Abstract
Patterns of medical resource use near the end of life may differ across modes of death. We characterized patterns of inpatient resource use and direct costs for patients with HF who died of sudden cardiac death (SCD), HF, other cardiovascular causes, or noncardiovascular causes during the last year of life. Data were from a randomized trial exercise training in patients with HF. Mode of death was adjudicated by an end point committee. We used generalized estimating equations to compare hospitalizations, inpatient days, and inpatient costs incurred during the final year of life among patients who died of different causes, adjusting for clinical and treatment characteristics. Of 2331 patients enrolled in the trial, 231 died after at least 1 year of follow-up with an adjudicated mode of death, including 72 of SCD, 80 of HF, 34 of other cardiovascular causes, and 45 of noncardiovascular causes. Patients who died of SCD were younger, had less severe HF, and incurred fewer hospitalizations, fewer inpatient days, and lower inpatient costs than patients who died of other causes. After adjustment for patient characteristics, inpatient resource use varied by 2 to 4 times across modes of death, suggesting that cost-effectiveness analyses of interventions that reduce mortality from SCD compared with other causes should incorporate mode-specific end-of-life costs. In conclusion, resource use and associated medical costs in the last year of life differed markedly among patients with HF who experienced SCD and patients who died of other causes.
Keywords: Costs and Cost Analysis, Death, Sudden, Cardiac, Heart Failure
Introduction
With the increasing costs associated with new technologies, one can expect mounting scrutiny of their cost-effectiveness and overall impact on health care spending. Understanding current resource use and cost patterns incurred by patients with heart failure (HF) near the end of life is critical to the development of high-quality economic models, which require accurate estimates of lifetime costs and survival associated with HF therapies. We compared patterns of inpatient resource use and direct medical costs among patients with sudden cardiac death (SCD) or who died of HF, other cardiovascular causes, or noncardiovascular causes. We hypothesized that patients with SCD would incur lower rates of inpatient medical resource use and lower costs compared with patients who died of other causes.
Methods
The study population was derived from HF-ACTION, a multicenter, randomized clinical trial of exercise training plus usual care vs usual care alone in patients with stable HF who were receiving state-of-the-art medical care.1,2 Exercise training consisted of 36 supervised sessions followed by home-based training. Eligible patients had NYHA class II to IV HF for at least 3 months and left ventricular ejection fraction ≤ 35% within 6 months of enrollment. Patients were ineligible if they recently had a major cardiovascular event or had a condition that could interfere with exercise training. A total of 2331 patients were enrolled between April 2003 and February 2007 and were followed for up to 4 years at 82 sites in the United States (n = 67), Canada (n = 9), and France (n = 6). The primary end point, a composite of all-cause mortality or hospitalization, occurred in 65% of patients assigned to exercise training vs 68% of patients assigned to usual care (hazard ratio [HR], 0.93; 95% confidence interval [CI], 0.84–1.02; P = .13).2
Some patient characteristics were collected only at baseline but did not necessarily represent characteristics of patients within the year before death (eg, blood pressure, comorbid conditions, ejection fraction). However, several important variables were collected routinely, including NYHA class, medications, and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. For these variables, we determined patient characteristics at the start of the year before death. We calculated age at 1 year before death.
In addition demographic, clinical, and laboratory characteristics, the trial collected a wide range of data on medical resource use quarterly for the first 2 years and annually thereafter, including all-cause hospitalizations, emergency department and urgent care visits, outpatient visits and procedures, and other institutional care. Dates for outpatient care, outpatient procedures, and care provided at non-acute care facilities were not collected; therefore, we limited our analysis to inpatient admissions, inpatient days, and inpatient costs (for which specific dates were available).
Inpatient costs were based primarily on event-level billing data, which were collected centrally for more than 80% of all hospitalizations reported during follow-up. Using these data, we estimated comprehensive costs of inpatient care by converting department-level charges to costs using cost-to-charge ratios generated from each hospital’s annual Medicare cost report.3 For remaining admissions for which bills were not available, we calculated inpatient costs by multiplying estimates of the median daily cost for each of 47 reasons for admission by length of stay for corresponding hospitalizations. We also assigned costs for physicians’ inpatient services and procedures throughout the follow-up period. Additional details about the costing methods have been described previously.3 We valued all costs in 2008 US dollars.
Mode of death was adjudicated by the trial’s end point committee, which was blinded to treatment assignment. Mode of death was assigned on the basis of the definitions below and using information from case report forms reporting death, including site investigator summaries of events, copies of pertinent hospital discharge or death summaries, and diagnostic studies (eg, computed tomographic scans, electrocardiograms, operative reports), and a summary of interviews conducted by the study site coordinator of family members or witnesses describing out-of-hospital deaths. These sources of information were reviewed by 2 members of the end point committee who had to independently agree on the mode of death. Mode of death was otherwise assigned by consensus of the entire committee.
Modes of death included SCD, HF, other cardiovascular causes, and noncardiovascular causes. Each mode was defined prospectively before the study. Sudden cardiac death was defined as an unexpected and otherwise unexplained death in a previously stable patient, including patients who were comatose and then died after attempted resuscitation. Patients in this category had recent human contact before the event. Patients who died but were out of human contact for prolonged or unknown periods were classified as unknown. Death from HF was defined as death from worsening or intractable HF, which generally occurred during hospitalization but could have occurred at home during hospice care. Terminal arrhythmias associated with HF deaths were classified as HF deaths. Other cardiovascular deaths were defined as being caused by a cardiovascular cause other than SCD or HF. Heart failure secondary to a recent myocardial infarction was classified as other cardiovascular death.
We excluded patients who died of unknown causes. To ensure that differences in available follow-up time did not influence descriptive estimates or comparisons of resource use or costs, we limited the analysis to patients who had at least 1 year of follow-up before death. We combined patients from both treatment groups for analysis.
We describe patient characteristics using means with SDs for continuous variables and frequencies for categorical variables. We compared baseline characteristics using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. We report proportions of patients hospitalized. To describe counts of medical resources and costs, we report means with SDs and medians with interquartile ranges.
We performed unadjusted and adjusted comparisons of medical resource use and costs incurred in the year before death using generalized linear regression models. To model counts of resource use throughout the year before death, we applied negative binomial distributions and log links. To model associations between cause of death and inpatient costs, we applied a generalized linear model specified with gamma variances and log links. Patients without an admission in the last year of life were assigned a cost of $1, because we modeled only inpatient costs. In unadjusted analyses, we included only covariates representing the cause of death.
We expected that differences in inpatient resource use and costs could differ across modes of death because of differences in disease severity or other factors. Therefore, we extended the regression models to include covariates. Before conducting multivariable regression analyses, we centered all continuous covariates on corresponding mean values and computed variance inflation factors to check for collinearity between covariates (factors for all covariates < 2). We limited selection of covariates to those that were significant in bivariable analyses at P < .10, because the samples were relatively small. These variables included NYHA class at 1 year before death and the following baseline variables: systolic blood pressure, ICD, biventricular pacemaker, blood urea nitrogen level, serum creatinine level, serum sodium level, left ventricular ejection fraction, and treatment with angiotensin-converting enzyme (ACE) inhibitors and β-blockers.
Results
During the follow-up period, 387/2331 (16%) patients died, of whom 48 died of unknown causes. Of the remaining 339 patients with an adjudicated reason for death, 231 (68.1%) had at least 12 months of follow-up before death. Approximately equal numbers of patients died of heart failure (n = 80) and SCD (n = 72). Another 34 patients died of other cardiovascular causes and 45 died of noncardiovascular causes. Based on the hospitalization dates and death dates available, 16.7% of patients with SCD and 58.5% of patients dying from non-SCD causes were in the hospital at the time of death.
Table 1 shows patient characteristics at the time of randomization for information collected only at baseline or 1 year before death for information collected repeatedly throughout the trial. Although patients who experienced SCD were approximately 5 years younger than patients who died of other causes (61 years vs 66 years; P = .06), they were similar with regard to sex and race.
Table 1.
Characteristics of the Study Population at Randomization or 1 Year Before Death
| Characteristic | Mode of Death | P Value* | |||
|---|---|---|---|---|---|
| Sudden Cardiac Death (n = 72) | Heart Failure (n = 80) | Other Cardiovascular (n = 34) | Noncardiovascular (n = 45) | ||
| Age, mean (SD), years† | 61 (13) | 66 (12.9) | 66 (12) | 66 (12) | .06 |
| Male | 60 (83%) | 62 (78%) | 27 (79%) | 36 (80%) | .84 |
| Race | .11 | ||||
| Black or African American | 29 (41%) | 24 (30%) | 10 (30%) | 16 (36%) | |
| White | 37 (53%) | 48 (60%) | 23 (70%) | 28 (64%) | |
| Other or missing | 4 (5.7%) | 8 (10%) | 0 | 0 | |
| NYHA class† | .005 | ||||
| I | 4 (6%) | 2 (3%) | 1 (3%) | 1 (2%) | |
| II | 28 (39%) | 12 (15%) | 10 (29%) | 13 (29%) | |
| III | 34 (47%) | 36 (45%) | 15 (44%) | 17 (38%) | |
| IV | 6 (8%) | 30 (38%) | 8 (24%) | 14 (31%) | |
| Ischemic etiology | 39 (54%) | 52 (65%) | 17 (50%) | 30 (66.67%) | .25 |
| Body mass index, mean (SD) | 31.6 (7.5) | 29.4 (7.4) | 30.3 (5.8) | 29.6 (5.3) | .27 |
| Systolic blood pressure, mean (SD), mm Hg | 113.4 (17.6) | 107.6 (17.9) | 111.2 (20.9) | 119.3 (15.7) | .001 |
| Diastolic blood pressure, mean (SD), mm Hg | 68.0 (11.6) | 67.1 (10.5) | 67.9 (10.7) | 69.9 (9.4) | .31 |
| Comorbid conditions | |||||
| Cancer in past 5 years | 2 (2.8%) | 4 (5%) | 2 (6%) | 2 (4.4%) | .87 |
| Chronic obstructive pulmonary disease | 14 (20%) | 9 (11%) | 0 (0%) | 10 (22%) | .02 |
| Diabetes mellitus | 25 (35%) | 26 (33%) | 17 (50%) | 17 (38%) | .34 |
| History of depression | 10 (14%) | 13 (16%) | 5 (15%) | 8 (18%) | .95 |
| History of stroke | 6 (8%) | 10 (13%) | 3 (9%) | 6 (13%) | .77 |
| Hyperlipidemia | 48 (67%) | 52 (65%) | 26 (76%) | 30 (67%) | .68 |
| Hypertension | 41 (58%) | 50 (63%) | 19 (56%) | 32 (71%) | .45 |
| Peripheral vascular disease | 9 (13%) | 7 (9%) | 4 (12%) | 4 (9%) | .87 |
| Hospitalized within 6 months of enrollment | 21 (29%) | 27 (34%) | 13 (38%) | 22 (49%) | .18 |
| Cardiac devices | |||||
| Implantable cardioverter-defibrillator | 16 (22%) | 45 (56%) | 18 (53%) | 22 (49%) | < .001 |
| Biventricular pacemaker | 6 (8%) | 19 (24%) | 10 (29%) | 8 (18%) | .03 |
| Pacemaker | 11 (15%) | 23 (29%) | 6 (18%) | 10 (22%) | .22 |
| Blood urea nitrogen, mean (SD), mg/dL | 25.5 (14.8) | 36.0 (21.8) | 30.0 (15.2) | 30.5 (18.7) | .008 |
| Creatinine, mean (SD), mg/dL | 1.4 (0.4) | 1.6 (0.6) | 1.8 (1.5) | 1.7 (1.4) | .01 |
| Sodium, mean (SD), mEq/L | 139.5 (3.0) | 138.0 (3.9) | 139.5 (3.3) | 139.7 (3.7) | .05 |
| Left ventricular ejection fraction, mean (SD), % | 24.3 (8.1) | 21.4 (6.6) | 22.7 (7.0) | 26.7 (7.8) | .001 |
| Exercise duration on cardiopulmonary exercise stress test, mean (SD), minutes | 8.5 (3.7) | 7.2 (2.8) | 7.7 (2.7) | 8.2 (3.3) | .19 |
| Peak VO2, mean (SD), ml/kg/min | 13.2 (3.9) | 11.6 (3.5) | 12.8 (3.1) | 12.6 (3.5) | .07 |
| KCCQ overall summary score, mean (SD)† | 64.7 (20.8) | 55.8 (25.0) | 65.6 (21.3) | 61.4 (23.4) | .12 |
| Medications† | |||||
| ACE inhibitor | 45 (63%) | 34 (43%) | 16 (47%) | 23 (51%) | .10 |
| Angiotensin receptor blocker | 13 (18%) | 11 (14%) | 7 (21%) | 6 (13%) | .73 |
| Antiarrhythmic agent | 22 (31%) | 33 (41%) | 10 (29%) | 14 (31%) | .43 |
| Aspirin | 42 (58%) | 41 (51%) | 20 (59%) | 26 (58%) | .79 |
| β-Blocker | 66 (92%) | 55 (69%) | 29 (85%) | 35 (78%) | .004 |
| Lipid-lowering agent | 45 (63%) | 45 (56%) | 22 (65%) | 21 (47%) | .30 |
| Loop diuretic | 51 (71%) | 69 (86%) | 26 (76%) | 37 (82%) | .12 |
| Potassium-sparing diuretic | 29 (40%) | 34 (43%) | 9 (26%) | 11 (24%) | .11 |
Abbreviation: KCCQ, Kansas City Cardiomyopathy Questionnaire.
P values from χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Measured at 1 year before death.
Medical histories were similar across groups, with the exception of patients who died of SCD or noncardiovascular causes, who were more likely to have chronic obstructive pulmonary disease (P = .02). Patients who experienced SCD were less likely to have an ICD or biventricular pacemaker before randomization. Several measures representing severity of HF indicated less severe heart failure in patients with SCD, including significantly lower blood urea nitrogen level, higher peak VO2, higher left ventricular ejection fraction, and fewer patients with NYHA class IV HF. Long-term medication use was similar across groups, except that β-blocker use was significantly higher among patients with SCD (92% vs 69% to 85%; P = .004).
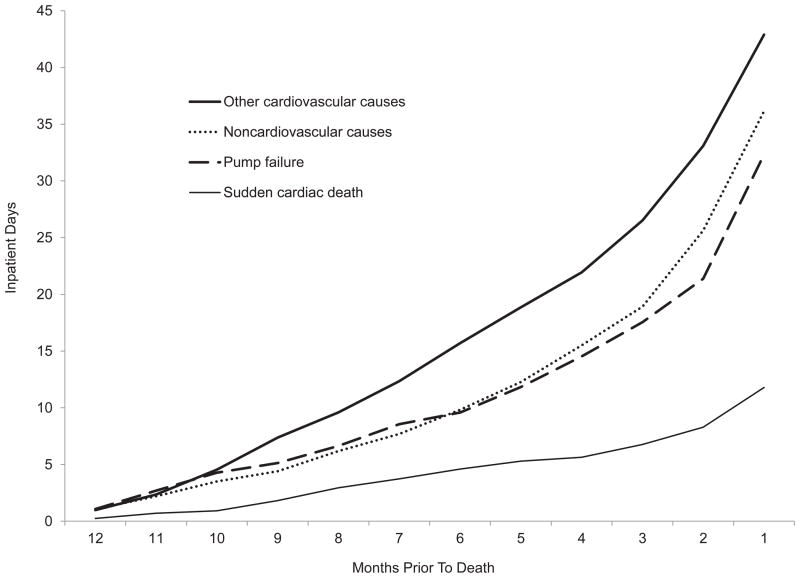
Approximately 6 of 10 patients who died of SCD were hospitalized in the previous year, compared with more than 9 of 10 patients who died of other causes (P < .05; Table 2). Patients with SCD had an average of 1.4 hospitalizations, whereas patients who died of other causes had twice as many (2.4 to 3.5; P < .05). The relative difference was larger with respect to the number of inpatient days in the last year of life, with 11.8 inpatient days for patients who experienced SCD and about 3 times as many for patients who died of other causes (32.3 to 42.8 days; P < .05). The sharpest increase in inpatient days occurred in the last 2 months of life (Figure 1).
Table 2.
Inpatient Resource Use and Costs Within 12 Months of Death
| Variable | Mode of Death | |||
|---|---|---|---|---|
| Sudden Cardiac Death (n = 72) | Heart Failure (n = 80) | Other Cardiovascular (n = 34) | Noncardiovascular (n = 45) | |
| Hospitalized at least once | 45 (63%) | 76 (95%)* | 31 (91%)* | 42 (93%)* |
| Number of hospitalizations | ||||
| Mean (SD) | 1.4 (1.6) | 3.5 (2.2)* | 2.4 (1.9)* | 3.3 (2.2)* |
| Median (interquartile range) | 1.0 (0.0–2.0) | 3.0 (2.0–5.0) | 2.0 (1.0–4.0) | 3.0 (2.0–4.0) |
| Number of inpatient days | ||||
| Mean (SD) | 11.8 (19.5) | 32.3 (28.5)* | 42.8 (55.0)* | 36.0 (37.1)* |
| Median (interquartile range) | 6.5 (0.0–12.5) | 26.5 (14.0–42.0) | 20.0 (5.0–60.0) | 27.0 (11.0–50.0) |
| Inpatient costs, $ | ||||
| Mean (SD) | 23,328 (48,003) | 70,224 (85,154)* | 119,374 (159,398)* | 76,313 (96,019)* |
| Median (interquartile range) | 7832 (0–23,750) | 46,756 (19,694–81,751) | 39,226 (8064–195,002) | 46,955 (17,043–91,272) |
P < .01. Sudden cardiac death served as the reference group. All other comparisons were P > .01.
Figure 1.
Cumulative Inpatient Days During 12 Months Before Death
Findings were consistent with respect to inpatient costs. Patients with SCD incurred mean costs of $23,328, and patients who died of heart failure or noncardiovascular causes incurred inpatient costs of $70,224 and $76,313, respectively (P < .05). Patients who died of other cardiovascular causes incurred the highest mean costs ($119,374), with 25% of patients incurring inpatient costs beyond $195,000. Median inpatient costs were similar among the non-SCD groups ($39,226 to $46,955) but were lower among those with SCD ($7832).
After adjustment for clinical and treatment characteristics, compared with patients with SCD, patients who died of other causes were hospitalized more frequently and for more days and incurred higher inpatient costs in the year before death (Table 3). Patients who died of non-SCD causes incurred 1.5 to 2.0 times more hospitalizations, 2.4 to 3.7-times more inpatient days, and 2.6 to 5.4 times higher inpatient costs in the last year of life (Table 3). Each pairwise comparison with the SCD group was statistically significant.
Table 3.
Results of the Multivariable Model for Inpatient Costs at 1 Year
| Variable | Hospital Admissions | Inpatient Days | Inpatient Costs | |||
|---|---|---|---|---|---|---|
| Intercept/Rate Ratio (95% CI)† | P Value | Intercept/Rate Ratio (95% CI)† | P Value | Intercept/Means Ratio (95% CI)† | P Value | |
| Intercept | 1.04 (0.66–1.63) | .88 | 8.63 (4.08–18.25) | < .001 | $15,215 ($4755–$4869) | < .001 |
| Cause of death | ||||||
| Sudden cardiac death | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| Heart failure | 1.82 (1.37–2.43) | < .001 | 2.41 (1.58–3.68) | < .001 | 2.56 (1.35–4.85) | .004 |
| Other cardiovascular | 1.52 (1.07–2.18) | .02 | 3.66 (2.09–6.42) | < .001 | 5.38 (2.21–13.09) | < .001 |
| Noncardiovascular | 1.99 (1.46–2.70) | < .001 | 2.77 (1.73–4.43) | < .001 | 3.18 (1.54–6.56) | .002 |
| Age, per 1 year increase* | 1.00 (0.99–1.01) | .94 | 1.01 (0.99–1.02) | .49 | 1.01 (0.98–1.03) | .67 |
| Male gender | 0.94 (0.73–1.21) | .62 | 1.21 (0.79–1.87) | .39 | 1.55 (0.80–3.00) | .19 |
| Race | ||||||
| Black or African American | 1.44 (1.13–1.82) | .003 | 1.84 (1.22–2.27) | .004 | 1.93 (1.02–3.64) | .04 |
| White | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| Other or missing | 1.11 (0.70–1.76) | .66 | 1.31 (0.54–3.17) | .54 | 1.46 (0.37–5.79) | .59 |
| NYHA class | ||||||
| I/II | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| III | 1.41 (1.09–1.83) | .009 | 1.07 (0.71–1.61) | .76 | 0.86 (0.46–1.58) | .62 |
| IV | 1.84 (1.40–2.41) | < .001 | 1.93 (1.24–2.99) | .003 | 2.02 (1.03–3.95) | .04 |
| LVEF, per 1% increase | 1.01 (1.00–1.02) | .20 | 1.01 (0.99–1.04) | .25 | 1.01 (0.97–1.05) | .56 |
| Systolic blood pressure, per 1 mm Hg increase | 1.00 (0.99–1.00) | .68 | 0.99 (0.98–1.00) | .10 | 0.99 (0.97–1.00) | .09 |
| Chronic obstructive pulmonary disease | 1.16 (0.88–1.53) | .29 | 1.05 (0.63–1.73) | .86 | 0.95 (0.44–2.03) | .89 |
| ICD or biventricular pacer | 1.42 (1.15–1.76) | .001 | 1.09 (0.77–1.55) | .61 | 1.18 (0.68–2.06) | .56 |
| Pacemaker | 0.97 (0.73–1.18) | .54 | 1.04 (0.69–1.57) | .84 | 1.11 (0.59–2.08) | .75 |
| Blood urea nitrogen, mg/dL | 1.00 (1.00–1.01) | .17 | 1.01 (1.00–1.02) | .21 | 1.01 (0.99–1.02) | .40 |
| Creatinine, mg/dL | 1.01 (0.90–1.13) | .89 | 1.06 (0.89–1.26) | .54 | 0.98 (0.77–1.25) | .88 |
| Sodium, mEq/L | 0.99 (0.96–1.02) | .38 | 1.01 (0.96–1.06) | .75 | 1.02 (0.94–1.09) | .68 |
| Peak VO2, mL/kg/min | 0.99 (0.95–1.02) | .32 | 0.99 (0.93–1.04) | .64 | 0.97 (0.89 –1 .06) | .55 |
| ACE inhibitor use* | 0.95 (0.77–1.17) | .63 | 0.85 (0.61–1.18) | .33 | 0.89 (0.53–1.48) | .65 |
| β-Blocker use* | 0.87 (0.69–1.09) | .22 | 0.70 (0.47–1.04) | .08 | 0.56 (0.30–1.03) | .06 |
Abbreviations: CI, confidence interval; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association.
Measured 1 year before death.
Intercept terms represent expected mean number of hospitalizations, inpatient days, and inpatient costs in patients with characteristics corresponding to the means of continuous covariates and the reference group for categorical variables. Rate ratios and means ratios corresponding to model covariates represent multiplicative effects of the covariates on the intercept terms with regard to the outcome measures.
After adjustment for cause of death and other factors listed in Table 3, patients with an ICD or biventricular pacemaker had 1.4 times more hospitalizations than patients without these devices. Two covariates were consistently associated with resource use and costs. Black or African American patients had 1.4 times more admissions, 1.8 times more inpatient days, and 1.9 times higher costs than similar white patients. Patients with NYHA class IV HF at the beginning of the last year of life incurred 1.8 to 2.0 times more admissions, inpatient days, and inpatient costs that patients with NYHA class II.
Discussion
Patterns of inpatient resource use and costs differed significantly between patients who died of SCD compared with other causes. More than one-third of patients who experienced SCD were not hospitalized in the year before death, compared with at least 9 of 10 patients who died of HF, other cardiovascular causes, or noncardiovascular causes. In addition, patients who experienced SCD were less than half as likely to have ICD or biventricular pacemaker before enrollment in HF-ACTION, compared with patients who died of other causes.
We also found consistent, independent associations between black or African American race and more hospitalizations, more inpatient days, and higher inpatient costs. Unroe et al4 also found that black patients near the end of life incurred approximately 20% higher costs than patients of other races. One contributing factor may be that black patients are less likely to receive hospice care5 and more likely to choose aggressive care6 over hospice. A recent study of nursing home residents with heart failure found that black patients were less likely than white patients to receive hospice.7 In HF-ACTION, black and white patients died in hospital in similar proportions (43% vs 46%). Other contributing factors are not clear. Some studies have found that black patients with HF more often receive care in lower-quality institutions8–10 and less often receive β-blockers and ACE inhibitors or angiotensin receptor blockers,11 which may be associated with higher risk for readmission and longer stays. However, these factors are inconsistent with the selection of sites participating in HF-ACTION and the high overall use of evidence-based medications.
Appropriate interpretation of the results must account for the patient population included in the study. HF-ACTION enrolled outpatients with medically stable heart failure and reduced left ventricular function who were willing to be randomly assigned to a structured exercise training program. Other studies of resource use and costs before death included either patients who were randomly assigned to receive medical treatment for end-stage HF12 or a broad range of Medicare beneficiaries with HF and either preserved or reduced left ventricular function.
Russo et al12 reported that 50.5% of estimated 2-year costs among patients with end-stage HF with reduced left ventricular function who were treated with medical management were expended over the final 6 months of life. In the last 2 years of life, these patients spent an average of 82.8 days in the hospital. If we assume that the patients incurred half of those days in the last 6 months of life, the estimate of inpatient days surpasses our estimate of 23 days among patients in our study who died of HF. This result may be attributable in part to aggressive medical therapy in the study by Russo et al12 among patients not assigned to mechanical assistance, because these patients were enrolled with more severe HF symptoms and the protocol specifically addressed this severity. Nevertheless, patients in both studies were treated by HF specialists and received high levels of guideline-recommended therapy. Our estimates appear to be consistent with those reported by Unroe et al.4 In their study of Medicare beneficiaries during the last 6 months of life, patients with HF spent an average of 21 days in the hospital, consistent with our estimates for patients who died of non-SCD causes.
Without access to dates of outpatient resource use, we could not distinguish reliably between resources use before versus during patients’ last year of life. The extent to which we have underestimated total costs is uncertain. Using administrative billing data, Russo et al12 found that inpatient costs represented approximately 80% of total costs during the last 2 years of life. Dunlay et al13 found that hospitalization costs represented approximately 80% of lifetime costs for patients with HF. However, Unroe et al4 found that nonphysician care outside of acute care institutions represented approximately 66% of total Medicare payments in the last year of life. We would argue, however, that the proportion of total costs for non-acute institutional care among patients in HF-ACTION was lower than reported for the Medicare population studied by Unroe et al.4 At the time of death, the mean age of patients in the Unroe et al4 study was 83 years, compared with 62 years for patients who died of SCD and 67 years for patients who died of other causes in our study. None of the patients in HF-ACTION were institutionalized at the time of randomization.
The availability of cause-specific end-of-life cost estimates can improve the validity of economic models designed to evaluate the cost-effectiveness of medical interventions in HF. The impact of accounting for mode of death when projecting costs in a cost-effectiveness analysis will depend on several factors. An intervention that reduces mortality from SCD will inversely affect the proportion of patients who die of other causes associated with higher costs. In such a case, assigning mode-specific costs would lead to higher cost-effectiveness ratios (ie, less cost-effective) than assigning costs averaged across non-SCD modes of death. However, reducing SCD will extend life expectancy, resulting in lower cost-effectiveness ratios (ie, more cost-effective). The balance of these countervailing forces will depend on the underlying health status of patients in whom SCD is prevented, with interventions that reduce the incidence of SCD being relatively more cost-effective for healthier patients with longer expected survival.
Acknowledgments
Funding/Support: This study was supported by grant 5R01NR011873-02 from the National Institute of Nursing Research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. HF-ACTION was funded by grants 5U01HL063747, 5U01HL066461, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, and 5U01HL064264 from the National Heart, Lung, and Blood Institute; and grants R37AG018915 and P60AG010484 from the National Institute on Aging.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00047437
Additional Contributions: Damon M. Seils, MA, Duke University, assisted with manuscript preparation. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA Piña IL; HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F Piña IL; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed SD, Whellan DJ, Li Y, Friedman JY, Stephen J, Ellis SJ, Piña IL, Settles SJ, Davidson-Ray L, Johnson JL, Cooper LS, O’Connor CM, Schulman KA. Economic evaluation of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) randomized controlled trial: an exercise training study of patients with chronic heart failure. Circ Cardiovasc Qual Outcomes. 2010;3:374–381. doi: 10.1161/CIRCOUTCOMES.109.907287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 5.Connor SR, Elwert F, Spence C, Christakis NA. Racial disparity in hospice use in the United States in 2002. Palliat Med. 2008;22:205–213. doi: 10.1177/0269216308089305. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KS, Kuchibhatla M, Tanis D, Tulsky JA. Racial differences in hospice revocation to pursue aggressive care. Arch Intern Med. 2008;168:218–224. doi: 10.1001/archinternmed.2007.36. [DOI] [PubMed] [Google Scholar]
- 7.Lepore MJ, Miller SC, Gozalo P. Hospice use among urban Black and White U.S. nursing home decedents in 2006. Gerontologist. 2011;51:251–260. doi: 10.1093/geront/gnq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasnain-Wynia R, Kang R, Landrum MB, Vogeli C, Baker DW, Weissman JS. Racial and ethnic disparities within and between hospitals for inpatient quality of care: an examination of patient-level Hospital Quality Alliance measures. J Health Care Poor Underserved. 2010;21:629–648. doi: 10.1353/hpu.0.0281. [DOI] [PubMed] [Google Scholar]
- 10.Hasnain-Wynia R, Baker DW, Nerenz D, Feinglass J, Beal AC, Landrum MB, Behal R, Weissman JS. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;25(167):1233–1239. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- 11.Bagchi AD, Stewart K, McLaughlin C, Higgins P, Croghan T. Treatment and outcomes for congestive heart failure by race/ethnicity in TRICARE. Med Care. 2011;49:489–495. doi: 10.1097/MLR.0b013e318207ef87. [DOI] [PubMed] [Google Scholar]
- 12.Russo MJ, Gelijns AC, Stevenson LW, Sampat B, Aaronson KD, Renlund DG, Ascheim DD, Hong KN, Oz MC, Moskowitz AJ, Rose EA, Miller LW REMATCH Investigators. The cost of medical management in advanced heart failure during the final two years of life. J Card Fail. 2008;14:651–658. doi: 10.1016/j.cardfail.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;1:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]