Abstract
Affinities of arylpiperazinylalkyl derivatives of imidazo[2,1-f]purine-2,4-dione and imidazolidine-2,4-dione for serotonin transporter and their acid–base properties were evaluated. The dissociation constant (pK a) of compounds 1–22 were determinated by potentiometric titration and calculated using pKalc 3.1 module of the Pallas system. The data from experimental methods and computational calculations were compared and suitable conclusions were reached.
Keywords: Dissociation constant; Imidazolidine-2,4-dione; Imidazo[2,1-f]purine-2,4-dione; Serotonin receptor 5-HT1A; Serotonin transporter
Introduction
Studies on major depression, anxiety, schizophrenia, mania, autism, obesity, and drug addiction have implicated the involvement of serotonergic (5-HT) abnormalities in these diseases. Serotonin acts via receptors which were classified into seven families (5-HT1–7) and at least 14 different subtypes (Barnes and Sharp, 1999; Filip et al., 2005; Hannon and Hoyer, 2008; Hoyer et al., 2002; Pauwels, 2003). The level of 5-HT in central nervous system (CNS) and regulation of its neurotransmission are connecting with serotonin transporter (SERT). This transporter is mediated extracellular uptake of serotonin from the synaptic clefts. The SERT protein belongs to the large family of transporters that are dependent on Na+ ions. Serotonin, Na+ and Cl− form a quaternary complex with the transporter before being co-transported across the plasma membrane, followed by counter transport of K+. At physiological pH = 7.4, serotonin is protonated and in the case of the SERT 5-HT accumulation was not affected by transmembrane pH differences (Rudnick et al., 1989; Forrest et al., 2007). Many drug molecules contain ionizable groups and hence penetrate across cell membranes, through pores and via active transport mechanism in a pK a dependent fashion, therefore pK a is an important factor on estimating the pharmacological behavior of drugs and their pharmacokinetic. This is particularly important in physiological systems, where ionization state will affect the rate at which the compound is able to diffuse across membranes and obstacles such as the blood–brain barrier (BBB) (Luan et al., 2005; Manallack, 2007).
Since the early seventies until today, a large number of selective SERT inhibitors (SSRIs) have been described. However, only 5 products are currently marketed: fluoxetine (Prozac®), escitalopram (Lexapro®, Cipralex®), sertraline (Zoloft®), paroxetine (Paxil®), and fluvoxamine (Fevarin®). A major limitation of SSRIs and that extends to all other classes of antidepressants as well, is the 2–6 weeks delay in onset of therapeutic activity. This lengthy time to achieve remission is suspected to result from indirect activation of somatodendric 5-HT1A autoreceptors (Chaput et al., 1986; Invernizzi et al., 1992; Invernizzi et al., 1996). The latest direction taken in antidepressant drug discovery has been to design ligands with multiple targets. Preclinical data obtained by co-administrating a SSRI with selective 5-HT1A antagonist, suggest that a single compound combining SSRIs with 5-HT1A antagonism should have a favorable therapeutic utility in the treatment (Artigas et al., 1996; Ballesteros and Callodo, 2004; Adell et al., 2005; Morphy and Rankovic, 2005; Millan, 2006). The most important class of 5-HT1A receptor ligands are derivatives of arylpiperazine. Simple arylpiperazines are non-selective ligands for 5-HT receptor. The good selectivity and affinity for 5-HT1A receptors show the majority of 4-substituted arylpiperazines. These derivatives contain a flexible aliphatic chain of different length (long-chain arylpiperazines, LCAPs), which connects the arylpiperazine fragment with second terminal pharmacophoric group (Lopez-Rodriguez et al., 2002; Paluchowska et al., 2007; Paluchowska et al., 2005). For several years our attention has been focused on developing LCAPs containing a different amide/imide terminal fragment. In our earlier study a series of arylpiperazinylalkyl derivatives with a complex terminal part based on the purine moiety had been synthesized. Compounds with pyrimido- and imidazo-[2,1-f]purine-2,4-dione fragment have been tested in vitro for their 5-HT1A and 5-HT2A receptor affinities and were potent 5-HT1A receptor ligands with K i within the range of 5.6–278 nM and demonstrated lack of affinity for 5-HT2A subtype (Zagórska et al., 2009). The majority of imidazolidine-2,4-dione derivatives displayed high affinity for 5-HT1A receptors (23–350 nM), and some of them exhibited significant affinity for 5-HT2A receptors (Czopek et al., 2010).
Continuing our research, we have been interested in affinities of arylpiperazinylalkyl derivatives of imidazo[2,1-f]purine-2,4-dione and imidazolidine-2,4-dione (hydantoin) for SERT and their acid-based properties presented as dissociation constant (pK a) values. The aqueous ionization constant of a molecule is denoted by its pK a values, where this constant is equivalent to the pH at which a given ionizable group on the molecule is half-ionized. In search of the structure activity relationship, the correlation with biological activity data with received pK a values, was done.
Methods and materials
Chemistry
The chemical structures of derivatives of the imidazo[2,1-f]purine-2,4-dione and imidazolidine-2,4-diones investigated are listed in the Table 1. The hydrochloride salts of investigated compound 1–22 of the analytical purity (mp, TLC, elemental analysis) were used for the potentiometric titration.
Table 1.
The structures of compounds 1–22, their SERT activity (pK
i), experimental and theoretical pK
a values 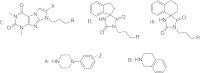
| Compd | Core | X | R | Z | pK i [SERT] | Exp pK a | pK a Pallas |
|---|---|---|---|---|---|---|---|
| 1 | I | H | A | H | 6.35 | 8.09 | 9.29 |
| 2 | I | H | A | 2–OCH3 | 6.95 | 8.19 | 9.29 |
| 3 | I | H | A | 3–Cl | 7.53 | 8.35 | 9.20 |
| 4 | I | CH3 | A | H | 4.95 | 7.55 | 9.29 |
| 5 | I | CH3 | A | 2–OCH3 | 5.09 | 7.61 | 9.29 |
| 6 | I | CH3 | A | 3–Cl | 7.52 | 8.12 | 9.20 |
| 7 | I | CH3 | A | 2,3–diCl | 7.25 | 7.61 | 9.20 |
| 8 | I | CH3 | B | – | 4.52 | 8.66 | 8.72 |
| 9 | I | C6H5 | A | H | 6.65 | 8.79 | 9.29 |
| 10 | I | C6H5 | A | 2–OCH3 | 4.69 | 8.44 | 9.29 |
| 11 | I | C6H5 | A | 3–Cl | 6.72 | 10.61 | 9.20 |
| 12 | I | C6H5 | B | H | 5.61 | 10.41 | 8.72 |
| 13 | II | – | A | H | 5.96 | 10.48 | 8.95 |
| 14 | II | – | A | 2–OCH3 | 5.96 | 9.60 | 8.95 |
| 15 | II | – | A | 3–Cl | 6.07 | 10.31 | 8.85 |
| 16 | II | – | A | 3–CF3 | 6.19 | 9.96 | 8.95 |
| 17 | II | – | B | – | Nd | 10.93 | 8.38 |
| 18 | III | – | A | H | 6.00 | 10.55 | 8.95 |
| 19 | III | – | A | 2–OCH3 | 6.01 | 10.32 | 8.95 |
| 20 | III | – | A | 3–Cl | 6.04 | 10.80 | 8.85 |
| 21 | III | – | A | 3–CF3 | 5.62 | 11.08 | 8.95 |
| 22 | III | – | B | – | 5.40 | 10.90 | 8.38 |
Compounds 1–12 were obtained in the cyclocondensation reaction of 7-acetic-8-bromotheophylline aldehyde, 7-acetonyl-8-bromotheophylline, and 7-phenacyl-8-bromotheophylline, with double amount of appropriate arylpiperazinylpropylamine, in boiling 2-methoxyethanol (Zagórska et al., 2009). Whereas initial spirohydantoins were prepared from appropriate ketone by the Buchere–Berg reaction with modification described by Goodson et al. (1960). Compounds 13–22 were obtained by a two-step procedure involving alkylation of hydantoin at N3 position and condensation of intermediates with the substituted arylpiperazine or tetrahydroizoquinoline. The synthesis and physicochemical properties are described elsewhere (Zagórska et al., 2009; Czopek et al., 2010). The synthetic procedures and physicochemical data of compounds 16, 17, 21, and 22 have not been published yet.
Pharmacology in vitro
The assay was performed according to the method of Owens et al. (1997) with slight modifications. [3H]-Citalopram (spec. act. 50 Ci/mmol, NEN Chemicals) was used for labeling 5-HT-transporter. Rat cerebral cortex was homogenized in 30 volumes of ice-cold 50 mM Tris–HCl containing 150 mM NaCl and 5 mM KCl, pH = 7.7 at 25°C and centrifuged at 20,000×g for 20 min. The supernatant was decanted and pellet was resuspended in 30 volumes of buffer and centrifuged again. The resulting pellet was resuspended in the same quantity of the buffer and centrifuged third time in the same conditions. 240 μl of the tissue suspension, 30 μl of 1 nM [3H]-citalopram, and 30 μl of the analyzed compound or 30 μl of 1 μM imipramine (displacer) were incubated at 22°C for 1 h. The concentrations of analyzed compounds ranged from 10−10 to 10−5 M. Incubations were terminated by vacuum filtration over Whatman GF/B filters and washed 5 times with 200 μl of ice-cold buffer. Radioactivity was measured in a MicroBeta TriLux– liquid scintillation counter (Perkin Elmer). All assays were done in duplicates.
Radioligand binding data were analyzed using iterative curve fitting routines (GraphPAD/Prism, Version 3.0 – San Diego, CA, USA). K i values were calculated from the Cheng–Prusoff equation (Cheng and Prusoff, 1973). The results of in vitro binding studies (pK i) of the compounds (1–22) are shown in Table 1.
Measurement of pKa
The pK a measurements were determined by potentiometric titration (alkalimetric), using a Compact Titrator Mettler Toledo G21 equipped with an integrated burette drive, and combined glass electrode DGi115-SC, compact rod stirrer, and 20 ml burette. Titrator was pre-programmed with standard tried-and-tested methods and calculations. The pH electrode was first calibrated with buffers (pH = 7.00 and pH = 9.00). Sample (5 × 10−5 M) were prepared in water solutions (between 10–20 ml). Typically, more than 120 pH readings were collected for each titration. The deionized water used for the aqueous solution was twice distilled, degassed, and filtered with a Hydrolab Polska HLP5s System. The 0.0512 M sodium hydroxide solution were prepared from substances delivered by POCH. The buffers pH = 7.00 and pH = 9.00 used for calibration were obtained from Beckman Coulter. The pK a were expressed as the mean of values of results from three titrations and are listed in Table 1.
The following equation was used for the calculation of the pK a values:
| 1 |
where Ct is a titrant concentration, Ca is a concentration of sample at each measured point.
Calculations
Calculations of pK a were performed using Pallas 3.1 (CompuDrug Chemistry Ltd, 1995). Program applied logarithm, adapted after Hammett and Taft takes into account all necessary electronic, steric, and other effects and relies on an extended database of almost a thousand equations.
Regression analysis was performed using the Statistica for Windows program (Statistica for Windows, version 9, Statsoft Inc.2009). The significance level of the performed calculations was above 95%.
Results and discussion
The library consisting of twenty two compounds was investigated. Based on their structural features, this library could be divided into two sublibraries: the first contained various arylpiperazinylpropyl derivatives of imidazo[2,1-f]theophylline, and the second derived from imidazolidine-2,4-dione. Comparing the affinity for SERT obtained for imidazo[2,1-f]purine-2,4-dione and respective imidazolidine-2,4-dione analogues revealed higher activity in the first mentioned series. The most potent SERT ligands were compounds 3, 6, and 7 with pK i within the range of 7.25–7.53, which were containing 2,3-dichloro or 3-chlorophenylpiperazine fragment in their structures. Compounds 1, 2, 9, 11, 12, 15, 16, 19, and 20 displayed moderate to very low affinity for the SERT (5.61–6.95), whereas other were practically devoid of any affinity.
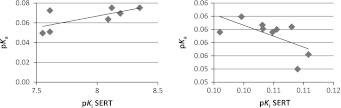
Furthermore experimental dissociation constants for investigated compounds were determined. It seems that the basicity of the compounds mainly depends on two basic nitrogen atoms in piperazine ring. In general, compounds containing a substituent at phenyl ring electro-attracting atom (Cl) or group (CF3) exhibited higher experimental pK a values than unsubstituted ones. In addition, the replacement of arylpiperazine fragment with tetrahydroisoquinoline in respective compounds caused increase of pK a values. The experimental pK a values are in range from 7.55 to 11.08, the detail data are presented in Table 1. The ranges of predicted pK a values of Pallas program are listed in Table 1. Unfortunately, the used program predicted no similar values to experimental pK a and could not diversify the acid–base properties of closely related compounds. In order to obtain more detailed relationships between acid–base properties of investigated compounds and the affinity of tested compounds to SERT, QSAR studies were undertaken. It was found linear correlation between affinities for SERT (pK i) and experimental pK a values (Fig. 1) but ratio of determination was moderate (R 2 = 0.48 for sublibraries 1 and R 2 = 0.38 for sublibraries 2).
Fig. 1.
Correlation between pK a values and pKi SERT of compounds 1–7 (left) and 13–22 (right)
Summarizing, two compounds 3 and 6 (derivatives of imidazo[2,1-f]purine-2,4-dione) are potent dual ligands for SERT and 5-HT1A receptor (pK i > 7.5) and were classified to the further pharmacological studies. The obtained results confirm that the applied potentiometric method is useful in characterization of the acid–base properties of closely related compounds contrary to values of pK a predicted by Pallas program. There is no correlation between values of pK a predicted by Pallas program and experimental. The moderate correlation between activity for SERT and pK a, indicating that acid–base properties are one of the important factors, which could influent and modify the activity for SERT.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adell A, Castro E, Celada P, Bortolozzi A, Pazos A, Artigas F. Strategies for producing faster acting antidepressants. Drug Discov Ther. 2005;10:578–585. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Ballesteros J, Callodo LF. Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomized controlled trials. J Affect Disord. 2004;79:137–147. doi: 10.1016/S0165-0327(02)00404-4. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montingny C, Blier P. Effects of selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Czopek A, Byrtus H, Kołaczkowski M, Pawłowski M, Dybała M, Nowak G, Tatarczyńska E, Wesołowska A, Chojnacka-Wójcik E. Synthesis and pharmacological evaluation of new 5-(cyclo)alkyl-5-phenyl- and 5-spiroimidazolidine-2, 4-dione derivatives. Novel 5-HT1A receptor agonist with potential antidepressant and anxiolytic activity. Eur J Med Chem. 2010;45:1295–1303. doi: 10.1016/j.ejmech.2009.11.053. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Gołda A. The serotoninergic system and its role in cocaine addiction. Pharmacol Rep. 2005;57:685–700. [PubMed] [Google Scholar]
- Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B. Identification of chloride ion binding site in Na+/Cl− dependent transporters. PNAS. 2007;104(31):12761–12766. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson LH, Honigberg IL, Lehman JJ, Burton WH. Potential growth antagonists. I. Hydantoins and disubstituted glycines. J Org Chem. 1960;25:1920–1924. doi: 10.1021/jo01081a024. [DOI] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Belli S, Samanin R. Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex. Brain Res. 1992;584:322–324. doi: 10.1016/0006-8993(92)90914-U. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Role of 5-HT1A receptors in the effects of acute and chronic fluoxetine on extracellular serotonin in the frontal cortex. Pharmacol Biochem Behav. 1996;54:143–147. doi: 10.1016/0091-3057(95)02159-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez ML, Ayala D, Benhamu B, Morcillo MJ, Viso A. Arylpiperazine derivatives acting at 5-HT1A receptors. Curr Med Chem. 2002;9:443–469. doi: 10.2174/0929867023371030. [DOI] [PubMed] [Google Scholar]
- Luan F, Ma W, Zhang H, Zhang X, Liu M, Hu Z, Fan B. Prediction of pKa for neutral and basic drugs based on radial basis function neural networks and their heuristic method. Pharm Res. 2005;22(9):1454–1460. doi: 10.1007/s11095-005-6246-8. [DOI] [PubMed] [Google Scholar]
- Manallack DT. The pKa distribution of drugs: application to drug discovery. Perspect Med Chem. 2007;1:25–38. [PMC free article] [PubMed] [Google Scholar]
- Millan PJ. Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110:135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005;48(21):6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283(3):1305–1322. [PubMed] [Google Scholar]
- Paluchowska MH, Bugno R, Bojarski A, Charakchieva-Minol S, Duszyńska B, Tatarczyńska E, Kłodzińska A, Stachowicz K, Chojnacka-Wójcik E. Novel, flexible, and conformationally defined analogs of gepirone: synthesis and 5-HT1A, 5-HT2A and D2 receptor activity. Bioorganic Med Chem. 2005;13:1195–1200. doi: 10.1016/j.bmc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Paluchowska MH, Bugno R, Duszyńska B, Tatarczyńska E, Nikiforuk A, Lenda T, Chojnacka-Wójcik E. The influence of modifications in imide fragment structure on 5-HT1A and 5-HT7 receptor affinity and in vivo pharmacological properties of some new 1-(m-trifluoromethylphenyl)piperazines. Bioorganic Med Chem. 2007;15:7116–7125. doi: 10.1016/j.bmc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ. 5-HT receptors and their ligands. Tocris Rev. 2003;25:1–10. [Google Scholar]
- Rudnick G, Kirk KL, Fishkes H, Schuldiner S. Zwitterionic and anionic forms of serotonin analog as transport substrates. J Biol Chem. 1989;264(25):14865–14868. [PubMed] [Google Scholar]
- Zagórska A, Jurczyk S, Pawłowski M, Dybała M, Nowak G, Tatarczyńska E, Nikiforuk A, Chojnacka-Wójcik E. Synthesis and preliminary pharmacological evaluation of imidazo[2, 1-f]purine-2, 4-dione derivatives. Eur J Med Chem. 2009;44:4288–4296. doi: 10.1016/j.ejmech.2009.07.014. [DOI] [PubMed] [Google Scholar]