome) is involved in NF-κB activation. Hayashi and Faustman suggested that a defect in NF-κB activity was linked to the lack of the immunoproteasome subunit β1i in genetically engineered animal models.6, 8 However, other investigators reported no apparent defect in NF-κB activation in cells lacking β1i,7 suggesting that the immunoproteasome is not involved in NF-κB activation. Given that β1i is a component of the fully assembled immunoproteasome core and is known to be required for the proper assembly of the immunoproteasome, conventional manipulation strategies of β1i at the gene (DNA or mRNA) level may complicate data interpretation.
To put this controversy to rest, we have investigated the role of the immunoproteasome in NF-κB activation using the small molecule immunoproteasome subunit-specific inhibitors UK-101 and LKS01, which target β1i and β5i, respectively. Specifically, we examined whether inhibition of β1i, β5i or both disrupt TNFα-induced activation of the canonical NF-κB pathway by measuring IκBα degradation, NF-κB nuclear localization, specific DNA-binding activity and transcriptional activity. Our results showed that targeted inhibition of β1i, β5i or both does not block TNFα-induced NF-κB activation in H23 and Panc-1 cells. However, we did observe an almost complete blockade of TNFα-stimulated activation of NF-κB by epoxomicin, a broadly acting proteasome inhibitor. Taken together, our findings suggest that the catalytic activity of the immunoproteasome subunits β1i and β5i is not required for TNFα-stimulated NF-κB activation in the H23 and Panc-1 adenocarcinoma cell line models.
Our current results obtained by a chemical approach are contradictory to previous reports claiming that the lack of β1i, β5i or both leads to defective NF-κB activation. These previous studies have all utilized non-cancerous cell lines or tissues and traditional genetic approaches for abolishing β1i expression and these differences may have contributed to apparent discrepancies. Specifically, these findings were obtained using the T2 cells (a human lymphocyte cell line) lacking both β1i and β5i,33 embryonic fibroblasts isolated from β1i-knockout mice8 or spleen tissues with impaired β1i expression from non-obese diabetic mice.6 Interestingly, a more recent report by Hensley et al.27 utilized purified B cells isolated from mice lacking β1i and demonstrated that NF-κB activation by lipopolysaccharide still takes place, but at a slightly delayed pace. These authors speculated that this slight delay in NF-κB signaling may be related to the formation of proteasomes with mixed compositions of the constitutive proteasome and immunoproteasome subunits.27 Indeed, an increasing body of evidence has been reported on the presence of mixed proteasomes and their functional implications in immune responses and numerous cellular signaling pathways.26 While it remains to be determined whether any mixed proteasomes containing β1i or β5i play a distinct role in NF-κB signaling, our results suggest that the immunoproteasome catalytic subunits β1i and β5i are not required for the activation of the canonical NF-κB pathway in H23 and Panc1 cells. In addition, our results showed that the nuclear translocation of p50 subunit may be slightly decreased with the targeted inhibition of β1i or β5i. These results may suggest a possibility that the immunoproteasome plays a modulatory role in the canonical NF-κB pathway. It should be noted that our current study primarily focused on canonical NF-κB pathway stimulated by TNFα in H23 and Panc-1 cells. Thus it remains to be determined whether the immunoproteasome contributes to canonical and noncanonical NF-κB activation across different cell types, in particular, in cells of high immunoproteasome expression/activity.
CONCLUSION
In this report, we showed that immunoproteasome-specific small molecule inhibitors (UK-101 and LKS01) do not block TNFα-induced IκBα degradation and subsequent NF-κB transcriptional activity. Our findings clearly support that the immunoproteasome does not play a crucial role in activation of the canonical NF-κB pathway.
EXPERIMENTAL
Cell culture and reagents
H23 (human lung adenocarcinoma) and Panc-1 (human pancreatic adenocarcinoma) cell lines were obtained from the American Type Culture Collection (ATCC) and maintained in RPMI 1640 and DMEM, respectively, supplemented with 10% FBS (HyClone). TNFα was purchased from Sigma. UK-101, LKS01 and epoxomicin were synthesized following a procedure similar to the one reported previously.29, 34
Chymotrypsin-like activity assay
The chymotrypsin-like (CT-L) activity of the proteasome was determined by the measurement of fluorescence generated from the cleavage of the fluorogenic substrate Succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Suc-LLVY-AMC), as described previously.35 Briefly, approximately 5,000 cells per well were plated onto 96 well plates and treated with the proteasome inhibitors UK-101 (1 μM), LKS01 (0.2 μM), epoxomicin (1 μM) or vehicle for 1.5 h. Following drug treatment, cells were rinsed with phosphate buffered saline (PBS) and lysed with passive lysis buffer (Promega). Subsequently, assay buffer (20 mM Tris/Cl, 0.5 mM EDTA, 0.035% SDS, pH 8.0) containing suc-LLVY-AMC was added and fluorescence signals (excitation at 360 nm and emission at 460 nm) were continuously monitored for 90 min using a microplate fluorescence reader (SpectraMax5, Molecular Devices). The reaction rates were determined from the initial slopes of the resulting reaction progress curves.
Preparation of cytoplasmic and nuclear extracts
Following drug treatment, cells were rinsed with PBS and collected by centrifugation. The resulting pellets were resuspended in ice-cold hypotonic lysis buffer (10 mM HEPES pH 7.9, 10 mM KCl, 3 mM MgCl2, 0.05% NP-40, 1 mM EDTA pH 8.0 containing a cocktail of protease inhibitors) and incubated for 30 min at 4 °C. The samples were centrifuged for 5 min at 3,000 rpm at 4 °C and the resulting supernatant was saved as the cytoplasmic extract. The pellet was rinsed with hypotonic lysis buffer and resuspended in gel shift lysis buffer (50 mM HEPES pH 7.9, 250 mM KCl, 0.1% NP-40, 0.1 mM EGTA pH 8.0, 0.1 mM EDTA pH 8.0, 10% glycerol containing a cocktail of protease inhibitors) and pulse sonicated and incubated on ice for 30 min. The samples were centrifuged at 14,000 rpm for 10 min at 4°C and the resulting supernatant was saved as the nuclear extract. The protein concentrations in these extracts were determined using the BCA assay (Bio-Rad).
Immunoblotting
Total cell lysates or cytosolic/nuclear extracts containing equivalent amounts of total protein were separated by SDS-PAGE and transferred to PVDF membranes. After blocking with 5% skim milk in Tris-buffered saline containing Tween 20 (TBS-T: 20 mM Tris-HCl, 137 mM NaCl, 0.05% Tween 20), the blots were incubated with TBS-T containing the appropriate primary and secondary antibodies conjugated with horseradish peroxidase (HRP), followed by enhanced chemiluminescence (ECL) detection. The following primary antibodies were used: β1i, β5i, β1, β5 (Thermo Fisher Scientific Inc); phospho-IκBα (Ser32), IκBα, NFκB (p65), histone 3 (Cell Signaling); NF-κB (p50, SantaCruz). β-actin (Cell Signaling) was used as a gel loading control.
Immunofluorescence
Cells were plated onto 8-well chamber slides and treated with UK-101 (1 μM), LKS01 (0.2 μM), epoxomicin (1 μM) or vehicle for 1.5 h. After extensive washing with PBS, the cells were then incubated with TNFα (15 ng/ml) for 15, 45 or 90 min. Cells were then fixed with 4% formaldehyde and permeabilized with 0.3% Triton X-100. After blocking, cells were incubated with NF-κB (p65) antibody for 2 h at room temperature, and subsequently incubated with anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen). Cells were mounted with Vectashield mounting medium containing 4′,6-diaminido-2-phenylindole (DAPI) (Vector Labs). Images were acquired using a Nikon Ti-U inverted fluorescence microscope.
Electrophoretic Mobility Shift Assay (EMSA)
Ten μg of nuclear extract was used for EMSA according to the manufacturer’s instructions (Affymetrix). Briefly, nuclear extracts containing equal amounts of protein for each sample were incubated with poly (dI-dC) and binding buffer for 5 min, followed by the addition of biotinylated NF-κB probe (Affymetrix). Binding reaction mixtures were incubated for 30 min at 15 °C. Protein-DNA complexes were separated on 6% native polyacrylamide gels in Tris-borate/EDTA buffer at 4°C. After electrophoresis, complexes were transferred to nylon membranes. Transferred oligos were immobilized by baking for 1 h at 80°C. For detection of bound probes, blots were blocked using a blocking buffer, followed by the addition of Streptavidin-HRP and detection using ECL according to the manufacturer’s instructions.
NF-κB reporter assay
The reporter plasmid pGL4.2[luc2P/NF-κB-RE/Hygro] containing five copies of an NF-κB response element (Promega) was used for the reporter assay. Cells were plated onto 24 well plates and transfected with the NF-κB reporter plasmid (360 ng/well) along with pRL-TK (40 ng/well, an internal control, Promega). The cells were cultured for 24 h after transfection and treated with UK-101 (1 μM), LKS01 (0.2 μM), epoxomicin (1 μM) or vehicle for 1.5 h. After extensive washing with PBS, cells were incubated with TNFα (15 ng/ml) for another 3 h before being harvested and assayed using the Dual Luciferase Reporter Assay kit (Promega).
Quantitative RT-PCR
Total RNA (0.5 μg) extracted using RNeasy Micro kit (Qiagen) was used to synthesize single-stranded cDNA using the iScript cDNA synthesis kit (Bio-Rad). The following primer sequences were used; for IκBα, sense 5′-GCTGAAGAAGGAGCGGCTACT-3′ and antisense 5′-TCGTACTCCTCGTCTTTCATGGA-3′; for β–actin, sense 5′-GCATCCTCACCCTGAAGTAC-3′ and antisense 5′-GATAGCACAGCCTGGATAGC-3′. Quantitative RT-PCR was performed in duplicate using iCycler with the iQ SYBR-green Supermix (Bio-Rad). The condition of quantitative RT-PCR was as follows: annealing at 60°C with 40 cycles for IκBα; annealing at 65°C with 40 cycles for β–actin. Expression was assessed by evaluating threshold cycle (Ct) values. The relative amount of mRNA expression was calculated by the comparative ΔΔCt method. 36
Supplementary Material
UK-101 and LKS01 selectively target immunoproteasome subunits β1i and β5i without influencing the constitutive proteasome subunits β1 and β5 in Panc-1 cells. Treatment with UK-101 (1 μM) or LKS01 (0.2 μM) selectively inhibits the β1i and β5i subunits, respectively, as shown by the increased molecular sizes resulting from covalent modifications of β1i or β5i, but no changes in the β1 or β5 subunits. In contrast, epoxomicin, an inhibitor targeting both the constitutive proteasome and immunoproteasome, covalently modifies the β1i and β5i subunits as well as the β5 subunit.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not affect TNFα-stimulated phosphorylation and degradation of IκBα in Panc-1 cells. (A) In Panc-1 cells pretreated with vehicle (DMSO), TNFα (15 ng/ml) was able to stimulate IκBα degradation when monitored by immunoblotting over 90 min. However, the expoxomicin pretreatment almost completely abolished TNFα-stimulated degradation of IκBα. (B) Pretreatment with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination, did not have a negative impact on TNFα-stimulated degradation of IκBα. Densitometric analyses were performed on the intensities of IκBα and β-actin bands and the calculated extent of IκBα degradation is shown in bar graphs.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not affect TNFα-stimulated translocation of NF-κB into the nucleus in Panc-1 cells. (A) TNFα-stimulated translocation of NF-κB (p65) to the nucleus was examined 90 min following TNFα addition. (B) Addition of TNFα (15 ng/ml, 90 min) led to nuclear translocation of NF-κB (p65) in Panc-1cells pretreated with UK-101 (1μM) or LKS01 (0.2 μM), either individually or in combination.
Acknowledgments
We thank the members of the Lee and Kim labs for their helpful comments and discussion. This work is supported by grants from the NIH (R01CA128903, R15CA156601, K.B.K and W.L.) and a grant from the Kentucky Lung Cancer Research Program (W.L. and K.B.K). J. T. H. is grateful for the Korea Research Foundation (MRC, R13-2008-001-00000-00) for financial support.
References
- 1.Vallabhapurapu S, Karin M. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 2.Hayden MS, Ghosh S. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Karin M, Delhase M. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Ben-Neriah Y. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 5.Karin M. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Faustman D. Mol Cell Biol. 1999;19:8646–8659. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Runnels HA, Watkins WA, Monaco JJ. Nat Med. 2000;6:1064–1065. doi: 10.1038/80349. discussion 1065–1066. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Faustman DL. Cancer Res. 2002;62:24–27. [PubMed] [Google Scholar]
- 9.Hayashi T, Kodama S, Faustman DL. Nat Med. 2000;6:1065–1066. doi: 10.1038/80353. [DOI] [PubMed] [Google Scholar]
- 10.Kessler BM, Lennon-Dumenil AM, Shinohara ML, Lipes MA, Ploegh HL. Nat Med. 2000;6:1064. doi: 10.1038/80346. discussion 1065–1066. [DOI] [PubMed] [Google Scholar]
- 11.Petroski MD. BMC Biochem. 2008;9(Suppl 1):S7. doi: 10.1186/1471-2091-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques AJ, Palanimurugan R, Matias AC, Ramos PC, Dohmen RJ. Chem Rev. 2009;109:1509–1536. doi: 10.1021/cr8004857. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinova IM, Tsimokha AS, Mittenberg AG. Int Rev Cell Mol Biol. 2008;267:59–124. doi: 10.1016/S1937-6448(08)00602-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee W, Kim KB. Curr Top Med Chem. 2011;11:2923–2930. doi: 10.2174/156802611798281348. [DOI] [PubMed] [Google Scholar]
- 15.Navon A, Ciechanover A. J Biol Chem. 2009;284:33713–33718. doi: 10.1074/jbc.R109.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angeles A, Fung G, Luo H. Front Biosci. 2012;17:1904–1916. doi: 10.2741/4027. [DOI] [PubMed] [Google Scholar]
- 17.Yewdell JW. Immunol Rev. 2005;207:8–18. doi: 10.1111/j.0105-2896.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 18.Caudill CM, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. J Immunol. 2006;176:4075–4082. doi: 10.4049/jimmunol.176.7.4075. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber SL. Bioorg Med Chem. 1998;6:1127–1152. doi: 10.1016/s0968-0896(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 20.Crews CM, Mohan R. Curr Opin Chem Biol. 2000;4:47–53. doi: 10.1016/s1367-5931(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 21.Crews CM, Splittgerber U. Trends Biochem Sci. 1999;24:317–320. doi: 10.1016/s0968-0004(99)01425-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim KB, Myung J, Sin N, Crews CM. Bioorg Med Chem Lett. 1999;9:3335–3340. doi: 10.1016/s0960-894x(99)00612-5. [DOI] [PubMed] [Google Scholar]
- 23.Meng L, Kwok BH, Sin N, Crews CM. Cancer Res. 1999;59:2798–2801. [PubMed] [Google Scholar]
- 24.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh JR, Mohan R, Crews CM. Proc Natl Acad Sci U S A. 2000;97:12782–12787. doi: 10.1073/pnas.97.23.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillaume B, Chapiro J, Stroobant V, Colau D, Van Holle B, Parvizi G, Bousquet-Dubouch MP, Theate I, Parmentier N, Van den Eynde BJ. Proc Natl Acad Sci U S A. 2010;107:18599–18604. doi: 10.1073/pnas.1009778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensley SE, Zanker D, Dolan BP, David A, Hickman HD, Embry AC, Skon CN, Grebe KM, Griffin TA, Chen W, Bennink JR, Yewdell JW. J Immunol. 2010;184:4115–4122. doi: 10.4049/jimmunol.0903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groll M, Kim KB, Kairies N, Huber R, Crews CM. J Am Chem Soc. 2000;122:1237–1238. [Google Scholar]
- 29.Ho YK, Bargagna-Mohan P, Wehenkel M, Mohan R, Kim KB. Chem Biol. 2007;14:419–430. doi: 10.1016/j.chembiol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei B, Abdul Hameed MD, Hamza A, Wehenkel M, Muzyka JL, Yao XJ, Kim KB, Zhan CG. J Phys Chem B. 2010;114:12333–12339. doi: 10.1021/jp1058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma LK, Lee NR, Jang ER, Lee W, Kim KB. Development of activity-based fluorescent probes targeting the immunoproteasome. Denver, Colorado: 2011. [Google Scholar]
- 32.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD, Anderson KC. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi T, Faustman D. J Biol Chem. 2000;275:5238–5247. doi: 10.1074/jbc.275.7.5238. [DOI] [PubMed] [Google Scholar]
- 34.Kim KB, Fonseca FN, Crews CM. Methods Enzymol. 2005;399:585–609. doi: 10.1016/S0076-6879(05)99039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roccaro AM, Sacco A, Aujay M, Ngo HT, Azab AK, Azab F, Quang P, Maiso P, Runnels J, Anderson KC, Demo S, Ghobrial IM. Blood. 2010;115:4051–4060. doi: 10.1182/blood-2009-09-243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Livak KJ. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UK-101 and LKS01 selectively target immunoproteasome subunits β1i and β5i without influencing the constitutive proteasome subunits β1 and β5 in Panc-1 cells. Treatment with UK-101 (1 μM) or LKS01 (0.2 μM) selectively inhibits the β1i and β5i subunits, respectively, as shown by the increased molecular sizes resulting from covalent modifications of β1i or β5i, but no changes in the β1 or β5 subunits. In contrast, epoxomicin, an inhibitor targeting both the constitutive proteasome and immunoproteasome, covalently modifies the β1i and β5i subunits as well as the β5 subunit.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not affect TNFα-stimulated phosphorylation and degradation of IκBα in Panc-1 cells. (A) In Panc-1 cells pretreated with vehicle (DMSO), TNFα (15 ng/ml) was able to stimulate IκBα degradation when monitored by immunoblotting over 90 min. However, the expoxomicin pretreatment almost completely abolished TNFα-stimulated degradation of IκBα. (B) Pretreatment with UK-101 (1 μM) or LKS01 (0.2 μM), either individually or in combination, did not have a negative impact on TNFα-stimulated degradation of IκBα. Densitometric analyses were performed on the intensities of IκBα and β-actin bands and the calculated extent of IκBα degradation is shown in bar graphs.
Selective inhibition of β1i, β5i or both using UK-101 or LKS01 does not affect TNFα-stimulated translocation of NF-κB into the nucleus in Panc-1 cells. (A) TNFα-stimulated translocation of NF-κB (p65) to the nucleus was examined 90 min following TNFα addition. (B) Addition of TNFα (15 ng/ml, 90 min) led to nuclear translocation of NF-κB (p65) in Panc-1cells pretreated with UK-101 (1μM) or LKS01 (0.2 μM), either individually or in combination.