Abstract
Aminoethylcysteine ketimine decarboxylated dimer [AECK-DD; systematic name: 1,2–3,4–5,6–7,8-octahydro-1,8a-diaza-4,6-dithiafluoren-9(8aH)-one] is a previously described metabolite of cysteamine that has been reported to be present in mammalian brain, urine, plasma, cells in culture and vegetables, and to possess potent anti-oxidative properties. Here, we describe a stable-isotope GC-MS/MS method for specific and sensitive determination of AECK-DD in biological samples. 13C2-AECK-DD was synthesized and used as the internal standard. Derivatization was carried out by N-pentafluorobenzylation with pentafluorobenzyl bromide in acetonitrile. Quantification was performed by selected-reaction monitoring of the mass transitions m/z 328 to m/z 268 for AECK-DD and m/z 330 to m/z 270 for 13C2-AECK-DD in the electron-capture negative-ion chemical ionization mode. The procedure was systematically validated for human plasma and urine samples. AECK-DD was not detectable in human plasma above ~ 4 nM, but was present in urine samples of healthy humans at a maximal concentration of 46 nM. AECK-DD was detectable in rat brain at very low levels of about 8 pmol/g wet weight. Higher levels of AECK-DD were detected in mouse brain (~1 nmol/g wet weight). Among nine dietary vegetables evaluated, only shallots were found to contain trace amounts of AECK-DD (~ 6.8 pmol/g fresh tissue).
Keywords: AECK-DD, Brain, Cysteamine, GC-MS/MS, Organic cation transporters, Validation, Vegetables
Introduction
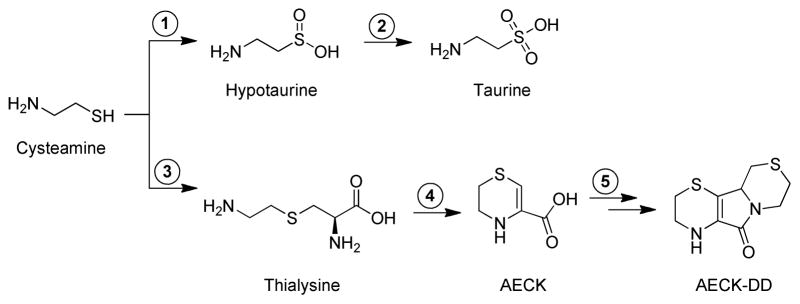
Cysteamine [HSCH2CH2NH2] has received considerable biomedical attention (see the discussion). Thus, a discussion of its metabolism is in order. The major route for metabolism of cysteamine is thought to be via conversion to hypotaurine, followed by oxidation to taurine, as shown in the upper pathway in Fig. 1. However, another pathway has been postulated to occur in mammals and plants [3–18]. In this pathway, cysteamine is converted to thialysine (aminoethylcysteine) by a β-replacement mechanism with cysteine or serine catalyzed by cystathionine β-synthase. Thialysine, in turn, is a substrate of bovine kidney [4], bovine liver [4] and human kidney (unpublished data) glutamine transaminases. The resulting α-keto acid cyclizes to a structure containing an internal ketimine, given the trivial name aminoethylcysteine ketimine (AECK)2 [3,4]. AECK (systematic name: tetrahydro-2H-1,4-thiazine-3-carboxylic acid) and its reduced form (5,6-dihydro-2H-1,4-thiazine-3-carboxylic acid) have been reported to be present in brain in μM-levels [5,6]. A tri-cyclic compound, resulting from the spontaneous dimerization and decarboxylation of AECK, given the trivial name aminoethylcysteine ketimine decarboxylated dimer (AECK-DD) [systematic name: 1,2–3,4–5,6–7,8-octahydro-1,8a-diaza-4,6-dithiafluoren-9(8aH)-one] has also been reported to be present in human urine [7], human plasma [8,9], bovine brain [10] and cells in culture [9]. The proposed metabolic pathway leading to the formation of thialysine, AECK, 1,4-thiomorpholine-3-carboxylate and AECK-DD from cysteamine is shown in the lower pathway of Fig. 1. AECK-DD has also been reported to be present in a wide variety of vegetables [11]. Thus, it was suggested that a portion of the AECK-DD previously reported to be present in brain may have been derived, at least in part, from dietary sources [11]. In addition to dietary sources, our previous findings show that AECK-DD can be detected, albeit at low concentrations,3 in brain of rats that were gavage-fed cysteamine [19], suggesting de novo biosynthesis.
Fig. 1.
Theoretical pathways for cysteamine metabolism. In the upper pathway, cysteamine is oxidized to hypotaurine by a highly specific cysteamine dioxygenase (step 1) [1,2]. In the second step, hypotaurine is oxidized to taurine. It is not yet clear whether the second step is enzyme catalyzed [1,2]. In the lower pathway, proposed by Cavallini and colleagues [3–17], the first step (step 3) is catalyzed by cystathionine β-synthase. In this reaction, cysteamine is postulated to replace L-homocysteine as a substrate [i.e., cysteamine + L-serine → L-thialysine + H2O]. In agreement with this hypothesis it was later shown by Shen et al. that human cystathionine β-synthase catalyzes an efficient β replacement reaction with L-serine and cysteamine [18]. Thialysine (aminoethyl-L-cysteine) is transaminated to the corresponding α-keto acid, which cyclizes to form a six-membered ring containing an internal ketimine (aminoethyl cysteine ketimine, AECK) (step 4). The ketimine form of AECK is in tautomeric equilibrium with an enamine structure. The latter predominates at neutral pH [6], and is shown in the figure. AECK dimerizes and oxidizes with the loss of one CO2 per dimer to generate aminoethyl cysteine ketimine decarboxylated dimer (AECK-DD) presumably through non-enzymatic reactions (step 5).
AECK-DD has been reported to be an anti-oxidant at μM-concentrations and it has been suggested that this compound may be active in vivo [12–16]. Given this suggestion, and the considerable interest in the scientific and lay communities regarding natural anti-oxidants as possible therapeutic agents and as research tools, we were interested in developing a highly sensitive analytical approach for the measurement of AECK-DD. In general, for gas chromatography-tandem mass spectrometry (GC-MS/MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques, the most reliable method for minimizing the occurrence of false positive results is to use as an internal standard (IS) an isotopically labeled form of the analyte under study. In the present report, we describe the development, validation and application of a GC-MS/MS method that uses 13C2-AECK-DD as the IS for the measurement of AECK-DD in biological samples. Derivatization (N-pentafluorobenzylation) of AECK-DD was accomplished with pentafluorobenzyl bromide (PFB-Br). Herein, we report methods for the measurement of AECK-DD in rodent brain, human plasma, human urine and various vegetables and discuss the biological relevance of the findings.
Materials and Methods
Materials
Cysteamine-HCl (used in the synthesis of AECK-DD) and octane sulfonic acid were obtained from Sigma Aldrich (St. Louis, MO). The cysteamine-HCl used in animal experiments was obtained from Aldrich (Milwaukee, WI). OmniSolvR solvents (acetonitrile and N,N-dimethylformamide) were HPLC grade and obtained from EM Science (Gibbstown, NJ). Unlabeled AECK-DD was synthesized by the method of Pinto et al. [19] based on the method of Antonucci et al. [17]. 2,3,4,5,6-Pentafluorobenzyl bromide and N,N-diisopropylethylamine were purchased from Sigma-Aldrich (Munich, Germany). The silylation reagent N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) was obtained from Pierce/Thermo Fisher Scientific (Rockford, IL, USA). Organic solvents used in GC-MS/MS were purchased from Mallinckrodt Baker (Griesheim, Germany). All glass vials used for derivatization and chromatography were obtained from Macherey-Nagel (Düren, Germany). Polypropylene tubes and test tubes for blood sampling were manufactured by Sarstedt (Nümbrecht, Germany).
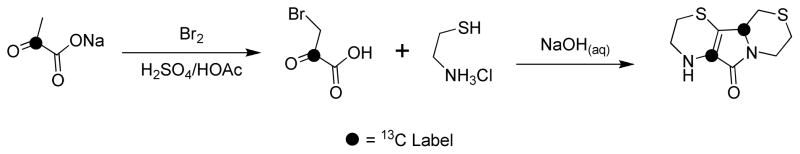
Synthesis of AECK-DD-5a,9a-13C2
13C-Labeled AECK-DD was synthesized essentially as described by Antonucci et al. [17] except that [2-13C]pyruvate was used in place of unlabeled pyruvate. The procedure is as follows. A slurry of sodium [2-13C]pyruvate (214.3 mg, 1.930 mmol, Sigma-Aldrich, USA) was stirred in glacial acetic acid (1 mL) with gentle heating by means of a heat gun to facilitate dissolution. To the solution was added Br2 (99 μL, 0.97 mmol) and the resultant solution was stirred at 90 °C for 2 to 3 min, followed by the dropwise addition of concentrated H2SO4 (106 μL, 1.93 mmol). The solution was stirred for approximately 5 min, followed by the addition of Br2 (99 μL, 0.97 mmol) and a further stirring for 30 min. The solution was cooled by means of an external ice bath and 6 M NaOH (802 μL, 4.81 mmol) was added, followed by a solution of cysteamine hydrochloride (219.3 mg, 1.930 mmol) in 1 M NaOH. The resultant solution was stirred at ambient temperature overnight followed by heating to approximately 100 °C until light bubbling and boiling occurred. The solution was then maintained at this temperature for 20 min. The solution was cooled using an external ice bath and the pH was adjusted to 8 using 6 M NaOH. Reaction products were extracted three times with ethyl acetate, the organic phases were pooled, dried over anhydrous sodium sulfate, filtered and the solvent was removed by rotary evaporation. The residue was chromatographed on Silica Gel 60 (0.060–0.2 mm, 70–230 mesh; Alfa-Aesar) to afford AECK-DD-5a,9a-13C2 (10.4 mg, 2.3 % yield). The low yield of the reaction is most likely due to the bromination/hydrolysis step (Hell-Volhard-Zelinsky reaction) as shown by the fact that the yield of unlabeled AECK-DD is much better (in the range of 50%) when this step is avoided and the starting material is commercial bromopyruvate. However, since doubly labeled bromopyruvate is not available commercially, it was necessary to use doubly labeled pyruvate as the starting material. The labeled carbons in AECK-DD-5a,9a-13C2 are shown in Fig. 2.
Fig. 2.
Synthetic sequence for the preparation of AECK-DD-5a,9a-13C2 (13C2-AECK-DD).
Preparation of brain tissue obtained from mice pretreated with cysteamine
The study was approved by the Subcommittee for Animal Studies and the Research and Development Committee of VA Long Beach Healthcare System. Wild-type (1.5 and 6 month of age) and OCT1/2 (organic cation transporters 1 and 2) knock out (KO) mice (1.5 and 6 months of age, FVB background, Taconic) were administered cysteamine-HCl (125 mg/100 g) by gavage. The FVB (Friend Virus B-type) mouse is an inbred strain that is used for transgenic analyses. The procedure for generating the OCT1/2 KO mice has recently been reported [20–22]. Two hours later, the animals were euthanized by CO2 inhalation, followed by cervical dislocation. The brains were then removed, frozen in liquid nitrogen and stored at −80 °C. Frozen brain samples were shipped on dry ice to the Institute of Clinical Pharmacology, Hannover Medical School (Hannover, Germany), where the analysis of brain samples was performed. In brief, brain pieces (89 – 181 mg) thawed on ice were treated with 250 μL ice-cold Tris buffer (100 mM, pH 7.6) per 100 mg tissue. The buffer contained 13C2-AECK-DD, EDTA and dithiothreitol (DTT) such that the final content and concentrations were 0.5 pmol 13C2-AECK-DD/mg wet weight tissue and 10 mM EDTA and DTT, respectively. The samples were homogenized in pre-cooled Precellys tubes in the presence of ceramic beads (1.4-mm diameter) on a Precellys 24 Dual homogenizer (1 × 26 s, 6200 rpm, 5 °C). After centrifugation (800 × g, 5 min, 4 °C), the supernatant fractions were extracted by vortexing with ethyl acetate (1250 μL/100 mg tissue). Aliquots were removed and derivatized with PFB-Br, as described below. A similar procedure was used to analyze AECK-DD in brain from an untreated adult male Sprague-Dawley rat.
Human plasma and urine
Plasma and urine samples from healthy human subjects analyzed in the present study had been collected and aliquoted in previously conducted clinical studies after approval by the local Ethics Committee. Blood (8 mL) was drawn from the antecubital vein by means of EDTA- or citrate-containing monovettes (Sarstedt, Germany) and centrifuged immediately (800×g, 4 °C, 5 min). Plasma was portioned into 100-μL aliquots and stored frozen at −80 °C. Urine from spontaneous micturition was collected in 45-mL polypropylene tubes, aliquoted into 1 mL-aliquots and stored at −20 °C until analysis. After thawing in an ice bath, the samples were centrifuged (800 × g, 5 min, 4 °C) and 100-μL aliquots of the supernatant fractions were processed as described below.
Analysis of various vegetables
Fresh vegetables from a local market were cut into small pieces and 1-g portions were transferred into polypropylene tubes that contained ice-cold 100 mM phosphate buffered saline, pH 7, and 1 nmol of 13C2-AECK-DD. The samples were homogenized in sealed pre-cooled Precellys tubes in the presence of ceramic beads (1.4-mm diameter) on a Precellys 24 Dual homogenizer (2 × 10 s, 5000 rpm, 5 °C). After centrifugation, the supernatant fraction (about 1 mL) was extracted with ethyl acetate (2 mL). Subsequently, derivatization with PFB-Br was performed as described below.
Extraction and derivatization procedures
Unless otherwise specified, samples (100 μL) were spiked with 13C2-AECK-DD to reach the desired final concentrations (as specified in the Results section), ethyl acetate (500 μL) was added, and samples were mixed by vortexing for 2 min. Phase separation was achieved by centrifugation (800 × g, 5 min, 4 °C). Aliquots (~450 μL) of the organic phase were removed, dried over anhydrous Na2SO4 and the solvent was evaporated under a gentle stream of nitrogen gas. Residues were reconstituted in acetonitrile (100 μL), N,N-diisopropylethylamine (10 μL) and PFB-Br (10 μL, 30 vol% in anhydrous acetonitrile) were added and the reaction mixtures were incubated in sealed glass vials for 30 min at 30 °C. After derivatization, solvent and reagents were completely removed under a stream of nitrogen at room temperature and the residues were reconstituted in anhydrous dichloromethane (50 μL). Samples were analyzed immediately or after storage at 4 °C in sealed glass vials. Under these conditions, the PFB derivatives of AECK-DD and 13C2-AECK-DD were stable for several weeks.
GC-MS and GC-MS/MS conditions
Analyses were performed in electron-capture negative-ion chemical ionization (ECNICI) mode on a ThermoElectron TSQ 7000 triple-stage quadrupole mass spectrometer (Finnigan MAT, San Jose, CA) directly interfaced with a Trace 2000 series gas chromatograph equipped with an autosampler AS 2000 (CE Instruments Austin, TX). Chromatographic separation was performed on an OPTIMA-17MS fused silica column (30 m × 0.25 mm i.d.; 0.25 μm film thickness) from Macherey-Nagel (Düren, Germany). The following oven temperature program was used: 1 min at 80 °C, increasing to 260 °C at a rate of 10 °C/min, followed by an increase to 360 °C at a rate of 25 °C/min. The interface and ion-source were maintained at 320 °C and 180 °C, respectively. Electron energy and electron current were set to 200 eV and 300 μA, respectively. For quantitative analyses, the electron multiplier voltage was set to 2800 V. Methane (530 Pa) and argon (0.2 Pa pressure in the collision chamber) in GC-MS/MS were used as reagent and collision gases, respectively. The collision energy was set to 15 eV for quantitative analyses. Aliquots (1 μL) were injected in splitless mode by using a BEST PTV injector, starting at an injector temperature of 70 °C, which was increased to 280 °C at a rate of 10 °C/s. Quantitative measurements were conducted on the AECK-DD-PFB derivatives by selected-reaction monitoring (SRM) of the product ions generated by collision-induced dissociation (CID) of the precursor ions at [M – 2 × HF]−. The dwell time was 100 ms, unless otherwise specified. For instance, the mass transitions for AECK-DD were mass-to-charge ratio (m/z) 368 for AECK-DD and m/z 370 for 13C2-AECK-DD to the product ions at m/z 268 and m/z 270, respectively (see Results section).
AECK-DD concentrations (CA) were calculated by multiplying the peak area (PA) ratio of measured peak areas of the analyte (PAA) and the internal standard (PAIS) by the known concentration of the respective internal standard (CIS): CA = CIS × PAA/PAIS.
All analyses were performed in duplicate in two independent experiments, unless otherwise specified. GraphPad Prism 5 from GraphPad Software Inc. (La Jolla, CA, USA) was used for data analysis.
Statistics
Data are reported as the mean ± SD. The Mann-Whitney U test was used to determine significance values. A p ≤ 0.05 is considered significant.
Results
Mass spectrometric and chromatographic characterization of underivatized and derivatized AECK-DD
Analyses of underivatized AECK-DD and 13C2-AECK-DD were performed on an Agilent HP 6890 gas chromatograph equipped with a 5973 mass selective detector and an Agilent DB-23 (30 m × 0.25 mm, 0.25 μm i.d.) column. Oven temperature was held for 5 min at 30 °C, then increased to 280 °C at a rate of 20 °C/min and finally held for 7.5 min. Unlabeled AECK-DD and 13C2-AECK-DD emerged from the column practically at the same time (i.e., 18.3 min). The electron ionization (EI) mass spectra of underivatized, unlabeled and 13C2-AECK-DD are summarized in Table 1. These mass spectra and the almost identical retention times unequivocally identify these compounds as AECK-DD and 13C2-AECK-DD.
Table 1.
Electron ionization mass spectra of native non-derivatized unlabeled AECK-DD and 13C2-AECK-DDa
| m/z (intensity, %) Ion assignment | m/z (intensity, %) Ion assignment | ||
|---|---|---|---|
|
| |||
| AECK-DD | 13C2-AECK-DD | ||
| 228 (100) | M+• | 230 (100) | M+• |
| 200 (36) | [M–CO]+ • | 202 (39) | [M–CO]+ • |
| 154 (58) | [M–SCH2CH2N]+ • and [M–CH2CH2SCH2]+ • | 156 (29) 155 (77) |
[M–SCH2CH2N]+ • [M–CH2CH2S13CH2]+ • |
| 126 (21) | [M–SCH2CH2N–CO]+ • and [M–CH2CH2SCH2–CO]+ • | 128 (20) 127 (41) |
[M–SCH2CH2NCH2–CO]+• [M–CH2CH2S13CH2–CO]+ • |
| 99 (17) | [CH2CH2SCHCN]+• | 100 (26) | [CH2CH2S13CHCN]+ • |
| 71 (17) | [SCHCN]+ • | 72 (15) | [S13CHCN]+ • |
| 45 (17) | [SCH]+ • | 46 (23) | [SCH]+ • |
| 28 (15) | [CO]+ • and/or [CH2CH2]+• | 28 (16) | [CO]+ • and/or [CH2CH2]+• |
Only ions with an intensity of ≥ 15% relative to M+• are listed.
Although AECK-DD and 13C2-AECK-DD can be analyzed by GC-MS in the EI mode, chemical conversion of these compounds to electron-capturing derivatives and analysis in ECNICI mode should greatly increase the method sensitivity. AECK-DD (and 13C2-AECK-DD) possesses an exchangeable H atom on the secondary amine group and is thus amenable to derivatization. We tested several derivatization reagents and found the alkylating agent PFB-Br to be the most useful derivatization reagent. Because AECK-DD is easily extractable from aqueous solutions at neutral pH by organic solvents such as ethyl acetate, N-pentafluorobenzylation was not performed directly in the aqueous phase. Rather, the reaction was carried out in anhydrous acetonitrile in the presence of N,N-diisopropylethylamine as the catalyst under mild conditions (30 min, 30 °C) after ethyl acetate extraction.
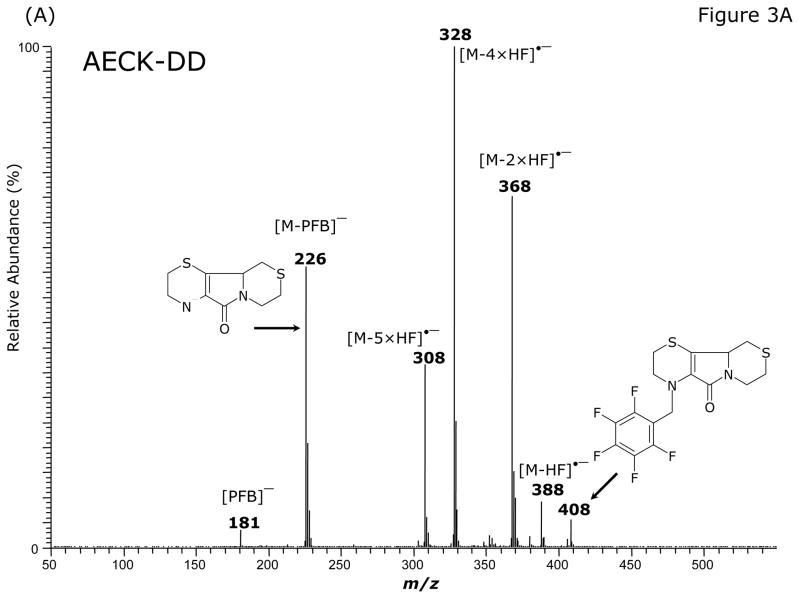
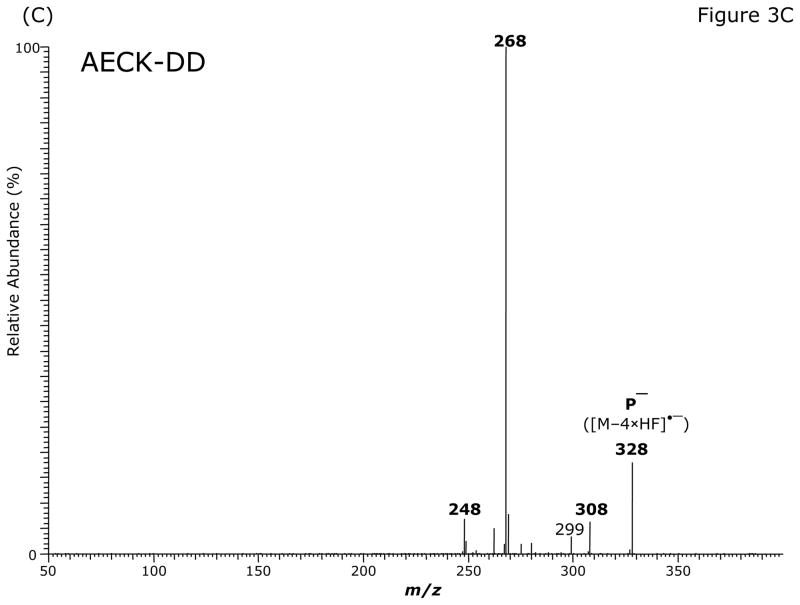
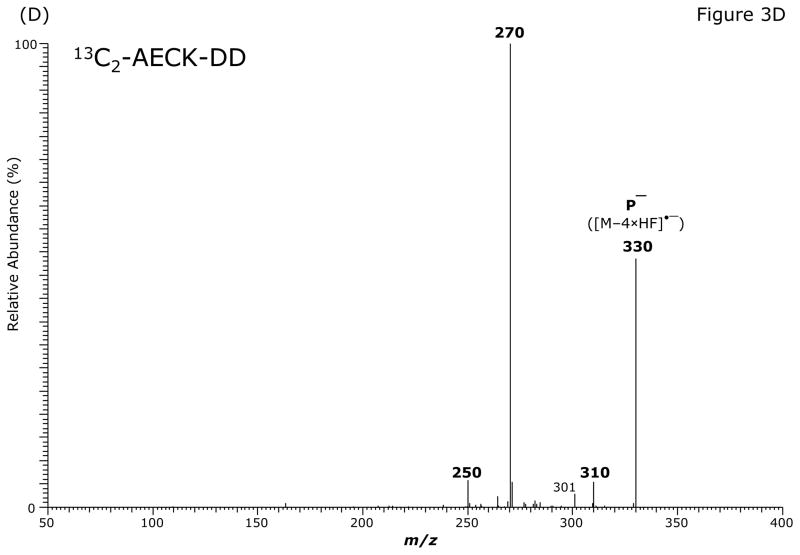
GC-MS analysis of the PFB-Br-derivatized unlabeled AECK-DD (Fig. 3A, Table 2) and 13C2-AECK-DD in the ECNICI mode each yielded a single GC peak at a retention time of 12.2 min with comparable mass spectra (Table 2). With the exception of the m/z 181 ion ([PFB]−) all the ions differed by 2 Da due to the two 13C atoms of 13C2-AECK-DD. These mass spectral data and the almost identical retention times of the PFB derivatives unequivocally identify these compounds as the N-pentafluorobenzyl derivatives of AECK-DD and 13C2-AECK-DD. The ions with m/z 226 and m/z 228 values represent the negatively charged AECK-DD and 13C2-AECK-DD molecules ([M–PFB]−), respectively. We observed the consecutive loss of one, two, four and five fluorine atoms of the PFB side chain as neutral HF (Table 2). Interestingly, consecutive loss of three HF did not occur during ECNICI of the PFB derivatives of AECK-DD and 13C2-AECK-DD. We therefore assume that m/z 328 does not originate from m/z 368 by loss of two HF molecules, but rather from an undetermined precursor. Extended loss of fluorine atoms is common in mono-PFB derivatives of various classes of compounds, including phenols and amines and requires opening and considerable rearrangement of the PFB ring [23–26].
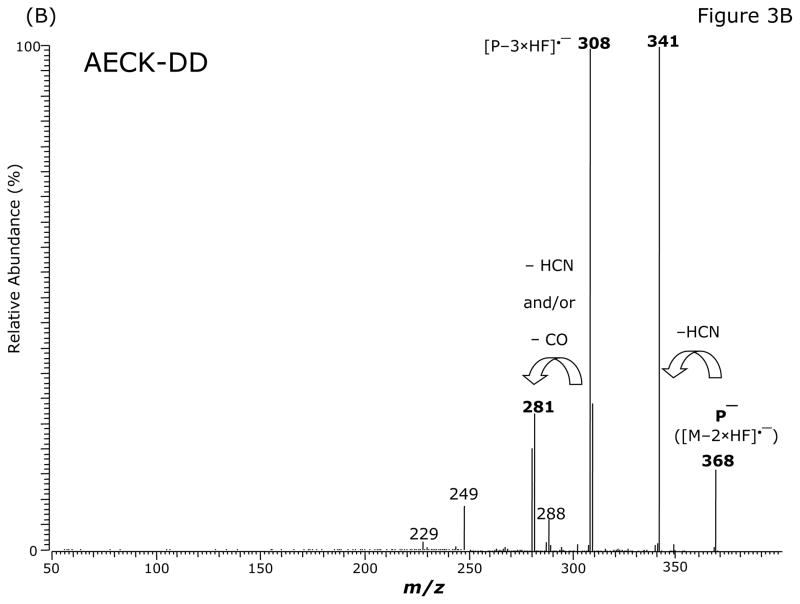
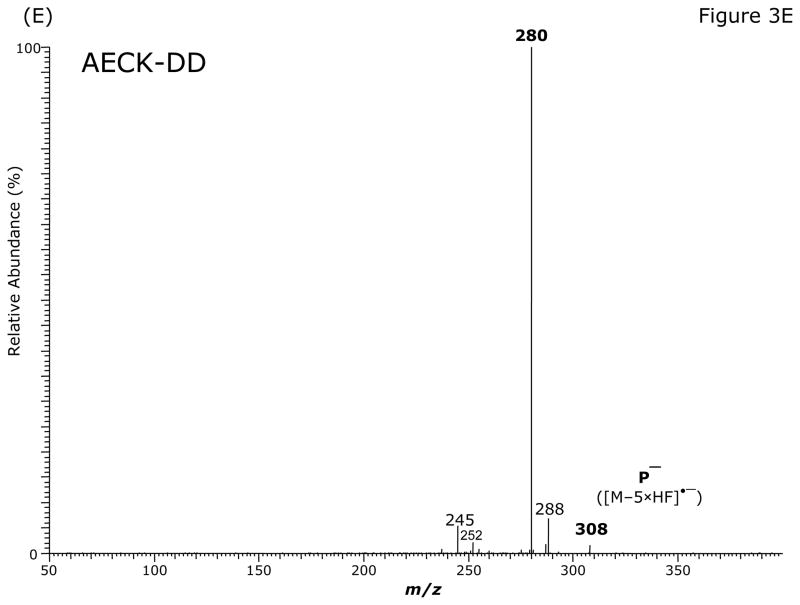
Fig. 3.
GC-MS (A) and GC-MS/MS spectra of the pentafluorobenzyl derivatives of AECK-DD (B, C, E) and 13C2-AECK-DD (D). Insets shows the proposed structures of the ions formed. P− indicates the respective precursor ion subjected to collision-induced dissociation.
Table 2.
Electron-capture negative-ion chemical ionization (ECNICI) mass spectra of the pentafluorobenzyl derivatives of unlabeled AECK-DD and 13C2-AECK-DD
| AECK-DD m/z (intensity, %) |
13C2-AECK-DD m/z (intensity, %) |
Ion assignment |
|---|---|---|
| 408 (6) | 410 (2) | M−• |
| 388 (9) | 390 (3) | [M–HF]−• |
| 368 (71) | 370 (49) | [M–2×HF]−• |
| 328 (100) | 330 (100) | [M–4×HF]−• |
| 308 (37) | 310 (27) | [M–5×HF]−• |
| 226 (56) | 228 (61) | [M–PFB]− |
| 181 (4) | 181 (10) | [PFB]− |
The intense ions with m/z 368 and m/z 370, m/z 328 and m/z 330, m/z 308 and m/z 310 and m/z 226 and m/z 228 were subjected to CID. Representative product ion mass spectra are shown in Fig. 3B to Fig. 3E. The CID of m/z 368 ([M–2 × HF]•−) generated m/z 341, possibly by neutral loss of an HCN molecule (27 Da) (Fig. 3B). The product ion m/z 281 could also originate from m/z 308 by neutral loss of an HCN molecule. Alternatively, because of the formation of the paired product ions m/z 309/m/z 308 and m/z 281/m/z 280, the latter could originate from neutral loss of CO (28 Da) from m/z 309 and m/z 308 (Fig. 3B). Interestingly, the ions m/z 341 and m/z 281 were absent in the mass spectrum of the PFB derivative of AECK-DD (Fig. 3A). The product ion mass spectrum observed from CID of m/z 368 suggests that m/z 308/m/z 309 do not originate from m/z 341. The CID of the most intense ion with m/z 328 in the mass spectrum of the PFB derivative of AECK-DD resulted in the production of an intense product ion at m/z 268 (Fig. 3C). The CID of the most intense ion with m/z 330 in the mass spectrum of the PFB derivative of 13C2-AECK-DD resulted in the production of an intense product ion at m/z 270 and other less intense ions (Fig. 3D), each differing by 2 Da from those obtained from the CID of m/z 328 for AECK-DD due to the two 13C atoms of 13C2-AECK-DD. The CID of m/z 328 of the PFB derivative of AECK-DD resulted in the production of an intense product ion at m/z 280 (Fig. 3E), presumably by neutral loss of CO (28 Da). It is worth mentioning that no product ions were observed at m/z 226 and m/z 228, which would correspond to the negatively charged AECK-DD and 13C2-AECK-DD ([M–PFB]−), respectively. This finding suggests that the CID of the above mentioned precursor ions results in opening of the PFB ring, thus avoiding formation of the PFB radical, which is an excellent leaving group. Finally, the CID of m/z 226 and m/z 228 at the same collision energy (15 eV) did not yield intense product ions (data not shown).
Standardization and utility of 13C2-AECK-DD as an internal standard for AECK-DD
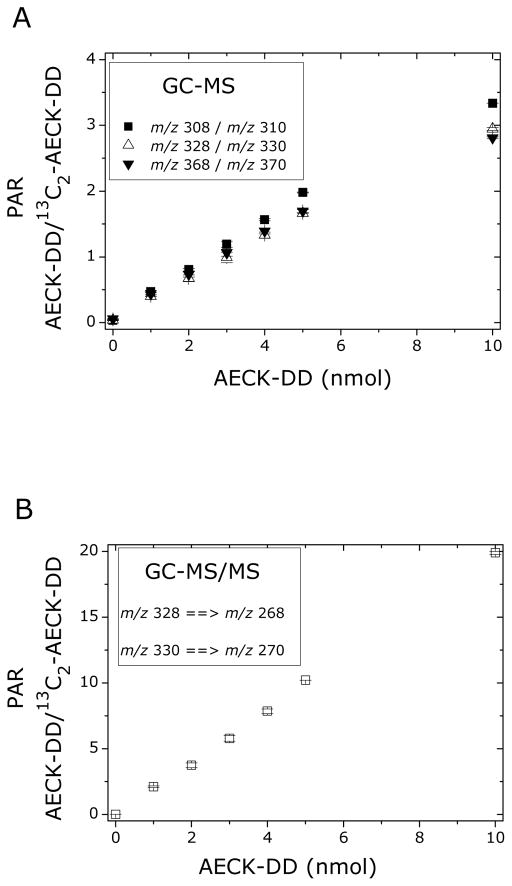
Both commercially available and newly synthesized stable-isotope labeled compounds require standardization prior to use in quantitative analysis [26]. Stock solutions of AECK-DD and 13C2-AECK-DD were prepared in acetonitrile by diluting a weighed amount of the synthetic standards. Dilutions were also prepared in acetonitrile. Varying amounts of AECK-DD (0, 1, 2, 3, 4, 5, 10 nmol) were derivatized with a fixed nominal amount of 13C2-AECK-DD (5 nmol) and analyzed by GC-MS in the selected-ion monitoring (SIM) mode, i.e., the ions m/z 308, m/z 328 and m/z 368 for AECK-DD, and the ions m/z 310, m/z 330 and 370 for 13C2-AECK-DD. The respective peak area ratios (PAR), y, for AECK-DD and 13C2-AECK-DD were calculated and plotted versus the AECK-DD amount, x. Linear regression equations were: y = 0.159 + 0.318x (r = 0.9976) for m/z 368 and m/z 370, y = 0.070 + 0.280x (r = 0.9976) for m/z 328 and m/z 330, and y = 0.040 + 0.323x (r = 0.9999) for m/z 308 and m/z 310. Thus, satisfactory linearity was observed for all ion pairs monitored, with the correlation coefficient increasing with decreasing size of the ions (Fig. 4A), presumably due to the decreasing contribution of naturally occurring 13C isotope in AECK-DD relative to that artificially incorporated into 13C2-AECK-DD. The theoretical slope of the regression equation is 0.200 1/nmol and corresponds to the reciprocal amount of 5 nmol of 13C2-AECK-DD. The experimentally observed slope values of 0.318, 0.280 and 0.323 1/nmol deviate from the theoretical slope value of 0.200 1/nmol, suggesting that the actual amount of 13C2-AECK-DD used deviates from the assumed amount of 5 nmol. As AECK-DD was quantitated by SRM of m/z 368 and m/z 370, the actual amount of 13C2-AECK-DD was calculated from the reciprocal of the slope value of 0.318 1/nmol, i.e., 1/0.318 = 3.14 nmol. Based on this finding, the concentration of 13C2-AECK-DD in the stock solution and their dilutions were corrected accordingly. AECK-DD is hygroscopic and the most likely explanation for the deviation in the observed slope values is the absorption of water acquired during shipping.
Fig. 4.
(A) Linear relationship between the peak area ratio (PAR) of AECK-DD to 13C2-AECK-DD after derivatization of the indicated amounts of AECK-DD with the fixed nominal amount of 5 nmol 13C2-AECK-DD and GC-MS analysis. (B) Linear relationship between the peak area ratio (PAR) of AECK-DD to 13C2-AECK-DD after derivatization of the indicated amounts of AECK-DD with the fixed corrected amount of 0.5 nmol 13C2-AECK-DD and GC-MS/MS analysis. Data are presented as mean ± SD, n = 4 (two sets of determinations each carried out in duplicate).
With the “corrected” 13C2-AECK-DD concentration in hand, we derivatized varying amounts of AECK-DD (0, 1, 2, 3, 4, 5, 10 nmol) with a fixed amount of 13C2-AECK-DD (0.5 nmol) and analyzed the samples by GC-MS/MS in the SRM mode, specifically the transition m/z 328 → m/z 268 for AECK-DD and m/z 330 → m/z 270 for 13C2-AECK-DD. Plotting the PAR of m/z 328 → m/z 268 to m/z 330 → m/z 270 (y) versus the AECK-DD amount (x) resulted in the regression equation y = 0.009 + 2.004x (r > 0.9999) (Fig. 4B). The experimentally observed slope is within experimental error identical to the theoretical slope of 1/0.5 = 2.000. Also, the y axis intercept is very low and indicates that 13C2-AECK-DD is almost completely free of unlabeled AECK-DD.
In summary, these findings show that 13C2-AECK-DD should be a useful IS for the quantitative analysis of AECK-DD by GC-MS and GC-MS/MS and that 13C2-AECK-DD is of such high isotopic purity that its use should allow quantitative determination of very low concentrations of AECK-DD in biological samples.
Validation of the method for AECK-DD in human plasma and urine samples
AECK-DD has been reported to be present in plasma of healthy normal subjects at a concentration range of between 513 and 768 ng/mL, i.e., between 2.25 and 2.86 μM, as measured by GC-MS [8], and between 106 and 520 ng/mL, corresponding to 0.46 and 1.86 μM, as measured by HPLC with electrochemical detection [9]. AECK-DD has also been reported to occur physiologically in human urine [8]. However, to our knowledge, no concentrations have been reported for urinary AECK-DD thus far. This information is the basis for the validation of the present method in human plasma and urine.
The GC-MS/MS method was validated for the quantitative determination of AECK-DD in plasma and urine of healthy humans in an extended (0 – 50 μM) and in a narrower (0 – 1000 nM) concentration range of added AECK-DD. The concentration of the internal standard 13C2-AECK-DD was 25 μM and 250 nM, respectively, in both the plasma and urine samples. Freshly prepared solutions of EDTA and citrate-treated plasma and urine, donated by two healthy subjects, were used in the method validation.
Plasma and urine samples (each 100 μL) were spiked with 13C2-AECK-DD and AECK-DD as indicated above and mixed by vortexing for 2 min with ethyl acetate (500 μL). After centrifugation (800 g, 5 min, 4 °C), the upper phase was removed and dried over anhydrous Na2SO4, the supernatant fraction was transferred to a conical glass vial and the solvent was evaporated by means of a gentle stream of nitrogen gas at room temperature. Subsequently, PFB-Br derivatization was performed as described in the experimental section, solvents and reagents were evaporated, the residue was reconstituted in CH2Cl2 (50 μL) and 1-μL aliquots were analyzed by GC-MS/MS with SRM of the transitions m/z 328 → m/z 268 for AECK-DD and m/z 330 → m/z 270 for 13C2-AECK-DD.
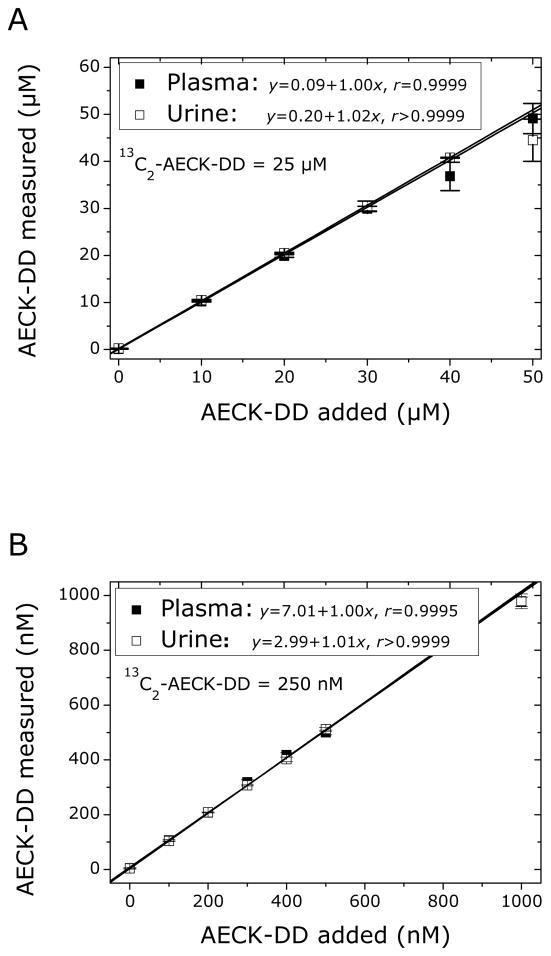
The results of the method validation experiments are shown in Fig. 5 and summarized in Appendix A. In both AECK-DD concentration ranges described above, linear relationships were observed between measured and added AECK-DD concentration in the plasma and urine samples analyzed. Mean recovery rate (accuracy) was close to 100% (see slope values in Fig. 5), and imprecision (RSD, %) was below 10.1% for spiked samples (see Appendix A).
Fig. 5.
Linear relationship between measured and added AECK-DD concentration in human plasma and urine samples from two healthy volunteers in an extended (A) and in a narrow (B) concentration range of AECK-DD. The final concentration of 13C2-AECK-DD added to the urine and plasma specimens as IS was 25 μM in (A) and 250 nM in (B). Plasma was obtained from blood anticoagulated with EDTA (A) or citrate (B). Data are presented as mean ± SD, n = 4 (two sets of determinations each carried out in duplicate).
Quantification of AECK-DD in plasma and urine of non-medicated healthy humans
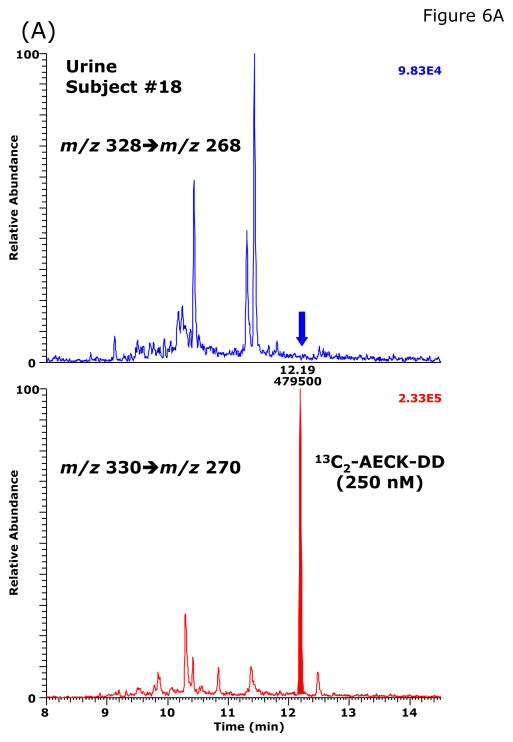
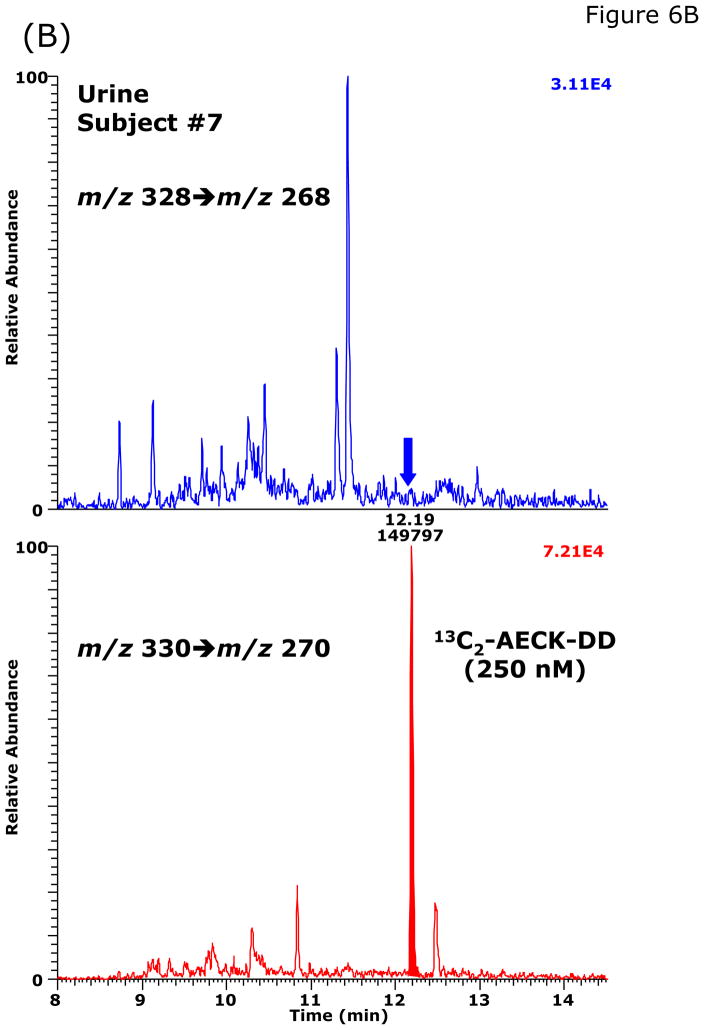
During method validation and in preliminary quantitative analyses, we found that the basal AECK-DD concentrations in plasma and urine of healthy subjects are much lower than those reported previously by Matarese et al. [8,9] (see above). Our validated GC-MS/MS method was used to measure basal excretion of AECK-DD in 24-h collected urine from 19 apparently healthy non-medicated volunteers (14 males and 5 females; Table 3). No AECK-DD was detected in the urine from two female volunteers above the limit of quantitation (LOQ) of the method, which was about 2 nM. In the urine samples from the other subjects, the AECK-DD concentration ranged from about 2 to 46 nM and from about 0.1 to 6 nmol AECK-DD per mmol creatinine (Table 3). Representative chromatograms from the GC-MS/MS analysis of AECK-DD in urine samples obtained from two healthy volunteers are shown in Fig. 6.
Table 3.
AECK-DD concentration in 24-h collected urine samples of nineteen healthy human subjects measured by GC-MS/MSa
| No | Gender | Age (years) | AECK-DD (nM) | Creatinine (mM) | AECK-DD/Creatinine (nmol/mmol) |
|---|---|---|---|---|---|
| 1 | Male | 29 | 45.7 | 11.5 | 3.97 |
| 2 | Female | 40 | <LOQb | 7.1 | <LOQb |
| 3 | Female | 28 | 14.4 | 6.6 | 2.18 |
| 4 | Male | 26 | 21.0 | 13.0 | 1.62 |
| 5 | Male | 60 | 3.73 | 18.5 | 0.20 |
| 6 | Female | 47 | 12.7 | 2.3 | 5.52 |
| 7 | Female | 40 | 3.31 | 8.1 | 0.41 |
| 8 | Male | 47 | 4.76 | 12.0 | 0.40 |
| 9 | Male | 42 | 9.65 | 2.97 | 3.25 |
| 10 | Male | 50 | 2.85 | 7.69 | 0.37 |
| 11 | Male | 34 | 19.1 | 3.22 | 5.93 |
| 12 | Male | 47 | 6.4 | 14.4 | 0.44 |
| 13 | Male | 41 | 22.9 | 5.93 | 3.86 |
| 14 | Male | 30 | 2.2 | 21.2 | 0.10 |
| 15 | Male | 42 | 14.9 | 12.4 | 1.20 |
| 16 | Male | 26 | 3.12 | 10.5 | 0.30 |
| 17 | Male | 47 | 5.1 | 22.5 | 0.23 |
| 18 | Female | 56 | <LOQ b | 6.02 | <LOQ b |
| 19 | Male | 52 | 6.1 | 21.5 | 0.28 |
| Mean ± SD | 41.3 ± 10.2 | 10.5 ± 11.1 | 10.9 ± 6.4 | 1.6 ± 2.0 | |
SRM of m/z 328 to m/z 268 for AECK-DD and m/z 330 to m/z 270 for 13C2-AECK-DD (IS added to a final concentration of 250 nM)
LOQ is about 2 nM.
Fig. 6.
Representative chromatograms from the GC-MS/MS analysis of AECK-DD in urine samples of two healthy volunteers: subjects #18 (A) and subject #7 (B). The concentration of 13C2-AECK-DD (IS) was 250 nM in each sample. The concentration of AECK-DD calculated to be present in these urine specimens were <LOQ and 3.31 nM, respectively.
We could not detect AECK-DD at concentrations above the method LOQ (~ 4 nM) in plasma freshly obtained from EDTA-anticoagulated blood analyzed by the present GC-MS/MS method. This finding is at variance with previous reports [8,9] that the concentration of AECK-DD in plasma of healthy normal subjects at basal concentrations ranges between about 0.5 μM and 2.9 μM.
Cerebral AECK-DD in cysteamine-treated mice
The concentration of cerebral AECK-DD was measured in six groups of mice sacrificed two hours after treatment. The six groups were as follows: 1) 2-month-old FVB mice gavaged with water; 2) 2-month FVB mice gavaged with an equal volume of cysteamine-HCl solution (125 mg/100 g body weight); 3) 2-month old FVB OCT1/2 KO mice gavaged with water; 4) 2-month old FVB OCT1/2 KO mice gavaged with an equal volume of cysteamine-HCl solution (125 mg/100 g body weight); 5) 1) 6-month-old FVB mice gavaged with water; 6) 6-month FVB mice gavaged with an equal volume of cysteamine-HCl solution (125 mg/100 g body weight). The results are shown in Table 4. AECK-DD was detected in all the control brains (i.e. 1a, 1b, 5a and 5b; 1.17 ± 18 nmol/g wet weight). On the other hand, AECK-DD could not be detected in brain tissue from 2 OCT1/2 KO mice gavage-treated with water (i.e. 3a, 3b) and in only one of two OCT1/2 KO mice treated with cysteamine (3d). These findings suggest that formation of AECK-DD from cysteamine may require the presence of OCT1, OCT2 or both. The level of AECK-DD was found to be lower in brain tissue obtained from cysteamine-treated FVB mice (2a, 2b, 2c, 6a, 6b, 6c). Assigning a value of zero to the non-detectable samples yields a concentration of cerebral AECK-DD in the treated group of 316 ± 129 pmol/g wet weight. A nonparametric (rank order) analysis (two-tailed Mann-Whitney U test) indicates a highly significant difference between the concentration of AECK-DD in brain samples from control FVB mice (1.17 ± 18 nmol/g wet weight; n = 4) and concentration of AECK-DD in brain samples from cysteamine-treated FVB mice (316 ± 129 pmol/g wet weight; n = 6); p = 0.013.
Table 4.
AECK-DD in mouse brain tissuea
| Identification number | AECK-DD (pmol/g wet weight) | Age (months), strain | Treatment |
|---|---|---|---|
| 1a | 2190 | 2 m, FVB (WT) | Water gavage |
| 1b | 729 | 2 m, FVB (WT) | Water gavage |
| 2a | N.D. | 2 m, FVB (WT) | Cysteamine gavage |
| 2b | 143 | 2 m, FVB (WT) | Cysteamine gavage |
| 2c | 93 | 2 m, FVB (WT) | Cysteamine gavage |
| 3a | N.D. | 2 m, FVB (OCT1/2 KO) | Water gavage |
| 3b | N.D. | 2 m, FVB (OCT1/2 KO) | Water gavage |
| 4a | N.D. | 2 m, FVB (OCT1/2 KO) | Cysteamine gavage |
| 4b | N.D. | 2 m, FVB (OCT1/2 KO) | Cysteamine gavage |
| 4c | 280 | 2 m, FVB (OCT1/2 KO) | Cysteamine gavage |
| 5a | 848 | 6 m, FVB (WT) | Water gavage |
| 5b | 925 | 6 m, FVB (WT) | Water gavage |
| 6a | N.D. | 6 m, FVB (WT) | Cysteamine gavage |
| 6b | N.D. | 6 m, FVB (WT) | Cysteamine gavage |
| 6c | 808 | 6 m, FVB (WT) | Cysteamine gavage |
Mice were sacrificed 2 h after gavage treatment with water or cysteamine. N.D., not detectable.
AECK-DD in rat brain
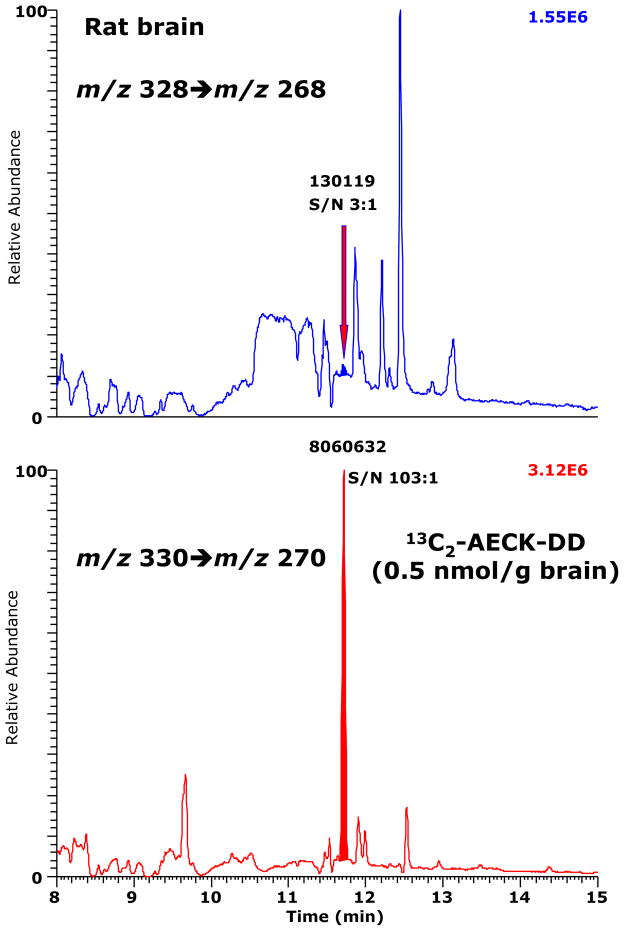
A chromatogram from the GC-MS/MS analysis of AECK-DD in brain tissue of an untreated rat is shown in Fig. 7. The AECK-DD content in this brain tissue is calculated to be 8 pmol/g tissue. The signal-to-noise (S/N) value of the peak of endogenous AECK-DD was 3:1. The S/N value of 0.5 nmol 13C2-AECK-DD/g tissue was 103:1 and suggests (see Ref. [25]) that about 15 pmol 13C2-AECK-DD added to 1 g tissue would be detectable by this method. The chromatogram shown in Fig. 7 indicates that AECK-DD occurs physiologically in rat brain, but its content is very low and difficult to quantify even with the present sensitive and specific GC-MS/MS method.
Fig. 7.
A chromatogram from the GC-MS/MS analysis of AECK-DD in the brain of an untreated adult male Sprague-Dawley rat. SRM of m/z 328 to m/z 268 for AECK-DD (upper panel) and of m/z 330 to m/z 270 for 13C2-AECK-DD (lower panel) was performed. 13C2-AECK-DD was added at a final content of 50 pmol per 100 mg tissue. The smaller retention time of the PFB derivatives of AECK-DD and 13C2-AECK-DD (11.7 min versus 12.2 min) are due to the shortening of GC column compared to previous analyses (e.g., Fig. 6).
Quantification of AECK-DD in vegetables
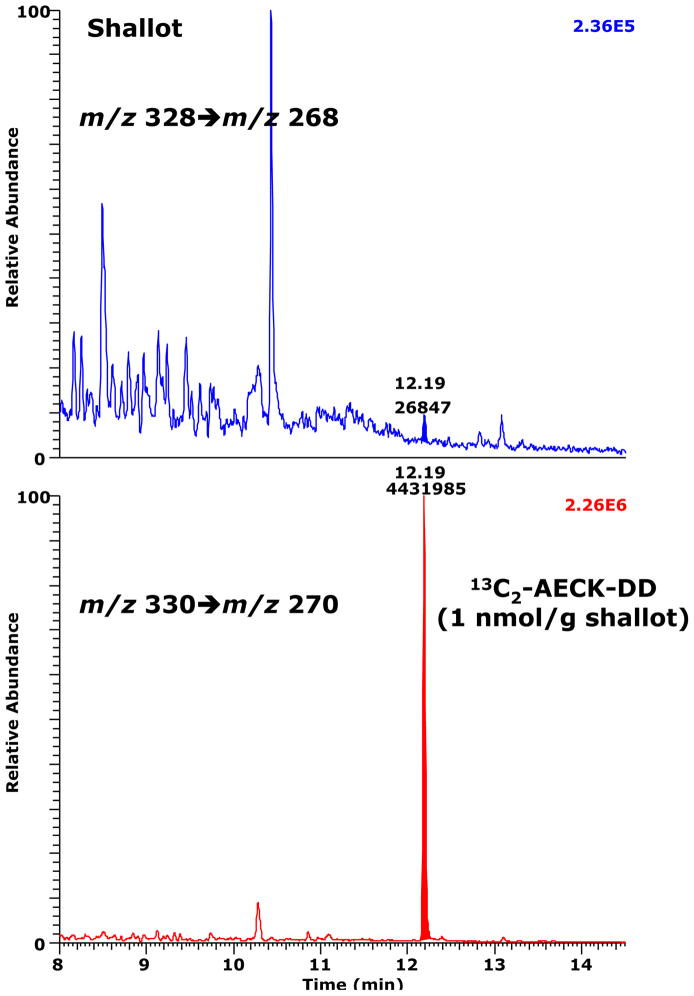
AECK-DD was previously reported to be present in various dietary vegetables, including garlic, spinach, tomato, asparagus, aubergine (egg plant), onion, pepper and courgette (zucchini), at concentrations ranging from 0.08 to 0.68 nmol per g vegetable, and, as noted in the Introduction, it has been hypothesized that vegetables may be an additional exogenous source for AECK-DD found in human and animal biological fluids and tissues [11]. We used a minor modification of the procedure, described above for the analysis of AECK-DD in human plasma and urine, to measure the AECK-DD content in the following fresh vegetables: carrot, courgette, cucumber, endive, garlic, napa cabbage, pepper, romaine lettuce and shallot. The amount of 13C2-AECK-DD (1000 pmol) added to the suspensions of each 1-g vegetable is of the same order of magnitude as that reported by Macone et al. for the concentration of unlabeled AECK-DD in a variety of vegetables [11]. Of the nine vegetables analyzed by the current procedure, only shallot contained detectable AECK-DD, but the concentration was very low (6.8 pmol per 1 g fresh weight) (Fig. 8). Even with this vegetable, however, the peak area of AECK-DD was only 0.68% of the peak area of 13C2-AECK-DD, which is of the same order of magnitude as the very small amount of unlabeled AECK-DD in the 13C2-AECK-DD preparation (Fig. 4B). Thus, our results do not support previous reports of the natural presence of appreciable concentrations of AECK-DD in dietary vegetables.
Fig. 8.
A chromatogram from the GC-MS/MS analysis of AECK-DD in fresh shallot. 13C2-AECK-DD was added to the shallot-containing 100 mM phosphate buffer, pH 7.4, at a final content of 1 nmol per 1 g shallot. SRM of m/z 328 to m/z 268 for AECK-DD (upper panel) and of m/z 330 to m/z 270 for 13C2-AECK-DD (lower panel) was performed. The AECK-DD content is estimated to be about 7 pmol/g shallot.
Discussion
Cysteamine (H2NCH2CH2SH; 2-mercaptoethylamine) is of broad biomedical interest. For example, several studies have shown that pharmacological doses of the disulfide form (cystamine, H2NCH2CH2SSCH2CH2NH2) significantly increase life expectancy in a mouse model of Huntington disease (surveyed in Ref. [27]). Cysteamine has been successfully employed for more than 30 years in the treatment of patients with cystinosis (reviewed in Ref. [28]). Cysteamine and phosphocysteamine may have utility in the design of treatments for Batten disease [29,30]. Amifostine (an S-phosphorylated N-substituted cysteamine) is a potent radioprotectant prodrug [31]. When administered in high doses as a gavage to rodents, cysteamine exhibits ulcerogenic properties and serves as a convenient model for studying both mechanisms of ulcer formation and treatment modalities (e.g., Ref. [32]). Lastly, in contrast to cysteamine, low levels of cystamine protect SHSY5Y cells against dopamine-induced macroautophagy [27].
Cysteamine is formally the decarboxylated analogue of cysteine. However, in mammals, cysteamine is not generated from cysteine by direct decarboxylation. Instead, it is derived from the metabolism of pantetheine, a component of coenzyme A, by the action of pantetheinase (EC 3.56.1.-) [33,34]. Pantetheinase is a GPI-anchored ectoenzyme that forms cysteamine following cleavage from pantetheine in order to recycle pantothenic acid. Thus, pantetheinase is the main source of cysteamine in tissues under physiological conditions [34]. Nevertheless, we have demonstrated that cysteamine can enter cells as free cysteamine in the brains of rats administered pharmacological doses of cyst(e)amine (see below). Moreover, our recent studies provide evidence that cysteamine can be transported into cells by the OCT1/2 transporters [22]. It is of interest that pantetheinase is identical to vanin-1, a membrane-bound protein that plays a role in thymic reconstitution following damage by irradiation [34]. Vanin-1/pantetheinase has been suggested to a) regulate glutathione (GSH)-dependent response to oxidative injury in tissues at the epithelial level [35]; b) exert a dominant control over innate immune responses by serving as an epithelial sensor of stress [36]; and c) mediate granuloma formation in response to Coxiella burretii infection [37].
Given the importance of cysteamine as a therapeutic agent, as a component of pantetheine (and pantetheine metabolism) and in sulfur metabolism, it is important to have reliable estimates of the in vivo levels of cysteamine and its metabolic fate. Unfortunately, the literature displays a wide discrepancy in reported values for cysteamine in a variety of tissues and body fluid, possibly due to inadequate analytical procedures in some cases (discussed in Ref. [19]). Most of the cysteamine released by the action of pantetheinase becomes covalently linked to proteins as mixed disulfides with cysteinyl moieties as a result of sulfide-disulfide exchange [1,38]. Best estimates are that the concentration of free cysteamine in tissues and body fluids is very low and often below the sensitivity of detection techniques (< 0.2 pmol/mg protein)3 [1,19, 38–40]. However, after administration of pharmacological doses of cysteamine [39] or cystamine [40] to rats, free cysteamine can be detected in the brain. For example, Ogony et al. [39] reported about 22 nmol of free cysteamine/mg protein in brain 30 min after intraperitoneal injection of 300 mg of cysteamine per kg body weight into adult rats. Bousqet et al. [40] reported peak levels of cysteamine in mouse brain of about 25, 30 and 110 pmol/kg 60 min after a single intraperitoneal injection of 10, 30 and 50 mg/kg of cystamine. Ogony et al. [39] and Bousquet et al. [40] used highly sensitive HPLC procedures to detect cysteamine previously derivatized with a fluorescent “tag” at the -SH moiety. We have used HPLC with CoulArray (coulometric) detection to analyze for redox-active compounds, including cysteamine, in rat tissues [19]. A major advantage of this technique is that prior derivatization is not necessary, provided the analyte is redox active. Using this technique, we reported that peak levels of cysteamine in the brains of rats gavage fed pharmacological doses of cysteamine-HCl over a period of 8 h were about 2.5 – 5.2 nmol/mg of protein [19].
Despite the fact that free cysteamine concentrations are normally very low, this compound is metabolized once released from disulfide linkage or when cyst (e)amine is administered to experimental animals in pharmacological doses. Evidence for a metabolic pathway for cysteamine is that mammalian tissues contain a dioxygenase that is highly specific for cysteamine [1,2]. The product of this reaction is hypotaurine. The generally accepted pathway for metabolism of cysteamine involves its oxidation to hypotaurine followed by oxidation of hypotaurine to taurine [18,41] (Fig. 1).
In mammals, cysteamine oxidation via the dioxygenase pathway is thought to occur predominantly in the liver [1]. Cysteamine dioxygenase is of low specific activity in the mammalian brain [1]. On the other hand, cystathionine β-synthase utilizes cysteamine as a substrate (as an alternative substrate to homocysteine) [33,41], and is of relatively high specific activity in rat brain [41]. Western blot analysis shows that the enzyme occurs in the hippocampus, forebrain and cerebellum [42]. When cysteamine replaces homocysteine as substrate, the product is L-thialysine (S-(2-aminoethyl)-L-cysteine) rather than cystathionine (Fig. 1). Thus, some investigators have suggested that cysteamine may be incorporated into L-thialysine by the action of cystathionine β-synthase, especially in the brain. L-Thialysine has been reported to be present in the urine of normal human adults at a concentration of about ~12.7 μg/mg creatinine (~88 nmol/mg creatinine) [43]. This finding suggests that L-thialysine is a normal metabolite. We have found variable, but low, levels of L-thialysine (below the level of detection to ~160 pmol/mg protein) in the brains of rats administered cysteamine by gavage feeding [19].
In the present work, we have developed, validated and applied a relatively straightforward, precise and accurate method for the quantitative determination of AECK-DD (a potential metabolite of thialysine) in a wide variety of biological tissues and fluids that includes plasma, urine, and brain tissue as well as in plant tissues. Our method has the following main advantages: a) we have synthesized a 13C-labeled AECK-DD (i.e., 13C2-AECK-DD) and validated its use as an internal standard (IS) and b) we have developed this methodology for use with GC-MS/MS technology, which possesses inherently high specificity and low susceptibility to interference by endogenous and exogenous analytes due to the tandem mass spectrometry approach. In contrast, other analytical approaches, including GC-MS, may be less specific and prone to interference by other analytes if chromatographic resolution is insufficient. Furthermore, the use of a stable-isotope labeled analogue as an internal standard provides a robustness that is often not available with other analytical techniques. Under the extraction conditions used in the present study, i.e., 100-μL aliquots of the biological samples and 500-μL aliquots of the organic solvent ethyl acetate, unlabeled and labeled AECK-DD were extracted quantitatively from buffered solutions, plasma and urine samples. The extraction yield of endogenous AECK-DD from tissue homogenates such as rat brain and vegetables is unknown. Nevertheless, the lipophilic nature of AECK-DD suggests that the extraction yield of tissue AECK-DD is presumably as high as that noted with plasma AECK-DD.
Based on previous articles that reported the physiological concentration of AECK-DD in human plasma to be in the lower μM-range [8,9], we validated our method first in the extended concentration range up to 50 μM in human plasma and urine. Upon the recognition that physiological AECK-DD concentrations are much lower than originally reported, we subsequently validated the GC-MS/MS method for an AECK-DD concentration range up to 1000 nM. Application of this method to urine and plasma samples of healthy volunteers confirmed the physiological occurrence of AECK-DD in human urine, but indicated that the previously reported values of AECK-DD concentrations in plasma are overestimates. Our study shows that the basal concentration of AECK-DD is not higher than 4 nM in human plasma, and ranges between the LOQ of the method (~ 2 nM) and 46 nM or between 0.1 and 6 nmol/mmol creatinine in human urine.
In the present work, we were able to detect AECK-DD in the brains of untreated mice of about 1 nmol/g wet weight (range not detectable to 2.19). Thus, AECK is present in mouse brain, but at low levels. It is of interest that AECK-DD was not detected in brain tissue obtained from two OCT1/2 KO mice (3a,3b) and from two OCT1/2 KO mice gavage treated with cysteamine (4a,4b); only a relatively low level was found in the brain of a third mouse gavage treated with cysteamine (4c) (Table 4). This finding is consistent with a role of the OCT1 and/or OCT2 transport in the cellular uptake of cysteamine.
In previous work, we were unable to assign a baseline value for AECK-DD for rat brain as measured by the HPLC/CoulArray detection technique [19]. The present work confirms this finding by a different analytical technique. Barely detectable levels were noted by GC-MS/MS (Fig. 7). However, after administration of pharmacological doses of cysteamine to the rats (250 mg/kg) we noted levels of brain AECK-DD of ~2 – 4 nmol/g wet weight by HPLC/CoulArray [19]. Based on this finding, we expected that there would be an increase in brain AECK-DD in the mice treated with cystamine. Thus, we were surprised to find that the level of mouse brain AECK-DD was actually significantly decreased in the cysteamine-treated animals compared to controls (compare 1a, 1b, 5a, 5b to 2a, 2b, 6a, 6b, 6c; Table 4; p = 0.03). This may suggest differences in the pharmacokinetics of cysteamine and AECK-DD clearance/metabolism between rats and mice. It is also possible that extremely high dose of cysteamine administered to the mice in the present study (125 mg/100 mg body weight) – about 5 times that administered to the rats (on a per weight basis) in the previous study – resulted in an inhibition of AECK-DD synthesis. Possibly, at very high levels cysteamine competes with the serine binding site on cystathionine β-synthase.
As noted above, in addition to in vivo pathways hypothesized to occur in humans leading to AECK-DD biosynthesis, some of the sulfur-containing AECK-DD has been suggested to originate from natural sources [9], particularly vegetables that are naturally rich in sulfur. Our study indicates that, of the vegetables analyzed only shallots exhibit a detectable source of AECK-DD, albeit at a very low concentration. Appreciable anti-oxidant properties of AECK-DD have been observed only in vitro at μM-concentrations (up to 250 μM), for instance, AECK-DD is protective against tert-butyl hydroperoxide-induced oxidative stress in human monocytic U937 cells [15].
The mechanisms by which AECK-DD may act as an antioxidant are unknown. Based on the ease of oxidation of sulfur, it is likely that the two sulfur atoms of AECK-DD react chemically with peroxynitrite, superoxide and hydrogen peroxide. However, to our knowledge this has not been demonstrated so far. AECK-DD has been reported to be a stronger antioxidant than the SH-containing N-acetylcysteine (NAC) [15]. However, in that study Mancone et al. compared the cell-permeable AECK-DD with the cell-impermeable (NAC). When one considers that the concentration of the most important intracellular antioxidants, namely GSH and ascorbate, are generally in the mM range, the very low content of AECK-DD in brain tissue, it is highly questionable that AECK-DD per se exhibits biological significances as an anti-oxidant. Given its tissue concentration in the sub- to low nanomolar range and its capacity as an effective antioxidant, AECK-DD may be an important candidate to consider as an inducer of the Keap1/Nrf2/ARE pathway that regulates expression of a network of ~100 genes associated with cytoprotection and inhibition of proinflammatory responses. Regarding induction of a variety of cytoprotective enzymes, a precedent for this potential effect of AECK-DD has been observed with a number of tricyclic and pentacyclic synthetic compounds that interact directly with specific sulfhydryl centers on Keap1 [44].
In summary, the GC-MS/MS method reported in this article is specific and sensitive and allows for accurate measurement of AECK-DD in human plasma and urine, in animal brain tissue and in vegetables. Although AECK-DD has been known for more than 50 years, its biochemistry and physiology are still incompletely understood. The GC-MS/MS method we report here should be a useful analytical tool for studying the occurrence and biological roles of AECK-DD, its precursors and putative metabolites. In such investigations, 13C2-AECK-DD will be a valuable non-radioactive IS.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants RO1 AG19589 and RO1 CA111842.
Footnotes
Abbreviations used: AECK, aminoethylcysteine ketimine; AECK-DD, aminoethylcysteine ketimine decarboxylated dimer; BSTFA, N,O-bis(trimethylsilyl)trifluoroacetamide; CID, collision-induced dissociation; DTT, dithiothreitol; ECNICI, electron-capture negative-ion chemical ionization; EI, electron ionization; FVB, Friend virus B-type; GC-MS, gas chromatography-mass spectrometry; GC-MS/MS, gas chromatography-tandem mass spectrometry; IS, internal standard; KO, knock out; LOD, limit of detection; LOQ, limit of quantitation; OCT, organic cation transporter; PAR, peak area ratio; PFB, pentafluorobenzyl; PFB-Br, pentafluorobenzyl bromide; RSD, relative standard deviation; SIM, selected-ion monitoring; SRM, selected-reaction monitoring.
In our previous work, we erroneously reported that the limit of detection for cysteamine by our HPLC/CoulArray detection technique was 0.2 nmol/mg protein [19]. The correct value should have been 0.2 pmol/mg protein, but the conclusion of this study, that cysteamine cannot be detected in rodent brain under normal physiological conditions by HPLC with CoulArray detection, remains unaltered.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coloso RM, Hirschberger LL, Dominy JE, Lee JI, Stipanuk MH. Cysteamine dioxygenase: evidence for the physiological conversion of cysteamine to hypotaurine in rat and mouse tissues. Adv Exp Med Biol. 2006;583:25–36. doi: 10.1007/978-0-387-33504-9_3. [DOI] [PubMed] [Google Scholar]
- 2.Dominy JE, Jr, Simmons CR, Hirschberger LL, Hwang J, Coloso RM, Stipanuk MH. Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J Biol Chem. 2007;282:25189–98. doi: 10.1074/jbc.M703089200. [DOI] [PubMed] [Google Scholar]
- 3.Ricci G, Nardini M, Federici G, Cavallini D. The transamination of L-cystathionine, L-cystine and related compounds by a bovine kidney transaminase. Eur J Biochem. 1986;157:57–63. doi: 10.1111/j.1432-1033.1986.tb09637.x. [DOI] [PubMed] [Google Scholar]
- 4.Coccia R, Foppoli C, Blarzino C, De Marco C, Pensa B. Transamination of some sulphur- or selenium-containing amino acids by bovine liver glutamine transaminase. Physiol Chem Phys Med NMR. 1992:313–321. [PubMed] [Google Scholar]
- 5.Nardini M, Matarese RM, Pecci L, Antonucci A, Ricci G, Cavallini D. Detection of 2H-1,4-thiazine-5,6-dihydro-3-carboxylic acid (aminoethylcysteine ketimine) in the bovine brain. Biochem Biophys Res Commun. 1990;166:1251–1256. doi: 10.1016/0006-291x(90)91000-i. [DOI] [PubMed] [Google Scholar]
- 6.Nardini M, Ricci G, Vesci L, Pecci L, Cavallini D. Bovine brain ketimine reductase. Biochim Biophys Acta. 1988;957:286–292. doi: 10.1016/0167-4838(88)90285-3. [DOI] [PubMed] [Google Scholar]
- 7.Matarese RM, Macone A, Maggio A, Cavallini D. Aminoethylcysteine ketimine decarboxylated dimer detected in normal human urine by gas-liquid chromatography, selected-ion monitoring and mass spectrometry. J Chromatogr B. 1996;683:269–272. doi: 10.1016/0378-4347(96)00105-3. [DOI] [PubMed] [Google Scholar]
- 8.Matarese RM, Macone A, Antonini R, Maggio A, Antonucci A. Identification of aminoethylcysteine ketimine decarboxylated dimer in human plasma. J Chromatogr B. 1999;732:137–144. doi: 10.1016/s0378-4347(99)00272-8. [DOI] [PubMed] [Google Scholar]
- 9.Nardini M, Macone A, Matarese RM. Determination of aminoethylcysteine ketimine decarboxylated dimer in human plasma and cultured cells by high-performance liquid chromatography with electrochemical detection. J Chromatogr B. 2003;795:319–327. doi: 10.1016/s1570-0232(03)00597-x. [DOI] [PubMed] [Google Scholar]
- 10.Matarese RM, Macone A, Crescentini G, Duprè S, Cavallini D. Detection of a decarboxylated dimer of aminoethylcysteine ketimine in bovine cerebellum. Neurochem Int. 1998;32:365–368. doi: 10.1016/s0197-0186(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 11.Macone A, Nardini M, Antonucci A, Maggio A, Matarese RM. Identification of aminoethylcysteine ketimine decarboxylated dimer, a natural antioxidant, in dietary vegetables. J Agric Food Chem. 2002;50:2169–2172. doi: 10.1021/jf0113934. [DOI] [PubMed] [Google Scholar]
- 12.Pecci L, Montefoschi G, Antonucci A, Cavallini D. Antioxidant properties of the decarboxylated dimer of aminoethylcysteine ketimine. Physiol Chem Phys Med NMR. 1995;27:223–229. [PubMed] [Google Scholar]
- 13.Matarese RM, Macone A, Fontana M, Duprè S, Cavallini D. Antioxidant activity of aminoethylcysteine ketimine decarboxylated dimer on copper-induced LDL oxidation. Biochem Mol Biol Int. 1998;46:829–837. doi: 10.1080/15216549800204372. [DOI] [PubMed] [Google Scholar]
- 14.Fontana M, Pecci L, Macone A, Cavallini D. Antioxidant properties of the decarboxylated dimer of aminoethylcysteine ketimine: assessment of its ability to scavenge peroxynitrite. Free Radic Res. 1998;29:435–440. doi: 10.1080/10715769800300481. [DOI] [PubMed] [Google Scholar]
- 15.Macone A, Matarese RM, Gentili V, Antonucci A, Duprè S, Nardini M. Effect of aminoethylcysteine ketimine decarboxylated dimer, a natural sulfur compound present in human plasma, on tert-butyl hydroperoxide-induced oxidative stress in human monocytic U937 cells. Free Radic Res. 2004;38:705–714. doi: 10.1080/10715760410001705159. [DOI] [PubMed] [Google Scholar]
- 16.Macone A, Fontana M, Barba M, Botta B, Nardini M, Ghirga F, Calcaterra A, Pecci L, Matarese RM. Antioxidant properties of aminoethylcysteine ketimine decarboxylated dimer: a review. Int J Mol Sci. 2011;12:3072–3084. doi: 10.3390/ijms12053072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonucci A, Pecci L, Coccia R, Fontana M, Cavallini D. The oxidation of aminoethylcysteine ketimine dimer by oxygen reactive species. Amino Acids. 1994;7:83–88. doi: 10.1007/BF00808449. [DOI] [PubMed] [Google Scholar]
- 18.Shen W, McGath MK, Evande R, Berkowitz DB. A continuous spectrophotometric assay for human cystathionine beta-synthase. Anal Biochem. 2005;342:103–10. doi: 10.1016/j.ab.2005.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto JT, Khomenko T, Szabo S, McLaren GD, Denton TT, Krasnikov BF, Jeitner TM, Cooper AJL. Measurement of sulfur-containing compounds involved in the metabolism and transport of cysteamine and cystamine. Regional differences in cerebral metabolism. J Chromatogr B. 2009;877:3434–3441. doi: 10.1016/j.jchromb.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khomenko T, Pinto JT, Chen L, Deng X, Tolstanova G, Cooper AJL, Szabo S. Role of organic cation transporters (Oct1, Oct2 and Oct3) in the uptake of the duodenal ulcerogen cysteamine in rat duodenal mucosa and in rat small intestinal epithelial cell line IEC-6: Use of siRNA Technology. Gastroenterology. 2008;134:A–405. [Google Scholar]
- 21.Khomenko T, Paunovic B, Deng XM, Chen L, Szabo S. Inhibition of cysteamine-induced duodenal ulcers in Oct1/2 knockout mice. Gastroenterology. 2011;140(Suppl 1):S-317. [Google Scholar]
- 22.Khomenko T, Kolodney J, Pinto JT, McLaren GD, Deng X, Chen LL, Tolstanova G, Paunovic B, Krasnikov BF, Hoa N, Cooper AJ, Szabo SS. New mechanistic explanation for the localization of ulcers in the rat duodenum: Role of iron and selective uptake of cysteamine. Arch Biochem Biophys. 2012;525:60–70. doi: 10.1016/j.abb.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Tsikas D, Beckmann B, Gutzki F-M, Jordan J. Simultaneous gas chromatography-tandem mass spectrometry quantification of symmetric and asymmetric dimethylarginine in human urine. Anal Biochem. 2011;413:60–62. doi: 10.1016/j.ab.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Velásquez RD, Brunner G, Varrentrapp M, Tsikas D, Frölich JC. Helicobacter pylori produces histamine and spermidine. Z Gastroenterol. 1996;34:116–122. [PubMed] [Google Scholar]
- 25.Zoerner AA, Heusser K, Gutzki FM, Mitschke A, Tank J, Stichtenoth DO, Jordan J, Tsikas D. Unique pentafluorobenzylation and collision-induced dissociation for specific and accurate GC-MS/MS quantification of the catecholamine metabolite 3,4-dihydroxyphenylglycol (DHPG) in human urine. J Chromatogr B. 2011;879:1444–1456. doi: 10.1016/j.jchromb.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Tsikas D. De novo synthesis of trideuteromethyl esters of amino acids for use in GC-MS and GC-tandem MS exemplified for ADMA in human plasma and urine: standardization, validation, comparison and proof of evidence for their aptitude as internal standards. J Chromatogr B. 2009;877:2308–2320. doi: 10.1016/j.jchromb.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Jeitner TM, Pinto JT, Krasnikov BF, Horswill M, Cooper AJL. Transglutaminases and neurodegeneration. J Neurochem. 2009;109(Suppl 1):160–166. doi: 10.1111/j.1471-4159.2009.05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleta R, Gahl WA. Pharmacological treatment of nephropathic cystinosis with cysteamine. Expert Opin Pharmacother. 2004;5:2255–2262. doi: 10.1517/14656566.5.11.2255. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Butler JD, Levin SW, Wisniewski KE, Brooks SS, Mukherjee AB. Lysosomal ceroid depletion by drugs: therapeutic implications for a hereditary neurodegenerative disease of childhood. Nat Med. 2001;7:478–484. doi: 10.1038/86554. [DOI] [PubMed] [Google Scholar]
- 30.Lu JY, Hofmann SL. Inefficient cleavage of palmitoyl-protein thioesterase (PPT) substrates by aminothiols: implications for treatment of infantile neuronal ceroid lipofuscinosis. J Inherit Metab Dis. 2006;29:119–126. doi: 10.1007/s10545-006-0225-z. [DOI] [PubMed] [Google Scholar]
- 31.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 32.Khomenko T, Szabo S, Deng X, Ishikawa H, Anderson GJ, McLaren GD. Role of iron in the pathogenesis of cysteamine-induced duodenal ulceration in rats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1277–G1286. doi: 10.1152/ajpgi.90257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitari G, Maurizi G, Flati V, Ursini CL, Spera L, Duprè S, Cavallini D. Enzymatic synthesis of S-aminoethyl-L-cysteine from pantetheine. Biochim Biophys Acta. 1992;1116:27–33. doi: 10.1016/0304-4165(92)90124-d. [DOI] [PubMed] [Google Scholar]
- 34.Pitari G, Malergue F, Martin F, Philippe JM, Massucci MT, Chabret C, Maras B, Duprè S, Naquet P, Galland F. Pantetheinase activity of membrane-bound vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett. 2000;483:149–154. doi: 10.1016/s0014-5793(00)02110-4. [DOI] [PubMed] [Google Scholar]
- 35.Berruyer C, Martin FM, Castellano R, Macone A, Malergue F, Garrido-Urbani S, Millet V, Imbert J, Duprè S, Pitari G, Naquet P, Galland F. Vanin-1−/− mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Mol Cell Biol. 2004;24:7214–7224. doi: 10.1128/MCB.24.16.7214-7224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berruyer C, Pouyet L, Millet V, Martin FM, LeGoffic A, Canonici A, Garcia S, Bagnis C, Naquet P, Galland F. Vanin-1 licenses inflammatory mediator production by gut epithelial cells and controls colitis by antagonizing peroxisome proliferator-activated receptor γ activity. J Exp Med. 2006;203:2817–2827. doi: 10.1084/jem.20061640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meghari S, Berruyer C, Lepidi H, Galland F, Naquet P, Mege JL. Vanin-1 controls granuloma formation and macrophage polarization in Coxiella burnetii infection. Eur J Immunol. 2007;37:24–32. doi: 10.1002/eji.200636054. [DOI] [PubMed] [Google Scholar]
- 38.Duffel MW, Logan DL, Ziegler DM. Cysteamine and cystamine. Methods Enzymol. 1987;143:149–154. doi: 10.1016/0076-6879(87)43027-9. [DOI] [PubMed] [Google Scholar]
- 39.Ogony J, Mare S, Wu W, Ercal N. High performance liquid chromatography analysis of 2-mercaptoethylamine (cysteamine) in biological samples by derivatization with N-(1-pyrenyl) maleimide (NPM) using fluorescence detection. J Chromatogr B. 2006;843:57–62. doi: 10.1016/j.jchromb.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 40.Bousquet M, Gibrat C, Ouellet M, Rouillard C, Calon F, Cicchetti F. Cystamine metabolism and brain transport properties: clinical implications for neurodegenerative diseases. J Neurochem. 2010;114:1651–1658. doi: 10.1111/j.1471-4159.2010.06874.x. [DOI] [PubMed] [Google Scholar]
- 41.Rassin DK, Gaull GE. Subcellular distribution of enzymes of transmethylation and transsulphuration in rat brain. J Neurochem. 1975;24:969–978. doi: 10.1111/j.1471-4159.1975.tb03664.x. [DOI] [PubMed] [Google Scholar]
- 42.Régnier V, Billard JM, Gupta S, Potier B, Woerner S, Paly E, Ledru A, David S, Luilier S, Bizot JC, Vacano G, Kraus JP, Patterson D, Kruger WD, Delabar JM, London J. Brain phenotype of transgenic mice overexpressing cystathionine β-synthase. PLoS One. 2012;7:e29056. doi: 10.1371/journal.pone.0029056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S, Sugahara K, Zhang J, Ageta T, Kodama H, Fontana M, Duprè S. Simultaneous determination of urinary cystathionine, lanthionine, S-(2-aminoethyl)-L-cysteine and their cyclic compounds using liquid chromatography-mass spectrometry with atmospheric pressure chemical ionization. J Chromatogr B. 1997;698:301–307. doi: 10.1016/s0378-4347(97)00295-8. [DOI] [PubMed] [Google Scholar]
- 44.Kalra S, Knatko EV, Zhang Y, Honda T, Yamamoto M, Dinkova-Kostova AT. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): Implications for protection against UV radiation during thiopurine therapy. Cancer Prev Res. 2012;5:973–81. doi: 10.1158/1940-6207.CAPR-12-0041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.