Abstract
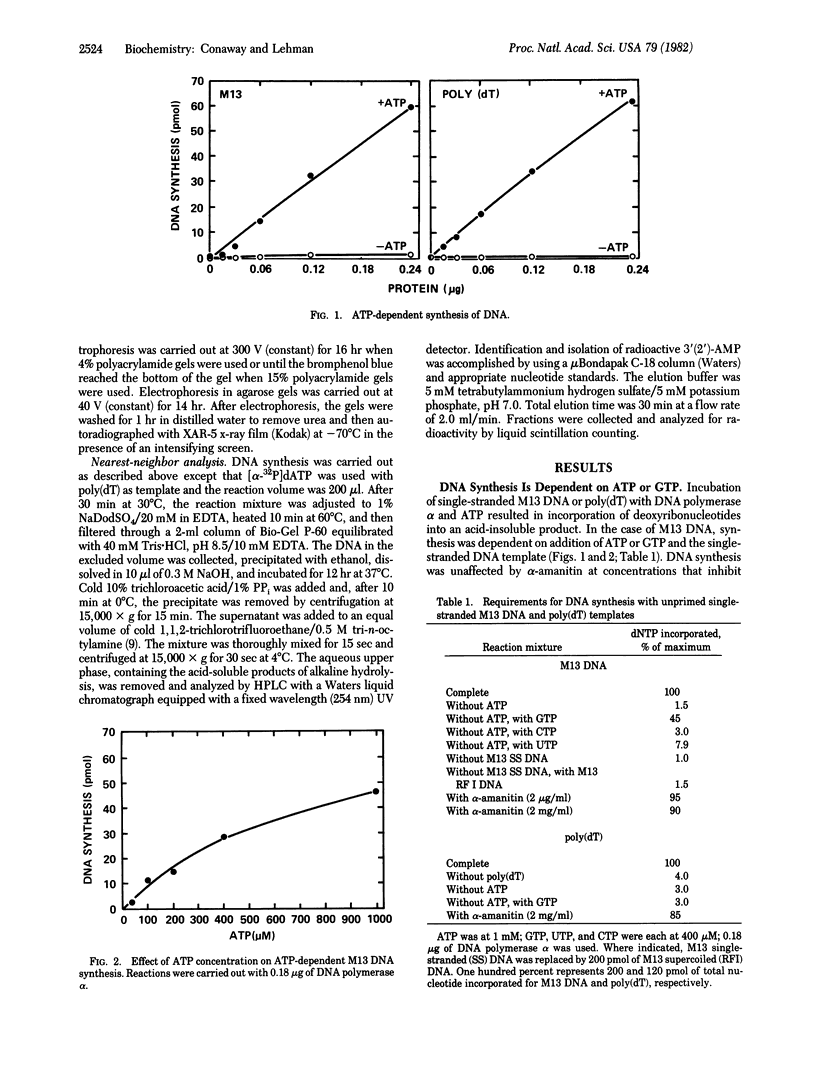
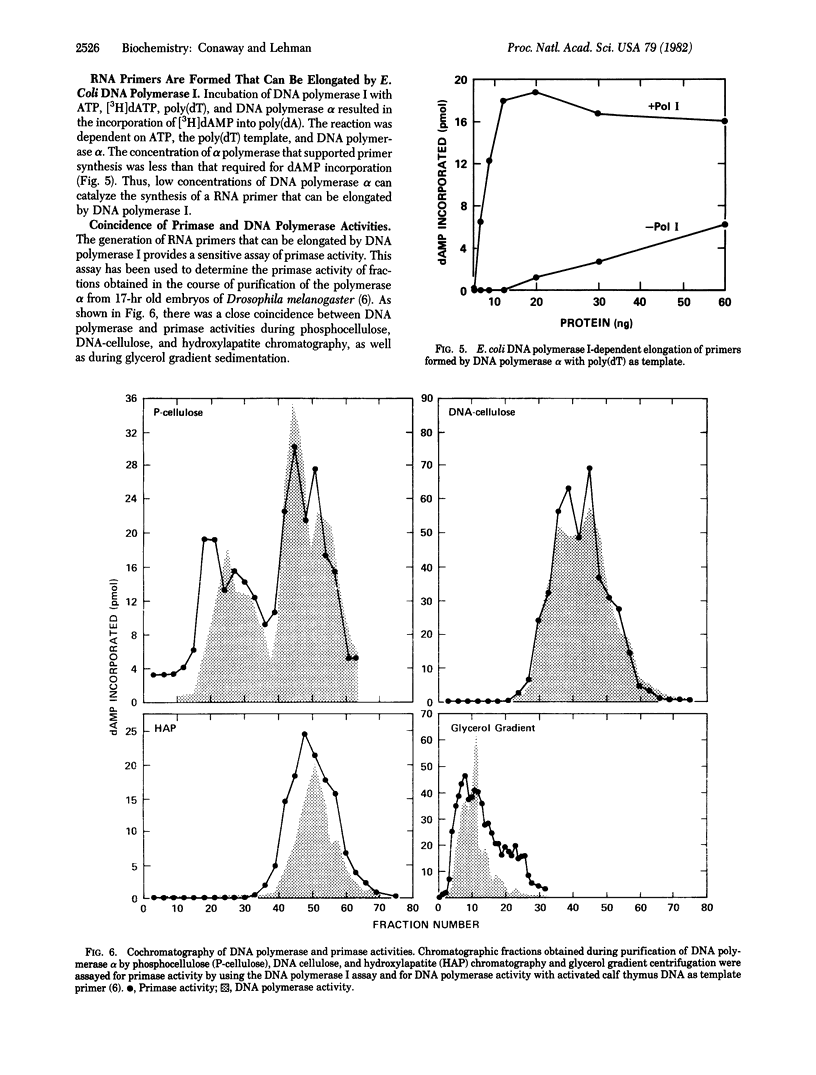
Preparations of DNA polymerase alpha from early embryos of Drosophila melanogaster catalyze the ATP-dependent synthesis of DNA with single-stranded M13 DNA or poly(dT) templates. In the case of M13 DNA, GTP, but not UTP or CTP, can replace ATP. The reaction is completely dependent on added template and is not inhibited by alpha-amanitin. Alkaline hydrolysis of the product synthesized in the presence of [alpha-32P]dATP and poly(dT) generates 32P-labeled 3'(2') adenylate, showing that a covalent ribo-deoxynucleotide linkage is formed. Furthermore, incorporation of ribonucleotides occurs at the 5' end of the newly synthesized polynucleotide chain. These findings are consistent with the hypothesis that a ribo-oligonucleotide primer is synthesized by primase action and subsequently elongated by DNA polymerase. Under the appropriate conditions, DNA polymerase I from Escherichia coli can elongate primers formed by primase in the presence of ATP and poly(dT). Primase activity copurifies with DNA polymerase alpha and may be part of the multisubunit polymerase molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kaguni L. S., Clayton D. A. Template-directed pausing in in vitro DNA synthesis by DNA polymerase a from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Feb;79(4):983–987. doi: 10.1073/pnas.79.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]






