Abstract
Exogenous collagen cross-linking has been investigated as method of reinforcing scleral biomechanics, with the goal of counteracting scleral weakening that occurs at the onset of myopia. This study uses whole globe inflation testing to investigate the biomechanical effect of treating posterior sclera with the collagen cross-linking agents methylglyoxal and genipin. Pairs of porcine eyes were treated in four ways. Three groups involved 1% methylglyoxal: two-hour (Group 1) or thirty-minute (Group II) incubation of the whole globe, and thirty-minute incubation of only the posterior sclera of the intact eye (Group III). Group IV consisted of a thirty-minute incubation of the posterior sclera in 1% genipin. Following treatment, each eye was subjected to inflation testing under physiological pressure levels (0-150 mmHg); four strain markers on the posterior pole were tracked, providing displacement measurements in two directions. Results were used to derive load versus deformation behavior and to calculate stiffness at 0.25% strain (toe stiffness) and at peak strain (peak stiffness). Toe stiffness of Group 1 was 4.8 and 1.3 times greater than controls (sagittal and transverse directions, respectively: 5.23 ± 0.39 vs. 0.90 ± 0.08 mHg, P < 0.001; and 3.41 ± 0.19 vs. 1.51 ± 0.22 mHg, P < 0.01; values in mean ± SE). Group II was 7.4 and 4.3 times stiffer than controls (sagittal and transverse directions, respectively: 5.26 ± 0.49 vs. 0.63 ± 0.10 mHg, P < 0.02; and 3.44 ± 0.44 vs. 0.65 ± 0.07 mHg, P < 0.003). Group III was 3.6 and 3.4 times stiffer than controls (sagittal and transverse directions, respectively: 5.21 ± 0.39 vs. 1.13 ± 0.31 mHg, P < 0.01; and 4.94 ± 1.48 vs. 1.13 ± 0.25, P < 0.01), while Group IV was 8.2 and 2.8 times stiffer than controls (sagittal and transverse: 12.36 ± 1.96 vs. 1.35 ± 0.14 mHg, P < 0.01; and 12.45 ± 1.34 vs. 3.27 ± 0.50 mHg, P < 0.05). In all groups, there was no significant difference in peak stiffness after scleral cross-linking (SXL). At low strain, the posterior sclera was stiffer in both measured directions following methylglyoxal and genipin treatments, however at peak strain the treated sclera was not stiffer. Additionally, the saturation level of scleral stiffening by methylglyoxal can be reached within thirty minutes of treatment.
Keywords: sclera, inflation, collagen cross-linking, methylglyoxal, genipin
1. Introduction
Progressive myopia is a major cause of vision impairment worldwide (McBrien and Gentle. 2003). This disease is characterized by axial elongation and abnormal ocular shape (McBrien et al. 2009, Rada et al. 2006). At the onset of myopia, remodeling leads to increased extensibility, especially at the posterior pole of the eye (Phillips et al. 2000, Siegwart and Norton. 1999). This weakening of sclera compromises eye shape and size; maintenance of proper eye size is a key responsibility of the sclera—the principal load-bearing tissue of the eye (McBrien and Gentle. 2003, Rada et al. 2006).
Exogenous collagen cross-linking has been suggested as a solution to prevent globe elongation. Compounds such as glutaraldehyde, glyceraldehyde, methylglyoxal, and genipin have been shown to increase ocular tissue stiffness (Avila and Navia. 2010, Mattson et al. 2010, Spoerl et al. 2005, Wollensak and Spoerl. 2004). Spoerl et al. previously performed uniaxial biomechanical testing to show that methylglyoxal is capable of stiffening sclera (Spoerl et al. 2005); however, multiaxial testing is more representative of sclera in its physiological state (Greene and McMahon. 1979). Furthermore, the treatment described involved a week-long incubation. In this study we aim to find a shorter treatment regimen that is more clinically relevant.
Of the many known cross-linking agents, methylglyoxal and genipin were chosen for cross-link efficacy with possibly less toxicity at low concentrations. Methylglyoxal (MG) is a naturally occurring Maillard intermediate (McLellan et al. 1992, Wells-Knecht et al. 1995). Genipin (GP) is a natural collagen cross-linker obtained from geniposide, which is found in the fruit Gardenia jasminoides ellis. GP has been shown to have low toxicity (Chang et al. 2002, Huang et al. 1998, Sung et al. 1999b) as well as the potential to increase rigidity of porcine corneas (Avila and Navia. 2010). However, its effect on scleral biomechanics has not been reported.
In this study, the biomechanical effects of whole globe inflation following methylglyoxal and genipin treatment of the sclera are tested using paired porcine eyes, so that both control and treated eyes were from the same animal. We hypothesized that exogenous collagen cross-linking by these compounds would strengthen the posterior sclera by increasing scleral stiffness and decreasing peak strain at equivalent loads in both sagittal and transverse directions.
2. Methods
2.1 Tissue preparation
Twenty-seven pairs of enucleated porcine eyes (Visiontech Inc., Sunnyvale, TX) were used. Each pair of eyes came from one animal. Eyes were stored at 4°C and experimented on within 72 hours of enucleation. Adherent muscle was removed to expose the sclera. Posterior scleral thickness was measured using an ultrasound pachymeter (Pachette 3, DGH Technology, Inc., Exton, PA). Eye diameter was measured with a digital caliper (CD-8″ PS, Mitutoyo Corp., Japan).
In each pair of eyes, one eye was randomly chosen for incubation in 1% methylglyoxal (Sigma-Aldrich, St Louis, MO) or 1% genipin (Challenge Bioproducts Co., Taichung, Taiwan) in PBS at room temperature (20°C); the contralateral eye was incubated in a control solution of PBS. Four incubation conditions were tested: whole globe incubation in 1% MG for 120 minutes (Group I); whole globe incubation in 1% MG for 30 minutes (Group II); posterior pole (of intact eye) incubation in 1% MG for 30 minutes (Group III); posterior pole incubation in 1% GP for 30 minutes (Group IV). For local treatment of posterior sclera, a circular area (diameter of 6 mm) at the posterior pole was exposed to treatment solution for 30 minutes; an inverted tube filled with treatment solution rested on the sclera in order to limit the area of sclera exposed to the solution. No solution was observed leaking out of the tube. Following treatment, eyes were rinsed in PBS, then blotted dry.
2.2 Mechanical testing
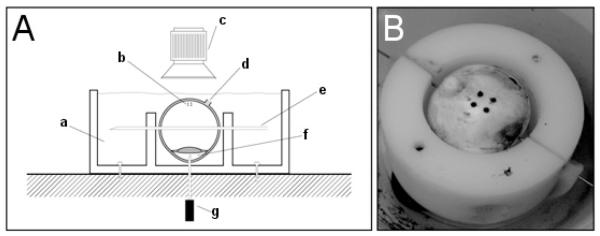
Scleral specimens were tested as described by Lari et al (Lari et al. 2012). The setup was as follows: whole globe inflation was achieved by inserting a 20-gauge inflation needle into the anterior chamber (Figure 1). This needle connected to a network of saline-filled tubing that involved a saline reservoir and pumping system to adjust saline column height to regulate IOP. The posterior pole of the eye was blotted dry and four beads were adhered to the surface forming a two by two square grid, with side lengths of 4 mm. The equator of the eye was skewered with a 22-gauge needle to restrict eye movement during testing. The eye was then placed in a custom inflation testing device with a mineral oil bath, maintained at 37°C. The intraocular pressure was gradually changed at a rate of 0.35 mmHg s-1 from 0 to 10 times normal IOP (physiologic porcine IOP assumed to be 15mmHg (Ruiz-Ederra et al. 2005); a maximum of 10xIOP was chosen because this level of pressure occurs upon eye rubbing (Coleman and Trokel. 1969). Therefore 10xIOP represented an upper bound of physiologically relevant pressure levels. Pressure was assumed to be equivalent throughout the eye; preliminary tonometry tests verified consistent pressures throughout the globe.
Figure 1.
Whole globe inflation setup (Lari et al. 2012). (A) Whole globe held in place by skewer and inflation needle with pressure being delivered via inflation needle in the anterior chamber; (a) oil bath (b) local strain targets (c) camera (d) optic nerve head (e) 22-gauge needle skewer (f) anterior chamber (g) 19-gauge needle to computer controlled pressure reservoir and (B) whole globe with local strain targets undergoing circumferential tension.
Each eye was preconditioned for 9 cycles to allow stress-strain curves to converge. Preconditioning criteria was defined to be met when maximum strain of the final preconditioning cycle was within 2% of the previous cycle. After valid preconditioning, the 10th cycle was used for data analysis. Quasi-static conditions (dε/dt < 0.001/s) were chosen to minimize strain rate dependent behavior.
During testing, strain targets were tracked every two seconds using a high precision CCTV (Panasonic WV-BD400, Matsushita Communication Industrial Co., Ltd., Japan) and custom software (LabView and IMAQ Vision, National Instruments, Austin, TX). Camera tracking occurred in two dimensions, although the globe inflated in three dimensions. (Note that arc strain and chord strain are geometrically equivalent and therefore do not affect our analysis.)
2.3 Data analysis
IOP-strain data were fit to an exponential curve:
| [Equation 1] |
where A and B are data fitting coefficients, ε is unitless strain, and IOP (mmHg) is pressure (Fung. 1993, Schultz et al. 2008). A Levenberg-Marquardt method was used in the data fitting algorithm to minimize variation in the curve fit (MATLAB, The Mathworks Inc., Natick, MA). The closeness of fit was demonstrated in our previous work (Lari et al. 2012).
Stiffness values at 0.25% strain and at peak strain were calculated. Toe stiffness of the IOP-strain relationship was calculated as the derivative of the fitted loading curve at 0.25% strain, which lies within the exponential “toe” region of all IOP-strain curves in this study; this region is presumed to represent the physiologic range in which tissues normally operate. Peak stiffness was calculated similarly but at peak strain.
Statistical comparisons of each mechanical parameter were made within eye pairs using the Wilcoxon rank-sum test.
3. Results
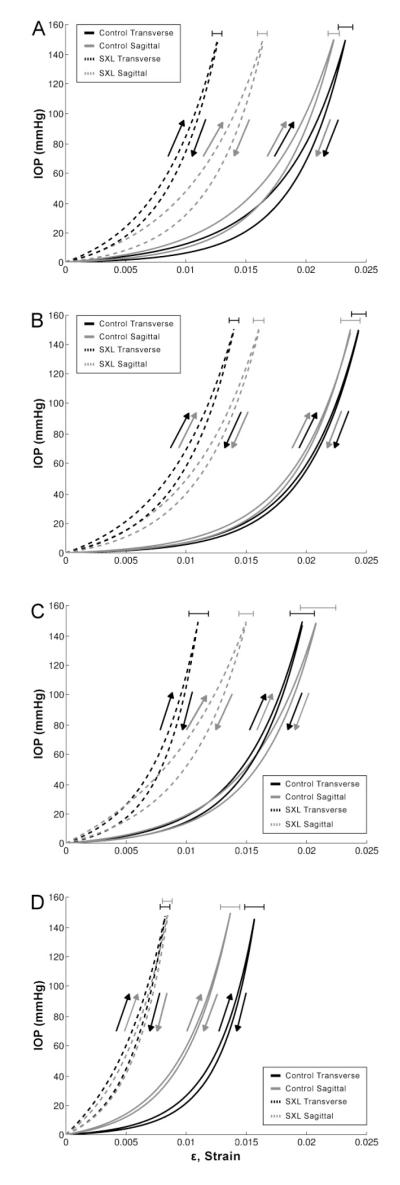
Composite tensile curves were created by averaging data from individual half-cycles to produce fitted curves (Figure 2). The exponential toe regions of the scleral cross-linking (SXL) curves are smaller than that of the controls in all cases. In particular, GP treatment (Group IV) produced the smallest toe region.
Figure 2.
Composite curve fits of inflation testing data from (A) Group I, (B) Group II, (C) Group III, (D) Group IV. Curves were generated by averaging the sum of the individual functions for each fit along the strain axis. Error bars show standard errors of peak strains
Group I: 1% MG, 120′ whole globe incubation
Group II: 1% MG, 30′ whole globe incubation
Group III: 1% MG, 30′ posterior pole incubation
Group IV: 1% GP, 30′ posterior pole incubation
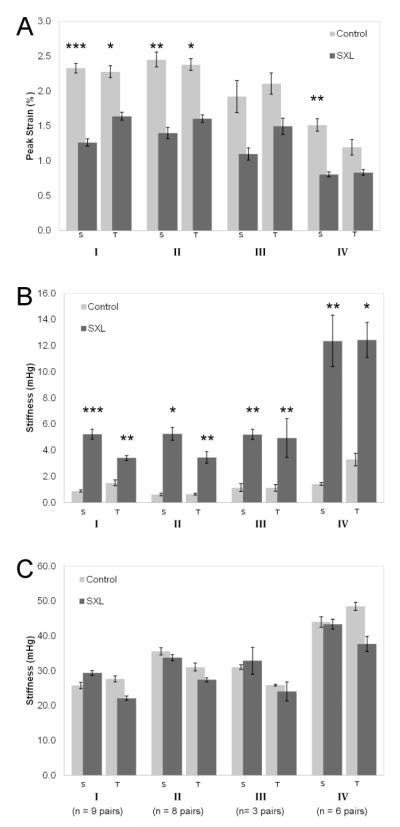
The mechanical parameters and percent differences between control and SXL are presented in Figure 3 and Table 1. SXL produced statistically significant differences in peak strain and toe stiffness in both sagittal and transverse directions in all groups, with the exception of peak strain in the transverse direction of the 1% GP group (Group IV) and peak strains in both directions in the pilot Group III (Fig. 3). Peak strain decreased by roughly 0.3-0.5 times in all SXL groups, while toe stiffness increased by a factor of 1.3 to 8 times (Table 1).
Figure 3.
Mechanical results of (A) peak strains, (B) toe stiffness at 0.25% strain, and (C) stiffness at peak strain S: sagittal direction; T: transverse direction
Group I: 1% MG, 120′ whole globe incubation
Group II: 1% MG, 30′ whole globe incubation
Group III: 1% MG, 30′ posterior pole incubation
Group IV: 1% GP, 30′ posterior pole incubation
Error bars show standard error.
* Statistically significant difference (P < 0.05), ** P < 0.01, *** P < 0.001
Table 1. Percent Differences of Mechanical Parameters.
| Peak Strain | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group I |
Group II |
Group III |
Group IV |
|||||
| S | T | S | T | S | T | S | T | |
| Percent Change |
45.7 ↓ | 28.0 ↓ | 43.0 ↓ | 32.6 ↓ | 42.9 ↓ | 29.1 ↓ | 47.4 ↓ | 38.0↓ |
| P value | < 0.001 | < 0.05 | < 0.01 | < 0.05 | ns | ns | < 0.01 | ns |
|
| ||||||||
| Stiffness at 0.25% Strain | ||||||||
|
| ||||||||
| I |
II |
III |
IV |
|||||
| S | T | S | T | S | T | S | T | |
|
| ||||||||
| Percent Change |
480 ↑ | 125 ↑ | 757 ↑ | 438 ↑ | 360 ↑ | 339 ↑ | 815 ↑ | 281 ↑ |
| P value | < 0.001 | < 0.01 | < 0.05 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.05 |
|
| ||||||||
| Stiffness at Peak Strain | ||||||||
|
| ||||||||
| I |
II |
III |
IV |
|||||
| S | T | S | T | S | T | S | T | |
|
| ||||||||
| Percent Change |
14 ↑ | 20 ↑ | 5 ↑ | 12 ↑ | 6 ↑ | 7 ↑ | 2 ↑ | 22 ↑ |
| P value | ns | ns | ns | ns | ns | ns | ns | ns |
S: sagittal direction; T: transverse direction
ns, not significant
Group I: 1% MG, 120′ whole globe incubation (n = 9 pairs)
Group II: 1% MG, 30′ whole globe incubation (n = 8 pairs)
Group III: 1% MG, 30′ posterior pole incubation (n = 3 pairs)
Group IV: 1% GP, 30′ posterior pole incubation (n = 6 pairs)
The MG treatments of Groups I and II produced similar percent decreases in peak strains—46% (2.33% ± 0.07% vs. 1.26% ± 0.05%, Group I) vs. 43% (2.45 ± 0.11% vs.1.40 ± 0.08%, Group II) in the sagittal direction and 28% (2.28 ± 0.08% vs. 1.64 ± 0.06%, Group I) vs. 33% (2.38 ± 0.09% vs. 1.61% ± 0.06%, Group II) in the transverse direction.
Toe stiffness of Group III SXL was 3.6 and 3.4 times greater than controls (sagittal and transverse directions, respectively: 5.21 ± 0.39 vs. 1.13 ± 0.31 mHg, P < 0.01; and 4.94 ± 1.48 vs. 1.13 ± 0.25, P < 0.01), while Group IV was 8.2 and 2.8 times stiffer than controls (sagittal and transverse: 12.36 ± 1.96 vs. 1.35 ± 0.14 mHg, P < 0.01; and 12.45 ± 1.34 vs. 3.27 ± 0.50 mHg, P < 0.05; values in mean ± SE; Table 1, Figure 3). However, peak stiffness of SXL in all groups were not significantly different than controls (Table 1 and Figure 3).
Scleral anisotropy was measured as the ratios of sagittal to transverse values of peak strain and toe stiffness. In Group I, the control group had similar peak strains in orthogonal directions, with a strain anisotropy value of 1.11 ± 0.05 (mean ± SE). The SXL group was not statistically different (0.08 ± 0.03, p = 0.08). The ratio of toe stiffness in orthogonal directions was 1.43 ± 0.16 vs. 1.69 ± 0.11 (control vs. SXL). There were no significant differences in anisotropy with SXL (p = 0.44). Likewise, no significant changes in anisotropy of strain or stiffness were found in Groups II, III or IV (not shown).
4. Discussion
The aim of this study was to investigate the mechanical consequence of chemically cross-linking posterior sclera under physiologic pressures. Our findings demonstrate a statistically significant strengthening of sclera at 0.25% strain in two dimensions following incubation in methylglyoxal and genipin. We assume this increased stiffness is due to an increased collagen cross-link density, which we have previously identified as a key determinant of scleral stiffness (Schultz et al. 2008). Moreover, this stiffening effect was accomplished following a treatment time of thirty minutes.
Groups I and II compare two incubation times of MG treatment. The extent of stiffening appears to reach a plateau within thirty minutes of treatment, suggesting that SXL reached a saturation point. This could mean that thirty minutes is sufficient time to elicit the maximum stiffening achievable by MG.
A pilot study of localized treatment with MG (Group III) was performed to rule out the possibility that whole globe incubation could stiffen the target sclera region more so than a local treatment of the posterior pole. Indeed, the results from Group III mimic those of Groups I and II. Therefore we proceeded to incubate Group IV (GP) in the same manner as Group III.
In Groups III and IV, tissues were subjected to similar regimens, with the exception of the chemical used—MG or GP. Toe stiffness of GP-treated sclera was greater in magnitude than that of MG, despite the fact that GP is naturally occurring cross-linking agent with low toxicity. Even beyond the realm of eye research, GP has been reported to be efficient at cross-linking collagenous materials and increasing tensile strength, toughness, and modulus (Chang et al. 2002, Slusarewicz et al. 2010, Sung et al. 1999b); notably, GP treatment of bovine pericardium, a collagenous tissue, has been shown to boost tensile strength and toughness more than glutaraldehyde and epoxy (Sung et al. 1999b).
In this study, SXL treatment appeared to make the sclera stiffer at low strain but not at peak strain. The apparent difference in toe stiffness and peak stiffness after SXL may be due to the difference in load-bearing of crimped and stretched collagen fibers. In the toe-region of the stress-strain curve, collagen fibers are in a crimped conformation. Thus, SXL-induced changes in the toe region could be caused by a difference in how the crimped collagen is unfolding. In other words, SXL may stabilize or reinforce collagen crimp, thereby increasing toe stiffness. However, at higher levels of strain, stiffness is instead dictated by axial loading of straightened fibers as collagen crimp unfolds with stretch.
Peak strains of scleral samples were near-isotropic, but there was anisotropy of toe stiffness. Anisotropy at low or physiologic IOP levels (corresponding to the toe region) may be due to the proximity of the strain targets to the immediate peripapillary region, where collagen fibers are predominantly oriented circumferential to the optic nerve head (Greene. 1980). The aligned fiber orientation may result in anisotropy: Girard et al.previously reported that at IOP levels of 4-5 mm Hg, peripapillary sclera demonstrated mechanical anisotropy (Girard et al. 2008). Also, there was no statistically significant difference in anisotropy after SXL treatment. This finding is difficult to compare to the literature due to a lack of comparable studies. A report on bovine pericardium found that the anisotropy of elastic modulus was eliminated after incubation in genipin (Sung et al. 1999a). However, a similar study on porcine aortic valves fixated with genipin found no change in anisotropy compared to fresh tissue (Sung et al. 1999b). Furthermore, Xu et al. found that chemical crosslinking of collagen gels with genipin did not affect mechanical anisotropy (Xu et al. 2011).
Methylglyoxal and genipin cross-link collagen via separate mechanisms. MG undergoes a Maillard reaction with free amino groups of proteins to produce a Schiff base; this reaction has been shown to be reversible and relatively unstable (Nagaraj et al. 1996). On the other hand, GP is presumed to cross-link free amino groups to create tertiary amine structures, thereby forming a network with cyclic structure within collagen fibers(Sung et al. 2001). (Tertiary amine structures are more stable than Schiff bases, which may be an important factor when considering long-term biomechanics (Sung et al. 2001)).
Exogenous SXL by methylglyoxal or genipin may offer a promising solution for prevention or slowing of the onset of progressive myopia. Scleral pathology, particularly at the posterior pole of the eye, is known to be a primary cause of permanent vision loss in myopes (McBrien et al. 2009). Due to the high incidence of pathological aberrations in this region, the posterior pole has been identified as a main target of cross-linking (Wollensak and Spoerl. 2004). As such, this study shows that significant stiffening of posterior sclera can be achieved in a timespan of thirty minutes, which is a viable time frame for a clinical procedure. However, the desired clinical effect size of scleral stiffening is not yet known. Animal models of myopia have been reported to display a 50% to 100% increase in creep rate compared to controls (Phillips et al. 2000).
In order for SXL treatment to be clinically relevant, the chemicals used and the cross-links formed must be biocompatible. Methylglyoxal is known to be toxic at a highconcentration, but its potential to damage ocular structures has not been studied. On the other hand, GP exhibits low cytotoxicity and forms biocompatible collagen cross-links in biological tissue (Huang et al. 1998), although its biocompatibility in ocular tissue has not yet been confirmed. Future toxicity studies must be performed to evaluate the hazards of working concentrations of these chemicals in vivo.
A potential side effect of exogenous chemical SXL may be decreased tissue permeability. We have previously reported that increased nonenzymatic cross-link density reduces solute diffusion and fluid flow through the sclera (Stewart et al. 2009). Future study of exogenous SXL must consider the level of permeability impairment induced by SXL treatment and proceed accordingly.
Although our whole globe inflation testing is more informative than uniaxial testing, we acknowledge a limitation of this study. The pressurizing needle was inserted into the aqueous of the anterior chamber of the eye to avoid potential clogging that would occur if the needle were inserted into the viscous vitreous in the posterior chamber. The concern with the tip’s position in the anterior chamber is whether the pressure adequately equalized between the anterior and posterior chamber. Preliminary tests using a tonometer verified consistent pressures throughout the globe. However, a manometer inserted at various locations within the globe would be necessary to confirm this definitively.
In summary, methylglyoxal and genipin appear to be promising agents for stiffening posterior sclera. The approach described in this study merits further investigation in an in vivo model. Furthermore, the safety of using these cross-linking agents in vivo must be evaluated.
Highlights.
-
-
The study evaluates scleral mechanics in porcine eyes after cross-linking (SXL)
-
-
Whole globe inflation testing confirms posterior stiffening with 30-min. treatment
-
-
Treatment of the posterior sclera alone is equivalent to whole eye treatment
-
-
Provides support for in vivo testing of posterior SXL for progressive myopia
Acknowledgments
Support: Knights Templar Eye Foundation; That Man May See, Inc. and Research to Prevent Blindness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: None
References
- Avila MY, Navia JL. Effect of genipin collagen crosslinking on porcine corneas. J. Cataract Refract. Surg. 2010;36:659–664. doi: 10.1016/j.jcrs.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Chang Y, Tsai CC, Liang HC. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin) Biomaterials. 2002;23:2447–2457. doi: 10.1016/s0142-9612(01)00379-9. [DOI] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch. Ophthalmol. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues. Second ed Springer-Verlag; New York: 1993. [Google Scholar]
- Girard MJ, Downs JC, Burgoyne CF, Suh JK. Experimental surface strain mapping of porcine peripapillary sclera due to elevations of intraocular pressure. J. Biomech. Eng. 2008;130:041017. doi: 10.1115/1.2948416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene PR. Mechanical considerations in myopia: relative effects of accommodation, convergence, intraocular pressure, and the extraocular muscles. Am. J. Optom. Physiol. Opt. 1980;57:902–914. [PubMed] [Google Scholar]
- Greene PR, McMahon TA. Scleral creep vs. temperature and pressure in vitro. Exp. Eye Res. 1979;29:527–537. doi: 10.1016/0014-4835(79)90153-2. [DOI] [PubMed] [Google Scholar]
- Huang LL, Sung HW, Tsai CC, Huang DM. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J. Biomed. Mater. Res. 1998;42:568–576. doi: 10.1002/(sici)1097-4636(19981215)42:4<568::aid-jbm13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Lari DR, Schultz DS, Wang AS, Lee OT, Stewart JM. Scleral mechanics: Comparing whole globe inflation and uniaxial testing. Exp. Eye Res. 2012;94:128–135. doi: 10.1016/j.exer.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MS, Huynh J, Wiseman ME, Coassin M, Kornfield JA, Schwartz D. An In Vitro Intact Globe Expansion Method for Evaluation of Cross-linking Treatments. Invest. Ophthalmol. Vis. Sci. 2010 doi: 10.1167/iovs.09-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog. Retin. Eye Res. 2003;22:307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom. Vis. Sci. 2009;86:E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- McLellan AC, Phillips SA, Thornalley PJ. The assay of methylglyoxal in biological systems by derivatization with 1,2-diamino-4,5-dimethoxybenzene. Anal. Biochem. 1992;206:17–23. doi: 10.1016/s0003-2697(05)80005-3. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J. Biol. Chem. 1996;271:19338–19345. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest. Ophthalmol. Vis. Sci. 2000;41:2028–2034. [PubMed] [Google Scholar]
- Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp. Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Garcia M, Hernandez M, Urcola H, Hernandez-Barbachano E, Araiz J, Vecino E. The pig eye as a novel model of glaucoma. Exp. Eye Res. 2005;81:561–569. doi: 10.1016/j.exer.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM. Structural factors that mediate scleral stiffness. Invest. Ophthalmol. Vis. Sci. 2008;49:4232–4236. doi: 10.1167/iovs.08-1970. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Slusarewicz P, Zhu K, Hedman T. Kinetic characterization and comparison of various protein crosslinking reagents for matrix modification. J. Mater. Sci. Mater. Med. 2010;21:1175–1181. doi: 10.1007/s10856-010-3986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerl E, Boehm AG, Pillunat LE. The influence of various substances on the biomechanical behavior of lamina cribrosa and peripapillary sclera. Invest. Ophthalmol. Vis. Sci. 2005;46:1286–1290. doi: 10.1167/iovs.04-0978. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Schultz DS, Lee OT, Trinidad ML. Exogenous collagen cross-linking reduces scleral permeability: modeling the effects of age-related cross-link accumulation. Invest. Ophthalmol. Vis. Sci. 2009;50:352–357. doi: 10.1167/iovs.08-2300. [DOI] [PubMed] [Google Scholar]
- Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J. Biomed. Mater. Res. 1999a;47:116–126. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sung HW, Huang RN, Huang LL, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999b;10:63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- Sung HW, Liang IL, Chen CN, Huang RN, Liang HF. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin) J. Biomed. Mater. Res. 2001;55:538–546. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J. Cataract Refract. Surg. 2004;30:689–695. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Xu B, Chow MJ, Zhang Y. Experimental and modeling study of collagen scaffolds with the effects of crosslinking and fiber alignment. Int. J. Biomater. 2011;2011:172389. doi: 10.1155/2011/172389. [DOI] [PMC free article] [PubMed] [Google Scholar]