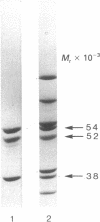
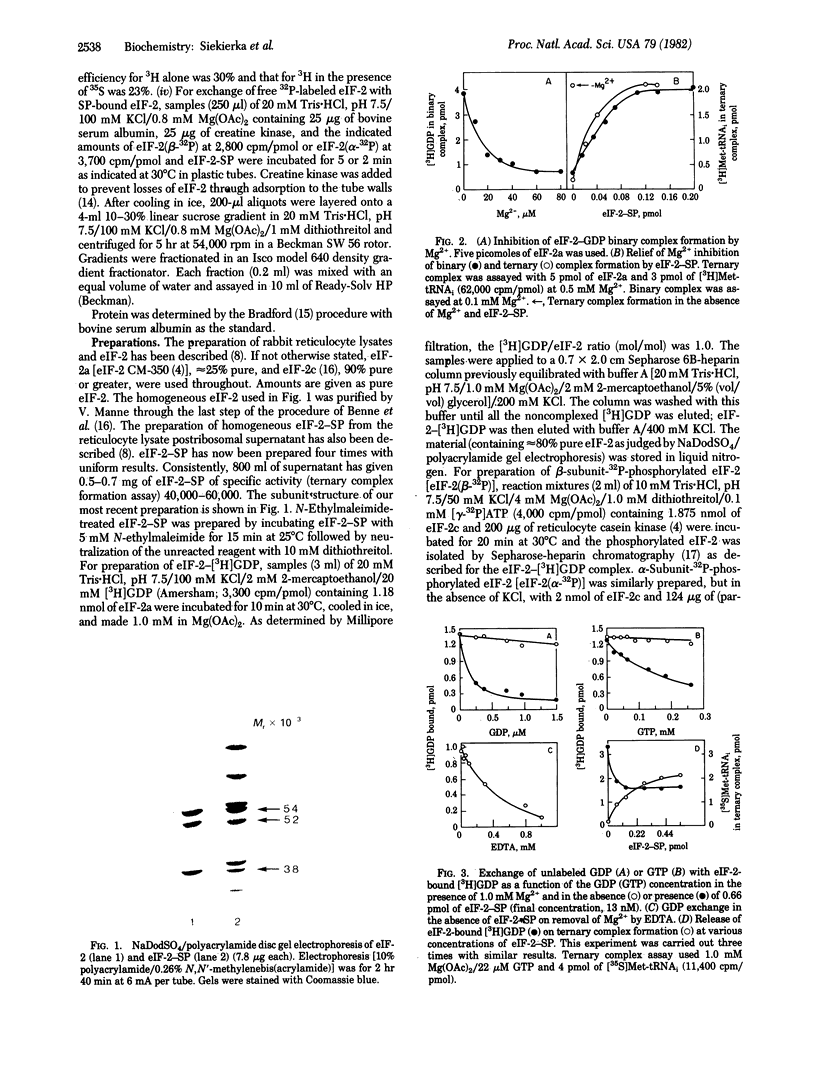
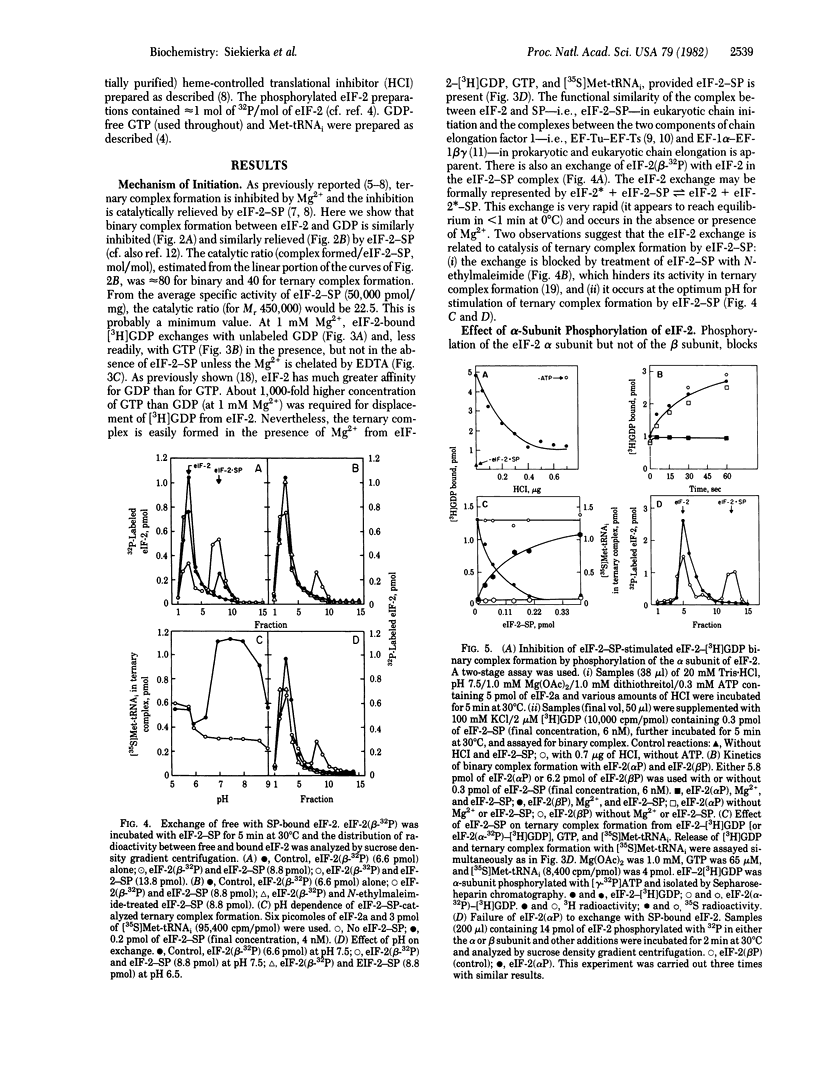
Abstract
Earlier, we isolated eukaryotic initiation factor 2 (eIF-2)-stimulating protein (SP) as a homogeneous complex with eIF-2 (eIF-2-SP) and showed that, in the presence of Mg2+, eIF-2-SP promotes formation of a ternary complex with GTP and eukaryotic initiator methionyl tRNA (Met-tRNAi) (eIF-2-GTP-Met-tRNAi) catalytically. We now show that SP-bound eIF-2 exchanges with eIF-2 (eIF-2 exchange). Furthermore, in the presence of Mg2+, eIF-2-SP catalyzes the exchange of eIF-2-bound [3H]GDP with unlabeled GDP or GTP (GDP exchange) and the release of [3H]GDP when the ternary complex is formed from eIF-2-[3H]GDP, GTP, and [35S]Met-tRNAi. All these reactions are blocked by alpha-subunit, but not by beta-subunit, phosphorylation of eIF-2. The eIF-2 and GDP exchanges are compatible with the reaction eIF-2-GDP + SP in equilibrium EIF-2-SP + GDP reminiscent of the exchange between the Tu and Ts components of prokaryotic elongation factor 1 (EF-Tu and EF-Ts, respectively) EF-Tu-GDP + EF-Ts in equilibrium EF-Tu-EF-Ts + GDP. Due to the high affinity of GDP (approximately 100 times greater than that of GDP) for eIF-2, 40S (eIF-2-GTP-Met-tRNAi-40S) to 80S (Met-tRNAi-mRNA-80S) initiation complex conversion, which is accompanied by GTP hydrolysis, probably releases eIF-2 as eIF-2-GDP. Our results suggest that, in the presence of Mg2+, GDP binding restricts the availability of eIF-2 for chain initiation and that SP relieves this restriction in a catalytic fashion, provided that the alpha subunit of eIF-2 is not phosphorylated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Amesz H., Hershey J. W., Voorma H. O. The activity of eukaryotic initiation factor eIF-2 in ternary complex formation with GTP and Met-tRNA. J Biol Chem. 1979 May 10;254(9):3201–3205. [PubMed] [Google Scholar]
- Benne R., Wong C., Luedi M., Hershey J. W. Purification and characterization of initiation factor IF-E2 from rabbit reticulocytes. J Biol Chem. 1976 Dec 10;251(23):7675–7681. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cherbas L., London I. M. On the mechanism of delayed inhibition of protein synthesis in heme-defecient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3506–3510. doi: 10.1073/pnas.73.10.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Das A., Ralston R. O., Grace M., Roy R., Ghosh-Dastidar P., Das H. K., Yaghmai B., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: mechanism of protein synthesis inhibition by heme-regulated inhibitor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5076–5079. doi: 10.1073/pnas.76.10.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J., Safer B. Use of heparin-Sepharose for the rapid isolation of initiation and elongation factors. Methods Enzymol. 1979;60:165–181. doi: 10.1016/s0076-6879(79)60014-9. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Interactions between the elongation factors: the displacement of GPD from the TU-GDP complex by factor Ts. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1016–1022. doi: 10.1016/0006-291x(70)90341-4. [DOI] [PubMed] [Google Scholar]
- Nagata S., Motoyoshi K., Iwasaki K. Interaction of subunits of polypeptide chain elongation factor I from pig liver. Formation of EF-1alpha.EF-1betagamma and EF-1beta complexes. J Biochem. 1978 Feb;83(2):423–429. doi: 10.1093/oxfordjournals.jbchem.a131929. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: additional initiation factor required for formation of ternary complex (eIF-2.GTP.Met-tRNAf) and demonstration of inhibitory effect of heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1079–1083. doi: 10.1073/pnas.76.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mitsui K. I., Ochoa S. Mode of action of the heme-controlled translational inhibitor: relationship of eukaryotic initiation factor 2-stimulating protein to translation restoring factor. Proc Natl Acad Sci U S A. 1981 Jan;78(1):220–223. doi: 10.1073/pnas.78.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Weissbach H., Ochoa S. Soluble factors required for eukaryotic protein synthesis. Annu Rev Biochem. 1976;45:191–216. doi: 10.1146/annurev.bi.45.070176.001203. [DOI] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1741–1745. doi: 10.1073/pnas.76.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor: stimulating protein acts at level of binary complex formation. Proc Natl Acad Sci U S A. 1979 May;76(5):2163–2164. doi: 10.1073/pnas.76.5.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]